Camostat mesylate therapy in critically ill patients with COVID-19 pneumonia
et al., Intensive Care Medicine, doi:10.1007/s00134-021-06395-1, Apr 2021
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 371 critically ill COVID-19 patients showing lower mortality with camostat mesylate treatment.
Study covers TMPRSS2 inhibitors and camostat.
|
risk of death, 69.0% lower, HR 0.31, p < 0.001, treatment 6 of 61 (9.8%), control 18 of 61 (29.5%), NNT 5.1, adjusted per study, propensity score matching, multivariable, Cox proportional hazards.
|
|
risk of mechanical ventilation, 10.0% lower, RR 0.90, p = 1.00, treatment 9 of 61 (14.8%), control 10 of 61 (16.4%), NNT 61, propensity score matching.
|
|
hospitalization time, 16.7% higher, relative time 1.17, p = 0.35, treatment 61, control 61, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sakr et al., 12 Apr 2021, retrospective, Germany, peer-reviewed, 11 authors, study period 16 March, 2020 - 19 July, 2020.
Contact: yasser.sakr@med.uni-jena.de (corresponding author).
Abstract: Intensive Care Med (2021) 47:707–709
https://doi.org/10.1007/s00134-021-06395-1
LETTER
Camostat mesylate therapy in critically ill
patients with COVID‑19 pneumonia
Yasser Sakr1* , Hatim Bensasi2, Ahmed Taha3, Michael Bauer1 and Khaled Ismail2 on behalf of the UAE-Jena
Research Group
© 2021 The Author(s)
Dear Editor,
Camostat mesylate inhibits several serine proteases
implicated in SARSCoV and SARS-CoV-2 virus-to-host
cell membrane fusion, such as transmembrane serine
protease (TMPRSS) 2, − 13, and − 11D/E/F [1–3]. In particular, inhibition of the SARS-CoV-2-activating host cell
TMPRSS2 have been shown to block SARS-CoV-2 entry
into the lung cells and represents, therefore, a possible
therapeutic option in patients with coronavirus disease
2019 (COVID-19) [4]. A preliminary observation suggested that camostat mesylate may be also effective to
treat the most advanced cases of COVID-19 with organ
dysfunction [5]; however, randomized clinical trials are
ongoing.
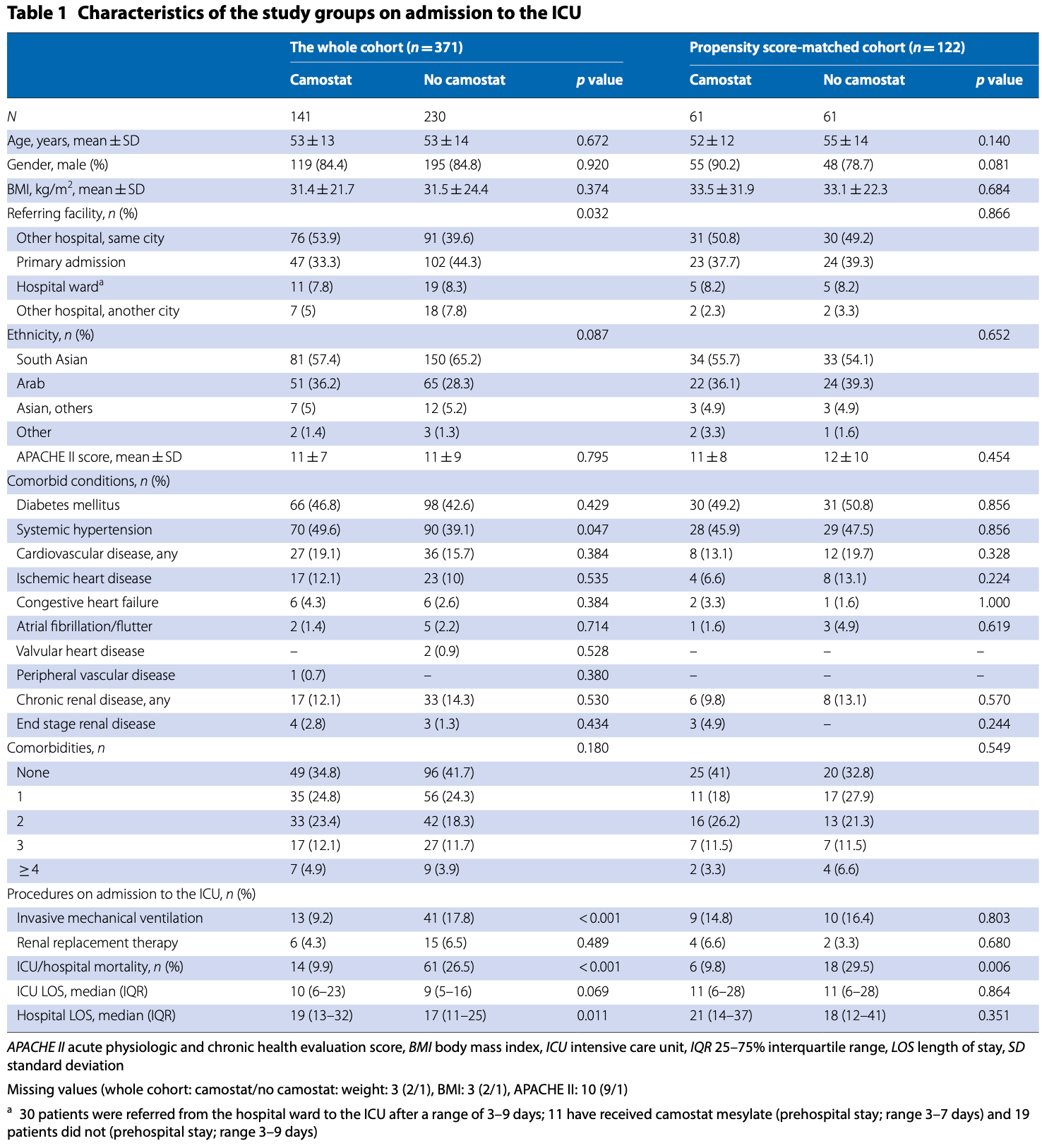
In a retrospective analysis of 371 adult patients
(> 18 years) admitted to the intensive care unit (ICU)
of Al Ain Hospital, Abu Dhabi, United Arabs Emirates
between March 16 and July 19, 2020 with COVID-19
pneumonia, we assessed whether treatment with camostat mesylate is associated with an improved outcome
(Figure S1–2, Supplementary material). Details of data
collection, patients’ management, and statistical methods
are presented in appendix S1 of the supplementary material. Off-label camostat mesylate (Foipan®, Osaka, Japan)
was given to 141 (38%) patients on admission to the ICU
(200 mg po TID) for 7 days (Table 1 and table S1–2 of the
Supplementary material). The overall ICU and hospital
*Correspondence: yasser.sakr@med.uni-jena.de
1
Department of Anesthesiology and Intensive Care Medicine, Jena
University Hospital, Am Klinikum 1, 07743 Jena, Germany
Full author information is available at the end of the article
The members of UAE-Jena Research Group are listed in the
acknowledgements.
lengths of stay were 9 (25–75% interquartile range 5–17)
and 18 (25–75% interquartile range 13–29) days, respectively, and ICU and hospital mortality rates were both
20.2% (n = 75). ICU/hospital mortality rate were lower
(9.9 vs. 26.5, p < 0.001); whereas, the hospital length
of stay was longer in patients who received camostat
mesylate than who did not (Table 1).
In a propensity score-adjusted multivariable Cox proportional hazard analysis in the whole cohort, camostat
mesylate therapy was independently associated with a
lower risk of in-hospital death, right censored at 60 days
(relative hazard 0.31, 95% confidence interval 0.15–0.60,
p = 0.001; table S3 and figure S3 of the supplementary
material). Moreover, after inversed propensity treatment
weight (IPTW)-adjustment and robust estimation using
generalized estimating equations, camostat mesylate
therapy was found to be independently associated with a
lower risk of in-hospital death (odds ratio 0.254; 95% confidence interval 0.108–0.595, p < 0.001). In 122 propensity
score-matched patients (61 pairs), ICU/hospital mortality rates (9.8 vs. 29.5, p = 0.006), the need for vasopressor
therapy (45.9 vs. 67.2%, p = 0.018) or invasive mechanical
ventilation (47.5 vs. 67.2%, p = 0.045) during the ICU stay
were lower; whereas, 60-day survival was higher (log rank
Chi2 = 18.6, p < 0.001) in patients treated with camostat
mesylate than those who were not (Table 1, tables S1–3
and figure S4 of the supplementary material). Nonetheless, despite of..
DOI record:
{
"DOI": "10.1007/s00134-021-06395-1",
"ISSN": [
"0342-4642",
"1432-1238"
],
"URL": "http://dx.doi.org/10.1007/s00134-021-06395-1",
"alternative-id": [
"6395"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "27 January 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "26 March 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "12 April 2021"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Conflicts of interest",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The authors declare that they do not have conflict of interests in relation to this manuscript."
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The off-label use of camostat mesylate was recommended by the Department of Health, Abu Dhabi (PO box 5674, Abu Dhabi, UAE). Informed consents for the off-label use of camostat mesylate were obtained on admission to the hospital according to the guidelines of UAE-Ministry of health. The analysis provided in the current manuscript was done retrospectively after obtaining the approval of the responsible institutional review board (Institutional review board of Department of Health, Abu Dhabi, PO box 5674, Abu Dhabi, UAE, application number: DOH/CVDC/2020/1669, August 8th 2020), which waived informed consent for data collection and analysis due to the retrospective nature of data collection."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-3163-1334",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sakr",
"given": "Yasser",
"sequence": "first"
},
{
"affiliation": [],
"family": "Bensasi",
"given": "Hatim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taha",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bauer",
"given": "Michael",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ismail",
"given": "Khaled",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belhaj",
"given": "Ghazala",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Afet",
"given": "Khaled M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Munde",
"given": "Dnyanwshwar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Monk",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buschbeck",
"given": "Samuel",
"sequence": "additional"
},
{
"affiliation": [],
"name": "the UAE-Jena Research Group",
"sequence": "additional"
}
],
"container-title": "Intensive Care Medicine",
"container-title-short": "Intensive Care Med",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
4,
12
]
],
"date-time": "2021-04-12T19:02:57Z",
"timestamp": 1618254177000
},
"deposited": {
"date-parts": [
[
2021,
6,
11
]
],
"date-time": "2021-06-11T08:03:13Z",
"timestamp": 1623398593000
},
"funder": [
{
"DOI": "10.13039/501100007653",
"doi-asserted-by": "crossref",
"name": "Universitätsklinikum Jena"
}
],
"indexed": {
"date-parts": [
[
2023,
8,
20
]
],
"date-time": "2023-08-20T02:38:15Z",
"timestamp": 1692499095399
},
"is-referenced-by-count": 17,
"issue": "6",
"issued": {
"date-parts": [
[
2021,
4,
12
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2021,
6
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
4,
12
]
],
"date-time": "2021-04-12T00:00:00Z",
"timestamp": 1618185600000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
4,
12
]
],
"date-time": "2021-04-12T00:00:00Z",
"timestamp": 1618185600000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1007/s00134-021-06395-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1007/s00134-021-06395-1/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1007/s00134-021-06395-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"page": "707-709",
"prefix": "10.1007",
"published": {
"date-parts": [
[
2021,
4,
12
]
]
},
"published-online": {
"date-parts": [
[
2021,
4,
12
]
]
},
"published-print": {
"date-parts": [
[
2021,
6
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.ebiom.2021.103255",
"author": "M Hoffmann",
"doi-asserted-by": "publisher",
"journal-title": "EBioMedicine",
"key": "6395_CR1",
"unstructured": "Hoffmann M, Hofmann-Winkler H, Smith JC, Kruger N, Sorensen LK, Sogaard OS, Hasselstrom JB, Winkler M, Hempel T, Raich L, Olsson S, Yamazoe T, Yamatsuta K, Mizuno H, Ludwig S, Noe F, Sheltzer JM, Kjolby M, Pohlmann S (2021) Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine https://doi.org/10.1016/j.ebiom.2021.103255",
"year": "2021"
},
{
"DOI": "10.1128/JVI.01890-13",
"author": "K Shirato",
"doi-asserted-by": "publisher",
"first-page": "12552",
"journal-title": "J Virol",
"key": "6395_CR2",
"unstructured": "Shirato K, Kawase M, Matsuyama S (2013) Middle east respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J Virol 87:12552–12561",
"volume": "87",
"year": "2013"
},
{
"DOI": "10.1128/JVI.00094-12",
"author": "M Kawase",
"doi-asserted-by": "publisher",
"first-page": "6537",
"journal-title": "J Virol",
"key": "6395_CR3",
"unstructured": "Kawase M, Shirato K, van der Hoek L, Taguchi F, Matsuyama S (2012) Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol 86:6537–6545",
"volume": "86",
"year": "2012"
},
{
"author": "M Hoffmann",
"first-page": "e278",
"issue": "271–280",
"journal-title": "Cell",
"key": "6395_CR4",
"unstructured": "Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(271–280):e278",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1097/CCE.0000000000000284",
"author": "H Hofmann-Winkler",
"doi-asserted-by": "publisher",
"first-page": "e0284",
"journal-title": "Crit Care Explor",
"key": "6395_CR5",
"unstructured": "Hofmann-Winkler H, Moerer O, Alt-Epping S, Brauer A, Buttner B, Muller M, Fricke T, Grundmann J, Harnisch LO, Heise D, Kernchen A, Pressler M, Stephani C, Tampe B, Kaul A, Gartner S, Kramer S, Pohlmann S, Winkler MS (2020) Camostat mesylate may reduce severity of Coronavirus disease 2019 sepsis: a first observation. Crit Care Explor 2:e0284",
"volume": "2",
"year": "2020"
}
],
"reference-count": 5,
"references-count": 5,
"relation": {},
"resource": {
"primary": {
"URL": "https://link.springer.com/10.1007/s00134-021-06395-1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Critical Care and Intensive Care Medicine"
],
"subtitle": [],
"title": "Camostat mesylate therapy in critically ill patients with COVID-19 pneumonia",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "47"
}
