Effect of bromhexine in hospitalized patients with COVID-19
et al., J. Investig. Med., doi:10.1136/jim-2020-001747, Mar 2021
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
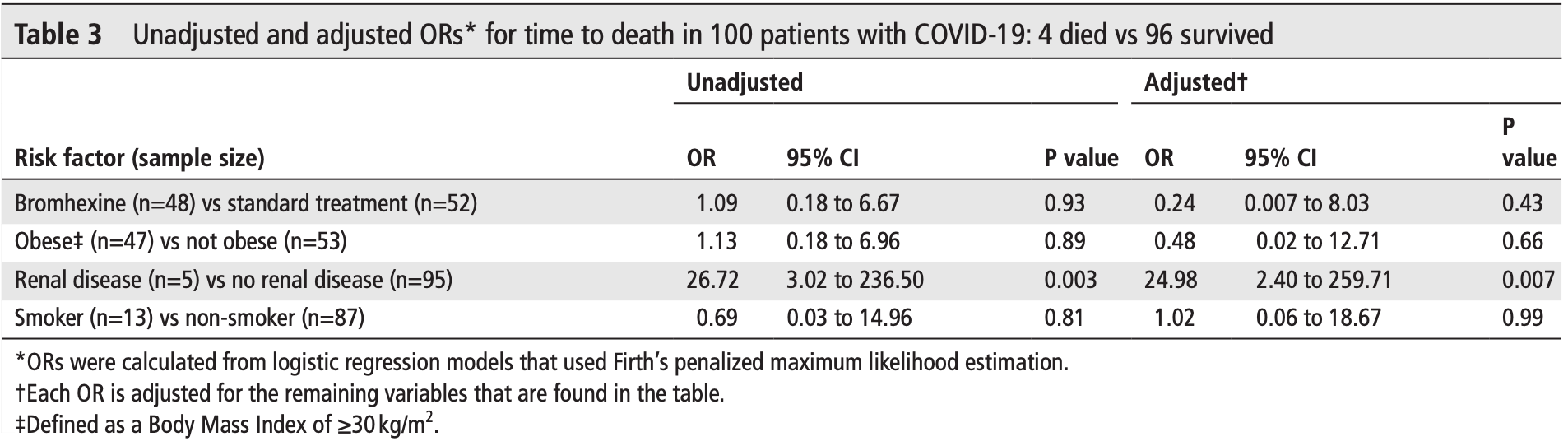
Small RCT with 100 patients, 48 with bromhexine added to SOC, showing slower viral- conversion but lower mortality and greater clinical improvement with bromhexine (not statistically significant with few deaths and very high recovery). The very large difference between unadjusted and adjusted results is due to much higher risk for patients with renal disease and the much higher prevalence of renal disease in the bromhexine group.
The study also shows 90% of patients in the control group had BMI ≥30 compared to 0% in the treatment group, suggesting a possible problem with randomization. Due to the imbalance between groups, results were adjusted for BMI>30, smoking, and renal disease.
11 patients were lost to followup in the treatment group compared to zero in the control group, perhaps in part due to faster recovery in the treatment group. 9 patients were excluded from the treatment group because they did not want to take bromhexine after discharge. Therefore up to 29% of treatment patients may have been excluded because they recovered quickly.
Viral load measured by PCR may not accurately reflect infectious virus measured by viral culture. Porter et al. show that viral load early in infection was correlated with infectious virus, but viral load late in infection could be high even with low or undetectable infectious virus. Assessing viral load later in infection may underestimate reductions in infectious virus with treatment.
Study covers TMPRSS2 inhibitors and bromhexine.
|
risk of death, 76.0% lower, OR 0.24, p = 0.43, treatment 48, control 52, adjusted per study, Table 3, RR approximated with OR.
|
|
risk of no improvement, 75.9% better, OR 0.24, p = 0.43, treatment 48, control 52, adjusted per study, inverted to make OR<1 favor treatment, Table 2, RR approximated with OR.
|
|
risk of no viral clearance, 74.5% higher, RR 1.75, p = 0.02, treatment 29 of 48 (60.4%), control 18 of 52 (34.6%), mid-recovery day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Tolouian et al., 15 Mar 2021, Randomized Controlled Trial, Iran, peer-reviewed, 7 authors.
Effect of bromhexine in hospitalized patients with COVID-19
Journal of Investigative Medicine, doi:10.1136/jim-2020-001747
Background Bromhexine is a potent inhibitor of transmembrane serine protease 2 and appears to have an antiviral effect in controlling influenza and parainfluenza infection; however, its efficacy in COVID-19 is controversial. Methods A group of hospitalized patients with confirmed COVID-19 pneumonia were randomized using 1:1 allocation to either standard treatment lopinavir/ritonavir and interferon beta-1a or bromhexine 8 mg four times a day in addition to standard therapy. The primary outcome was clinical improvement within 28 days, and the secondary outcome measures were time to hospital discharge, all-cause mortality, duration of mechanical ventilation, the temporal trend in 2019-nCoV reverse transcription-polymerase chain reaction positivity and the frequency of adverse drug events within 28 days from the start of medication. Results A total of 111 patients were enrolled in this randomized clinical trial and data from 100 patients (48 patients in the treatment arm and 52 patients in the control arm) were analyzed. There was no significant difference in the primary outcome of this study, which was clinical improvement. There was no significant difference in the average time to hospital discharge between the two arms. There were also no differences observed in the mean intensive care unit stay, frequency of intermittent mandatory ventilation, duration of supplemental oxygenation or risk of death by day 28 noted between the two arms. Conclusion Bromhexine is not an effective treatment for hospitalized patients with COVID-19. The potential prevention benefits of bromhexine in asymptomatic postexposure or with mild infection managed in the community remain to be determined.
Competing interests None declared. Patient consent for publication Not required.
Ethics approval The study was approved by the ethics committee of the Shahid Beheshti University of Medical Sciences, Tehran, Iran (IR.SBMU.NRITLD. REC.1399.142). Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement All data relevant to the study are included in the article or uploaded as supplementary information. The deidentified subject data are available in a secure space under the control of corresponding author [ fzh. dastan@ gmail. com]. This article is made freely available for use in accordance with BMJ's website terms and conditions for the duration of the covid-19 pandemic or until otherwise determined by BMJ. You may use, download and print the article for any lawful, non-commercial purpose (including text and data mining) provided that all copyright notices and trade marks are retained.
ORCID iDs Ramin Tolouian http:// orcid. org/ 0000-0003-2242-9310 Zuber D Mulla http:// orcid. org/ 0000-0003-1670-5702
References
Ansarin, Tolouian, Ardalan, Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: a randomized clinical trial, BioImpacts
Barzegar, Ghadipasha, Rezaei, New hope for treatment of respiratory involvement following COVID-19 by bromhexine, J Nephropharmacol
Böttcher, Matrosovich, Beyerle, Proteolytic activation of influenza viruses by serine proteases TMPRSS2 and HAT from human airway epithelium, J Virol, doi:10.1128/JVI.01118-06
Chegini, Bolurian, Mojtahed, High risk individuals in COVID-19 pandemic; an updated review, Immunopathol Persa
Cheng, Zhou, To, Identification of TMPRSS2 as a Susceptibility Gene for Severe 2009 Pandemic A(H1N1) Influenza and A(H7N9) Influenza, J Infect Dis, doi:10.1093/infdis/jiv246
Depfenhart, De Villiers, Lemperle, Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy?, Intern Emerg Med, doi:10.1007/s11739-020-02383-3
Fernandez, Mulla, Avoiding sparse data bias: an example from gynecologic oncology, J Registry Manag
Flythe, Assimon, Tugman, Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States, Am J Kidney Dis, doi:10.1053/j.ajkd.2020.09.003
Gansevoort, Hilbrands, CKD is a key risk factor for COVID-19 mortality, Nat Rev Nephrol, doi:10.1038/s41581-020-00349-4
Glowacka, Bertram, Müller, Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response, J Virol, doi:10.1128/JVI.02232-10
Group, Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, The Lancet
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hörnich, Großkopf, Schlagowski, SARS-CoV-2 differs from SARS-CoV in the requirements for receptor expression and proteolytic activation to trigger cell-cell fusion and is not inhibited by bromhexine
Jahromi, Mazloom, Ballard, What the European and American health care systems can learn from China COVID-19 epidemic; action planning using purpose designed medical telecommunication, courier services, home-based quarantine, and COVID-19 walk-in centers, Immunopathol Persa, doi:10.34172/ipp.2020.17
Karanicolas, Farrokhyar, Bhandari, Practical tips for surgical research: blinding: who, what, when, why, how?, Can J Surg
Pan, Peto, WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for Covid-19 -Interim WHO Solidarity Trial Results, N Engl J Med, doi:10.1056/NEJMoa2023184
Ray, Schull, Vermeulen, Association Between ABO and Rh Blood Groups and SARS-CoV-2 Infection or Severe COVID-19 Illness : A Population-Based Cohort Study, Ann Intern Med
Sagawa, Inoue, Takano, Use of protease inhibitors for the prevention of COVID-19, Prev Med, doi:10.1016/j.ypmed.2020.106280
Tartof, Qian, Hong, Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization, Ann Intern Med, doi:10.7326/M20-3742
Tolouian, Mulla, Controversy with bromhexine in COVID-19; where we stand, Immunopathol Persa
Tolouian, Tolouian, Ardalan, Blocking serine protease (TMPRSS2) by bromhexine; looking at potential treatment to prevent COVID-19 infection, Marshall Journal of Medicine, doi:10.33470/2379-9536.1286
Zanasi, Mazzolini, Kantar, A reappraisal of the mucoactive activity and clinical efficacy of bromhexine, Multidiscip Respir Med, doi:10.1186/s40248-017-0088-1
DOI record:
{
"DOI": "10.1136/jim-2020-001747",
"ISSN": [
"1081-5589",
"1708-8267"
],
"URL": "http://dx.doi.org/10.1136/jim-2020-001747",
"abstract": "<jats:p> Background: Bromhexine is a potent inhibitor of transmembrane serine protease 2 and appears to have an antiviral effect in controlling influenza and parainfluenza infection; however, its efficacy in COVID-19 is controversial. </jats:p><jats:p> Methods: A group of hospitalized patients with confirmed COVID-19 pneumonia were randomized using 1:1 allocation to either standard treatment lopinavir/ritonavir and interferon beta-1a or bromhexine 8 mg four times a day in addition to standard therapy. The primary outcome was clinical improvement within 28 days, and the secondary outcome measures were time to hospital discharge, all-cause mortality, duration of mechanical ventilation, the temporal trend in 2019-nCoV reverse transcription-polymerase chain reaction positivity and the frequency of adverse drug events within 28 days from the start of medication. </jats:p><jats:p> Results: A total of 111 patients were enrolled in this randomized clinical trial and data from 100 patients (48 patients in the treatment arm and 52 patients in the control arm) were analyzed. There was no significant difference in the primary outcome of this study, which was clinical improvement. There was no significant difference in the average time to hospital discharge between the two arms. There were also no differences observed in the mean intensive care unit stay, frequency of intermittent mandatory ventilation, duration of supplemental oxygenation or risk of death by day 28 noted between the two arms. </jats:p><jats:p> Conclusion: Bromhexine is not an effective treatment for hospitalized patients with COVID-19. The potential prevention benefits of bromhexine in asymptomatic postexposure or with mild infection managed in the community remain to be determined. </jats:p>",
"alternative-id": [
"10.1136/jim-2020-001747"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-2242-9310",
"affiliation": [
{
"name": "Renal Section, Southern Arizona VA Health Care System, University of Arizona, Tucson, Arizona, USA"
}
],
"authenticated-orcid": false,
"family": "Tolouian",
"given": "Ramin",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-1670-5702",
"affiliation": [
{
"name": "Department of Obstetrics and Gynecology, and Office of Faculty Development, Paul L. Foster School of Medicine, Texas Tech University Health Sciences Center El Paso, El Paso, Texas, USA"
}
],
"authenticated-orcid": false,
"family": "Mulla",
"given": "Zuber D",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Chronic Respiratory Diseases Research Center (CRDRC), National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran"
}
],
"family": "Jamaati",
"given": "Hamidreza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases and Tropical Medicine Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran"
}
],
"family": "Babamahmoodi",
"given": "Abdolreza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Tuberculosis and Epidemiology Research Center, National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Masih Daneshvari Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran"
}
],
"family": "Marjani",
"given": "Majid",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Chronic Respiratory Diseases Research Center (CRDRC), National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran"
}
],
"family": "Eskandari",
"given": "Raha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Pharmacy, School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran"
}
],
"family": "Dastan",
"given": "Farzaneh",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04355026",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "Journal of Investigative Medicine",
"container-title-short": "Journal of Investigative Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2021,
3,
15
]
],
"date-time": "2021-03-15T15:40:14Z",
"timestamp": 1615822814000
},
"deposited": {
"date-parts": [
[
2023,
10,
12
]
],
"date-time": "2023-10-12T10:23:16Z",
"timestamp": 1697106196000
},
"indexed": {
"date-parts": [
[
2024,
3,
16
]
],
"date-time": "2024-03-16T23:56:11Z",
"timestamp": 1710633371829
},
"is-referenced-by-count": 11,
"issue": "7",
"issued": {
"date-parts": [
[
2023,
10
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2023,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://journals.sagepub.com/page/policies/text-and-data-mining-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
1
]
],
"date-time": "2023-10-01T00:00:00Z",
"timestamp": 1696118400000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1136/jim-2020-001747",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1136/jim-2020-001747",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1136/jim-2020-001747",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"page": "691-699",
"prefix": "10.1177",
"published": {
"date-parts": [
[
2023,
10
]
]
},
"published-online": {
"date-parts": [
[
2023,
10,
12
]
]
},
"published-print": {
"date-parts": [
[
2023,
10
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.34172/ipp.2020.17",
"doi-asserted-by": "publisher",
"key": "bibr1-jim-2020-001747"
},
{
"DOI": "10.1186/s40248-017-0088-1",
"doi-asserted-by": "publisher",
"key": "bibr2-jim-2020-001747"
},
{
"DOI": "10.1128/JVI.01118-06",
"doi-asserted-by": "publisher",
"key": "bibr3-jim-2020-001747"
},
{
"DOI": "10.1093/infdis/jiv246",
"doi-asserted-by": "publisher",
"key": "bibr4-jim-2020-001747"
},
{
"DOI": "10.33470/2379-9536.1286",
"doi-asserted-by": "publisher",
"key": "bibr5-jim-2020-001747"
},
{
"DOI": "10.1016/j.ypmed.2020.106280",
"doi-asserted-by": "publisher",
"key": "bibr6-jim-2020-001747"
},
{
"author": "Barzegar A",
"journal-title": "J Nephropharmacol",
"key": "bibr7-jim-2020-001747",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1007/s11739-020-02383-3",
"doi-asserted-by": "publisher",
"key": "bibr8-jim-2020-001747"
},
{
"DOI": "10.1128/JVI.02232-10",
"doi-asserted-by": "publisher",
"key": "bibr9-jim-2020-001747"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "bibr10-jim-2020-001747"
},
{
"DOI": "10.34172/bi.2020.27",
"doi-asserted-by": "publisher",
"key": "bibr11-jim-2020-001747"
},
{
"author": "Fernandez NP",
"journal-title": "J Registry Manag",
"key": "bibr12-jim-2020-001747",
"volume": "39",
"year": "2012"
},
{
"DOI": "10.7326/M20-3742",
"doi-asserted-by": "publisher",
"key": "bibr13-jim-2020-001747"
},
{
"author": "Chegini R",
"journal-title": "Immunopathol Persa",
"key": "bibr14-jim-2020-001747",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1053/j.ajkd.2020.09.003",
"doi-asserted-by": "publisher",
"key": "bibr15-jim-2020-001747"
},
{
"DOI": "10.1038/s41581-020-00349-4",
"doi-asserted-by": "publisher",
"key": "bibr16-jim-2020-001747"
},
{
"author": "Ray JG",
"journal-title": "Ann Intern Med",
"key": "bibr17-jim-2020-001747",
"year": "2020"
},
{
"author": "Group RC",
"journal-title": "The Lancet",
"key": "bibr18-jim-2020-001747",
"volume": "396",
"year": "2020"
},
{
"author": "Pan H",
"journal-title": "N Engl J Med",
"key": "bibr19-jim-2020-001747",
"volume": "384",
"year": "2021"
},
{
"author": "Tolouian R",
"journal-title": "Immunopathol Persa",
"key": "bibr20-jim-2020-001747",
"volume": "7",
"year": "2021"
},
{
"author": "Hörnich B",
"journal-title": "July",
"key": "bibr21-jim-2020-001747",
"year": "2020"
},
{
"author": "Karanicolas PJ",
"journal-title": "Can J Surg",
"key": "bibr22-jim-2020-001747",
"volume": "53",
"year": "2010"
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "http://journals.sagepub.com/doi/10.1136/jim-2020-001747"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Biochemistry, Genetics and Molecular Biology",
"General Medicine"
],
"subtitle": [],
"title": "Effect of bromhexine in hospitalized patients with COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "71"
}
