RAndomized Clinical Trial Of NAfamostat Mesylate, A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia
et al., Journal of Clinical Medicine, doi:10.3390/jcm12206618, RACONA, NCT04352400, Oct 2023
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 15 hospitalized COVID-19 patients showing a positive safety profile with nafamostat mesylate treatment. While the study was underpowered to detect differences in efficacy, Bayesian analysis suggested a signal for potential benefit (69-88% probability that nafamostat is effective depending on prior assumptions). Authors note that nafamostat, as a potent TMPRSS2 inhibitor with anticoagulant properties, could theoretically prevent virus entry into cells and complications like disseminated intravascular coagulation in COVID-19 patients. The study was significantly limited by small sample size due to recruitment challenges.
Study covers TMPRSS2 inhibitors and nafamostat.
|
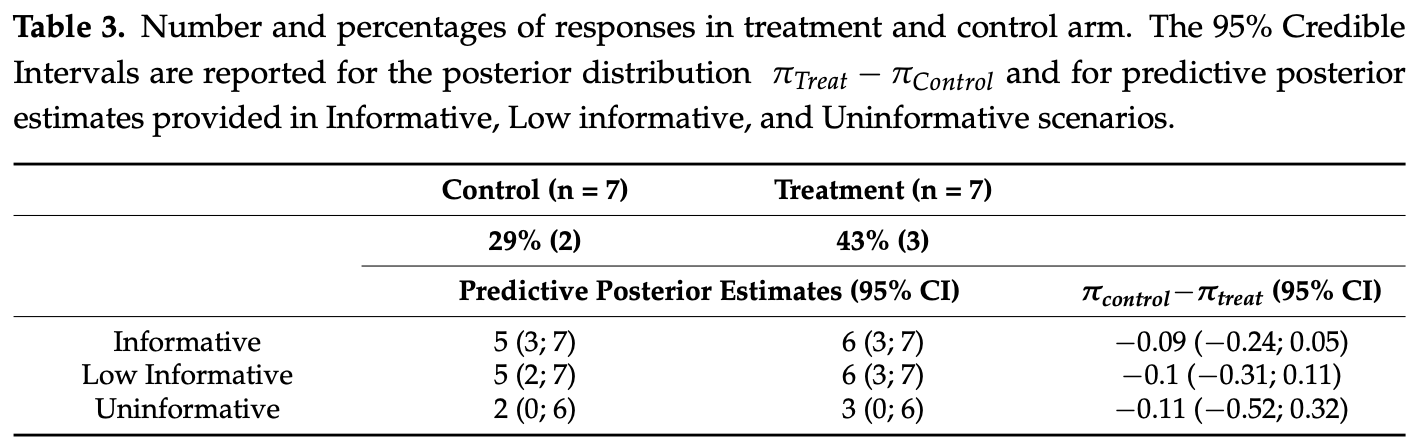
risk of death, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 7 (0.0%), control 1 of 7 (14.3%), NNT 7.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of no recovery, 20.0% lower, RR 0.80, p = 1.00, treatment 4 of 7 (57.1%), control 5 of 7 (71.4%), NNT 7.0, no improvement in WHO score of 2+ points.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Seccia et al., 19 Oct 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Italy, peer-reviewed, 15 authors, study period June 2021 - August 2022, trial NCT04352400 (history) (RACONA).
Contact: gianpaolo.rossi@unipd.it (corresponding author), teresamaria.seccia@unipd.it, tungalagtamir.shagjaa@studenti.unipd.it, sangaviola.md@gmail.com, margherita.morpurgo@unipd.it, sonia.faoro@aopd.veneto.it, francesca.venturini@aopd.veneto.it, girolama.iadicicco@aopd.veneto.it, sara.lococo@aopd.veneto.it, maria.mazzitelli@aopd.veneto.it, filippo.farnia@gmail.com, paola.fioretto@unipd.it, yusuke.kob@live.jp, dario.gregori@unipd.it.
RAndomized Clinical Trial Of NAfamostat Mesylate, A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia
Journal of Clinical Medicine, doi:10.3390/jcm12206618
Even though SARS-CoV-2 was declared by WHO as constituting no longer a public health emergency, the development of effective treatments against SARS-CoV-2 infection remains a critical issue to prevent complications, particularly in fragile patients. The protease inhibitor nafamostat, currently used in Japan and Korea for pancreatitis, owing to its anticoagulant properties for disseminated intravascular coagulation (DIC), is appealing for the treatment of COVID-19 infection, because it potently inhibits the transmembrane protease serine 2 (TMPRSS2) that, after virus binding to ACE-2, allows virus entry into the cells and replication. Moreover, it could prevent the DIC and pulmonary embolism frequently associated with COVID-19 infection. The goal of the RAndomized Clinical Trial Of NAfamostat (RACONA) study, designed as a prospective randomized, double-blind placebo-controlled clinical trial, was to investigate the efficacy and safety of nafamostat mesylate (0.10 mg/kg/h iv for 7 days), on top of the optimal treatment, in COVID-19 hospitalized patients. We could screen 131 patients, but due to the predefined strict inclusion and exclusion criteria, only 15 could be randomized to group 1 (n = 7) or group 2 (n = 8). The results of an ad interim safety analysis showed similar overall trends for variables evaluating renal function, coagulation, and inflammation. No adverse events, including hyperkalemia, were found to be associated with nafamostat. Thus, the RACONA study showed a good safety profile of nafamostat, suggesting that it could be usefully used in COVID-19 hospitalized patients.
Conflicts of Interest: The authors declare no conflict of interest.
References
Albert, Bayesian Computation with R
Aoyama, Ino, Ozeki, Oda, Sato et al., Pharmacological studies of FUT-175, nafamstat mesilate. I. Inhibition of protease activity in in vitro and in vivo experiments, Jpn. J. Pharmacol, doi:10.1254/jjp.35.203
Barry, Doing Bayesian Data Analysis: A Tutorial with R and BUGS, Eur. J. Psychol, doi:10.5964/ejop.v7i4.163
Botton, Jabagi, Bertrand, Baricault, Drouin et al., Risk for Myocardial Infarction, Stroke, and Pulmonary Embolism Following COVID-19 Vaccines in Adults Younger Than 75 Years in France, Ann. Intern. Med, doi:10.7326/M22-0988
Broglio, Randomization in clinical trials: Permuted blocks and stratification, JAMA, doi:10.1001/jama.2018.6360
Cao, Zhang, Yu, Shao, Chen et al., A method for quantifying the unstable and highly polar drug nafamostat mesilate in human plasma with optimized solid-phase extraction and ESI-MS detection: More accurate evaluation for pharmacokinetic study, Anal. Bioanal. Chem, doi:10.1007/s00216-008-2054-4
Chen, Ibrahim, Power prior distributions for regression models, Stat. Sci, doi:10.1214/ss/1009212673
Choi, Kang, Jang, Jung, Cho et al., Nafamostat Mesilate as an Anticoagulant during Continuous Renal Replacement Therapy in Patients with High Bleeding Risk: A Randomized Clinical Trial, Medicine, doi:10.1097/MD.0000000000002392
Chuenkitmongkol, Solante, Burhan, Chariyalertsak, Chiu et al., Expert review on global real-world vaccine effectiveness against SARS-CoV-2, Expert. Rev. Vaccines, doi:10.1080/14760584.2022.2092472
Dhawan, Sun Pharma Laboratories Limited
Eslamifar, Behzadifard, Soleimani, Behzadifard, Coagulation abnormalities in SARS-CoV-2 infection: Overexpression tissue factor, Thromb. J, doi:10.1186/s12959-020-00250-x
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hoffmann, Schroeder, Kleine-Weber, Müller, Drosten et al., Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19, Antimicrob. Agents Chemother, doi:10.1128/AAC.00754-20
Inokuchi, Kuno, Komiyama, Uda, Miyamoto et al., Association between Nafamostat Mesylate and In-Hospital Mortality in Patients with Coronavirus Disease 2019: A Multicenter Observational Study, J. Clin. Med, doi:10.3390/jcm11010116
Isgrò, Trillò, Russo, Tornesello, Buonaguro et al., Bimodal antibody-titer decline following BNT162b2 mRNA anti-SARS-CoV-2 vaccination in healthcare workers of the INT-IRCCS "Fondazione Pascale, Infect. Agents Cancer, doi:10.1186/s13027-022-00451-1
Iwashita, Kitamura, Narikiyo, Adachi, Shiraishi et al., Inhibition of prostasin secretion by serine protease inhibitors in the kidney, J. Am. Soc. Nephrol, doi:10.1097/01.ASN.0000043900.39397.48
Kastenhuber, Mercadante, Nilsson-Payant, Johnson, Jaimes et al., Coagulation factors directly cleave SARS-CoV-2 spike and enhance viral entry, Elife, doi:10.7554/eLife.77444
Kawasaki, Shimokawa, Miyaoka, Comparison of Three Calculation Methods for a Bayesian Inference of P(π1 > π2), J. Mod. Appl. Stat. Methods, doi:10.22237/jmasm/1383279240
Keaven Anderson, gsDesign: Group Sequential Design. 30-1
Kim, Korea, None
Knight, Walker, Ip, Cooper, Bolton et al., Association of COVID-19 with Major Arterial and Venous Thrombotic Diseases: A Population-Wide Cohort Study of 48 Million Adults in England and Wales, Circulation, doi:10.1161/CIRCULATIONAHA.122.060785
Lachin, Foulkes, Evaluation of Sample Size and Power for Analyses of Survival with Allowance for Nonuniform Patient Entry, Losses to Follow-Up, Noncompliance, and Stratification, Biometrics, doi:10.2307/2531201
Lesaffre, The Randomized Controlled Trial: Methodological Perspectives
Lunn, Spiegelhalter, Thomas, Best, The BUGS project: Evolution, critique and future directions, Stat. Med, doi:10.1002/sim.3680
Maruyama, Yoshida, Uchino, Yokoyama, Yamamoto et al., Nafamostat mesilate as an anticoagulant during continuous veno-venous hemodialysis: A three-year retrospective cohort study, Int. J. Artif. Organs, doi:10.5301/IJAO.2011.8535
Minakata, Fujiwara, Ikeda, Kawaguchi, Toda et al., Comparison of gabexate mesilate and nafamostat mesilate for disseminated intravascular coagulation associated with hematological malignancies, Int. J. Hematol, doi:10.1007/s12185-018-02567-w
Muto, Imai, Asano, Mechanisms of hyperkalemia caused by nafamostat mesilate, Gen. Pharmacol, doi:10.1016/0306-3623(95)00072-0
Nimishakavi, Raymond, Gruenert, Caughey, Divergent Inhibitor Susceptibility among Airway Lumen-Accessible Tryptic Proteases, PLoS ONE, doi:10.1371/journal.pone.0141169
Ota, Hara, Kanoh, Shinoda, Kawano et al., Acute eosinophilic pneumonia caused by camostat mesilate: The first case report, Respir. Med. Case Rep, doi:10.1016/j.rmcr.2016.06.005
Quinn, Gaughan, Bruce, Antonelli, O'connor et al., Randomised controlled trial of intravenous nafamostat mesylate in COVID pneumonitis: Phase 1b/2a experimental study to investigate safety, Pharmacokinetics and Pharmacodynamics, EBio Med, doi:10.1016/j.ebiom.2022.103856
Rossi, Sanga, Barton, Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients, eLife, doi:10.7554/eLife.57278
Soma, Fujii, Yoshifuji, Maruki, Itoh et al., Nafamostat Mesylate Monotherapy in Patients with Moderate COVID-19: A Single-Center, Retrospective Study, Jpn. J. Infect. Dis, doi:10.7883/yoken.JJID.2021.699
Team, A Language and Environment for Statistical Computing
Uchiba, Okajima, Abe, Okabe, Takatsuki, Effect of nafamostat mesilate, a synthetic protease inhibitor, on tissue factor-factor VIIa complex activity, Thromb. Res, doi:10.1016/0049-3848(94)90008-6
Vaduganathan, Vardeny, Michel, Mcmurray, Pfeffer et al., Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19, N. Engl. J. Med, doi:10.1056/NEJMsr2005760
Voigtlaender, Edler, Gerling, Schädler, Ondruschka et al., Thromboembolic events in deceased patients with proven SARS-CoV-2 infection: Frequency, characteristics and risk factors, Thromb. Res, doi:10.1016/j.thromres.2022.08.021
Xie, Prats-Uribe, Feng, Wang, Gill et al., Clinical and Genetic Risk Factors for Acute Incident Venous Thromboembolism in Ambulatory Patients with COVID-19, JAMA Intern. Med, doi:10.1001/jamainternmed.2022.3858
Yamamoto, Matsuyama, Li, Takeda, Kawaguchi et al., Identification of Nafamostat as a Potent Inhibitor of Middle East Respiratory Syndrome Coronavirus S Protein-Mediated Membrane Fusion Using the Split-Protein-Based Cell-Cell Fusion Assay, Antimicrob. Agents Chemother, doi:10.1128/AAC.01043-16
Yamaori, Fujiyama, Kushihara, Funahashi, Kimura et al., Involvement of Human Blood Arylesterases and Liver Microsomal Carboxylesterases in Nafamostat Hydrolysis, Drug Metab. Pharmacokinet, doi:10.2133/dmpk.21.147
Yang, Xie, Li, Understanding the mechanisms for COVID-19 vaccine's protection against infection and severe disease, Expert. Rev. Vaccines, doi:10.1080/14760584.2023.2174529
Yu, Li, Wan, Wang, Hu, Nafamostat mesilate for prevention of post-ERCP pancreatitis: A meta-analysis of prospective, randomized, controlled trials, Pancreas, doi:10.1097/MPA.0000000000000310
Zhang, Zhu, Cai, Lei, Qin et al., Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients with Hypertension Hospitalized With COVID-19, Circ. Res, doi:10.1161/CIRCRESAHA.120.317134
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
Zhuravel, Khmelnitskiy, Burlaka, Gritsan, Goloshchekin et al., Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: A randomised Phase II clinical trial, E Clin. Med, doi:10.1016/j.eclinm.2021.101169
DOI record:
{
"DOI": "10.3390/jcm12206618",
"ISSN": [
"2077-0383"
],
"URL": "http://dx.doi.org/10.3390/jcm12206618",
"abstract": "<jats:p>Even though SARS-CoV-2 was declared by WHO as constituting no longer a public health emergency, the development of effective treatments against SARS-CoV-2 infection remains a critical issue to prevent complications, particularly in fragile patients. The protease inhibitor nafamostat, currently used in Japan and Korea for pancreatitis, owing to its anticoagulant properties for disseminated intravascular coagulation (DIC), is appealing for the treatment of COVID-19 infection, because it potently inhibits the transmembrane protease serine 2 (TMPRSS2) that, after virus binding to ACE-2, allows virus entry into the cells and replication. Moreover, it could prevent the DIC and pulmonary embolism frequently associated with COVID-19 infection. The goal of the RAndomized Clinical Trial Of NAfamostat (RACONA) study, designed as a prospective randomized, double-blind placebo-controlled clinical trial, was to investigate the efficacy and safety of nafamostat mesylate (0.10 mg/kg/h iv for 7 days), on top of the optimal treatment, in COVID-19 hospitalized patients. We could screen 131 patients, but due to the predefined strict inclusion and exclusion criteria, only 15 could be randomized to group 1 (n = 7) or group 2 (n = 8). The results of an ad interim safety analysis showed similar overall trends for variables evaluating renal function, coagulation, and inflammation. No adverse events, including hyperkalemia, were found to be associated with nafamostat. Thus, the RACONA study showed a good safety profile of nafamostat, suggesting that it could be usefully used in COVID-19 hospitalized patients.</jats:p>",
"alternative-id": [
"jcm12206618"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-3639-4086",
"affiliation": [
{
"name": "Internal Emergency Medicine Unit, Specialized Center for Blood Pressure Disorders-Regione Veneto, Department of Medicine—DIMED, University of Padua, 35128 Padua, Italy"
}
],
"authenticated-orcid": false,
"family": "Seccia",
"given": "Teresa Maria",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0001-9877-1054",
"affiliation": [
{
"name": "Internal Emergency Medicine Unit, Specialized Center for Blood Pressure Disorders-Regione Veneto, Department of Medicine—DIMED, University of Padua, 35128 Padua, Italy"
}
],
"authenticated-orcid": false,
"family": "Shagjaa",
"given": "Tungalagtamir",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-8767-1995",
"affiliation": [
{
"name": "Department of Pharmaceutical & Pharmacological Sciences, University of Padua, 35131 Padua, Italy"
}
],
"authenticated-orcid": false,
"family": "Morpurgo",
"given": "Margherita",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3775-0011",
"affiliation": [
{
"name": "Internal Emergency Medicine Unit, Specialized Center for Blood Pressure Disorders-Regione Veneto, Department of Medicine—DIMED, University of Padua, 35128 Padua, Italy"
}
],
"authenticated-orcid": false,
"family": "Caroccia",
"given": "Brasilina",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-4735-1636",
"affiliation": [
{
"name": "Internal Emergency Medicine Unit, Specialized Center for Blood Pressure Disorders-Regione Veneto, Department of Medicine—DIMED, University of Padua, 35128 Padua, Italy"
}
],
"authenticated-orcid": false,
"family": "Sanga",
"given": "Viola",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmacy, University Hospital of Padua, 35126 Padua, Italy"
}
],
"family": "Faoro",
"given": "Sonia",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0396-4377",
"affiliation": [
{
"name": "Pharmacy, University Hospital of Padua, 35126 Padua, Italy"
}
],
"authenticated-orcid": false,
"family": "Venturini",
"given": "Francesca",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmacy, University Hospital of Padua, 35126 Padua, Italy"
}
],
"family": "Iadicicco",
"given": "Girolama",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pneumology, University Hospital of Padua, 35126 Padua, Italy"
}
],
"family": "Lococo",
"given": "Sara",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0263-0703",
"affiliation": [
{
"name": "Infectious Diseases, University Hospital of Padua, 35126 Padua, Italy"
}
],
"authenticated-orcid": false,
"family": "Mazzitelli",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine 3, University Hospital of Padua, 35128 Padua, Italy"
}
],
"family": "Farnia",
"given": "Filippo",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-3445-0387",
"affiliation": [
{
"name": "Internal Medicine 3, University Hospital of Padua, 35128 Padua, Italy"
}
],
"authenticated-orcid": false,
"family": "Fioretto",
"given": "Paola",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7996-3720",
"affiliation": [
{
"name": "Yokohama City University, Yokohama 236-0027, Japan"
}
],
"authenticated-orcid": false,
"family": "Kobayashi",
"given": "Yusuke",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7906-0580",
"affiliation": [
{
"name": "Biostatistics, Epidemiology and Public Health Unit, University of Padua, 35131 Padua, Italy"
}
],
"authenticated-orcid": false,
"family": "Gregori",
"given": "Dario",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7963-0931",
"affiliation": [
{
"name": "Internal Emergency Medicine Unit, Specialized Center for Blood Pressure Disorders-Regione Veneto, Department of Medicine—DIMED, University of Padua, 35128 Padua, Italy"
}
],
"authenticated-orcid": false,
"family": "Rossi",
"given": "Gian Paolo",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Medicine",
"container-title-short": "JCM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
10,
19
]
],
"date-time": "2023-10-19T09:43:28Z",
"timestamp": 1697708608000
},
"deposited": {
"date-parts": [
[
2023,
10,
19
]
],
"date-time": "2023-10-19T11:36:04Z",
"timestamp": 1697715364000
},
"funder": [
{
"award": [
"B/2020/0138"
],
"name": "Fondazione Compagnia San Paolo"
}
],
"indexed": {
"date-parts": [
[
2025,
2,
21
]
],
"date-time": "2025-02-21T19:30:54Z",
"timestamp": 1740166254218,
"version": "3.37.3"
},
"is-referenced-by-count": 4,
"issue": "20",
"issued": {
"date-parts": [
[
2023,
10,
19
]
]
},
"journal-issue": {
"issue": "20",
"published-online": {
"date-parts": [
[
2023,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
19
]
],
"date-time": "2023-10-19T00:00:00Z",
"timestamp": 1697673600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2077-0383/12/20/6618/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "6618",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
10,
19
]
]
},
"published-online": {
"date-parts": [
[
2023,
10,
19
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1186/s13027-022-00451-1",
"article-title": "Bimodal antibody-titer decline following BNT162b2 mRNA anti-SARS-CoV-2 vaccination in healthcare workers of the INT—IRCCS “Fondazione Pascale” Cancer Center (Naples, Italy)",
"author": "Russo",
"doi-asserted-by": "crossref",
"first-page": "40",
"journal-title": "Infect. Agents Cancer",
"key": "ref_1",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1080/14760584.2023.2174529",
"article-title": "Understanding the mechanisms for COVID-19 vaccine’s protection against infection and severe disease",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "186",
"journal-title": "Expert. Rev. Vaccines",
"key": "ref_2",
"volume": "22",
"year": "2023"
},
{
"DOI": "10.1080/14760584.2022.2092472",
"article-title": "Expert review on global real-world vaccine effectiveness against SARS-CoV-2",
"author": "Chuenkitmongkol",
"doi-asserted-by": "crossref",
"first-page": "1255",
"journal-title": "Expert. Rev. Vaccines",
"key": "ref_3",
"volume": "21",
"year": "2022"
},
{
"DOI": "10.7326/M22-0988",
"article-title": "Risk for Myocardial Infarction, Stroke, and Pulmonary Embolism Following COVID-19 Vaccines in Adults Younger Than 75 Years in France",
"author": "Botton",
"doi-asserted-by": "crossref",
"first-page": "1250",
"journal-title": "Ann. Intern. Med.",
"key": "ref_4",
"volume": "175",
"year": "2022"
},
{
"DOI": "10.1001/jamainternmed.2022.3858",
"article-title": "Clinical and Genetic Risk Factors for Acute Incident Venous Thromboembolism in Ambulatory Patients with COVID-19",
"author": "Xie",
"doi-asserted-by": "crossref",
"first-page": "1063",
"journal-title": "JAMA Intern. Med.",
"key": "ref_5",
"volume": "182",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "ref_6",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.thromres.2022.08.021",
"article-title": "Thromboembolic events in deceased patients with proven SARS-CoV-2 infection: Frequency, characteristics and risk factors",
"author": "Voigtlaender",
"doi-asserted-by": "crossref",
"first-page": "171",
"journal-title": "Thromb. Res.",
"key": "ref_7",
"volume": "218",
"year": "2022"
},
{
"DOI": "10.1161/CIRCULATIONAHA.122.060785",
"article-title": "Association of COVID-19 with Major Arterial and Venous Thrombotic Diseases: A Population-Wide Cohort Study of 48 Million Adults in England and Wales",
"author": "Knight",
"doi-asserted-by": "crossref",
"first-page": "892",
"journal-title": "Circulation",
"key": "ref_8",
"volume": "146",
"year": "2022"
},
{
"DOI": "10.1186/s12959-020-00250-x",
"article-title": "Coagulation abnormalities in SARS-CoV-2 infection: Overexpression tissue factor",
"author": "Eslamifar",
"doi-asserted-by": "crossref",
"first-page": "38",
"journal-title": "Thromb. J.",
"key": "ref_9",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1128/AAC.01043-16",
"article-title": "Identification of Nafamostat as a Potent Inhibitor of Middle East Respiratory Syndrome Coronavirus S Protein-Mediated Membrane Fusion Using the Split-Protein-Based Cell-Cell Fusion Assay",
"author": "Yamamoto",
"doi-asserted-by": "crossref",
"first-page": "6532",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_10",
"volume": "60",
"year": "2016"
},
{
"DOI": "10.5301/IJAO.2011.8535",
"article-title": "Nafamostat mesilate as an anticoagulant during continuous veno-venous hemodialysis: A three-year retrospective cohort study",
"author": "Maruyama",
"doi-asserted-by": "crossref",
"first-page": "571",
"journal-title": "Int. J. Artif. Organs",
"key": "ref_11",
"volume": "34",
"year": "2011"
},
{
"DOI": "10.1097/MD.0000000000002392",
"article-title": "Nafamostat Mesilate as an Anticoagulant during Continuous Renal Replacement Therapy in Patients with High Bleeding Risk: A Randomized Clinical Trial",
"author": "Choi",
"doi-asserted-by": "crossref",
"first-page": "e2392",
"journal-title": "Medicine",
"key": "ref_12",
"volume": "94",
"year": "2015"
},
{
"DOI": "10.1097/MPA.0000000000000310",
"article-title": "Nafamostat mesilate for prevention of post-ERCP pancreatitis: A meta-analysis of prospective, randomized, controlled trials",
"author": "Yu",
"doi-asserted-by": "crossref",
"first-page": "561",
"journal-title": "Pancreas",
"key": "ref_13",
"volume": "44",
"year": "2015"
},
{
"DOI": "10.1007/s12185-018-02567-w",
"article-title": "Comparison of gabexate mesilate and nafamostat mesilate for disseminated intravascular coagulation associated with hematological malignancies",
"author": "Minakata",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Int. J. Hematol.",
"key": "ref_14",
"volume": "109",
"year": "2019"
},
{
"DOI": "10.7554/eLife.77444",
"article-title": "Coagulation factors directly cleave SARS-CoV-2 spike and enhance viral entry",
"author": "Kastenhuber",
"doi-asserted-by": "crossref",
"first-page": "e77444",
"journal-title": "Elife",
"key": "ref_15",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1254/jjp.35.203",
"article-title": "Pharmacological studies of FUT-175, nafamstat mesilate. I. Inhibition of protease activity in in vitro and in vivo experiments",
"author": "Aoyama",
"doi-asserted-by": "crossref",
"first-page": "203",
"journal-title": "Jpn. J. Pharmacol.",
"key": "ref_16",
"volume": "35",
"year": "1984"
},
{
"DOI": "10.1016/0049-3848(94)90008-6",
"article-title": "Effect of nafamostat mesilate, a synthetic protease inhibitor, on tissue factor-factor VIIa complex activity",
"author": "Uchiba",
"doi-asserted-by": "crossref",
"first-page": "155",
"journal-title": "Thromb. Res.",
"key": "ref_17",
"volume": "74",
"year": "1994"
},
{
"DOI": "10.1007/978-0-387-92298-0",
"doi-asserted-by": "crossref",
"key": "ref_18",
"unstructured": "Albert, J. (2009). Bayesian Computation with R, Springer."
},
{
"DOI": "10.22237/jmasm/1383279240",
"article-title": "Comparison of Three Calculation Methods for a Bayesian Inference of P(π1 > π2)",
"author": "Kawasaki",
"doi-asserted-by": "crossref",
"first-page": "256",
"journal-title": "J. Mod. Appl. Stat. Methods",
"key": "ref_19",
"volume": "12",
"year": "2013"
},
{
"DOI": "10.5964/ejop.v7i4.163",
"article-title": "Doing Bayesian Data Analysis: A Tutorial with R and BUGS",
"author": "Barry",
"doi-asserted-by": "crossref",
"first-page": "778",
"journal-title": "Eur. J. Psychol.",
"key": "ref_20",
"volume": "7",
"year": "2011"
},
{
"DOI": "10.1002/sim.3680",
"article-title": "The BUGS project: Evolution, critique and future directions",
"author": "Lunn",
"doi-asserted-by": "crossref",
"first-page": "3049",
"journal-title": "Stat. Med.",
"key": "ref_21",
"volume": "28",
"year": "2009"
},
{
"key": "ref_22",
"unstructured": "R Development Core Team (2020, August 02). R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/."
},
{
"DOI": "10.1214/ss/1009212673",
"article-title": "Power prior distributions for regression models",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "46",
"journal-title": "Stat. Sci.",
"key": "ref_23",
"volume": "15",
"year": "2000"
},
{
"article-title": "Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: A randomised Phase II clinical trial",
"author": "Zhuravel",
"first-page": "101169",
"journal-title": "E Clin. Med.",
"key": "ref_24",
"volume": "41",
"year": "2021"
},
{
"DOI": "10.1201/b16018",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "Gelman, A., Carlin, J.B., Stern, H.S., Dunson, D.B., Vehtari, A., and Rubin, D.B. (2013). Bayesian Data Analysis, Chapman and Hall/CRC."
},
{
"article-title": "Acute eosinophilic pneumonia caused by camostat mesilate: The first case report",
"author": "Ota",
"first-page": "21",
"journal-title": "Respir. Med. Case Rep.",
"key": "ref_26",
"volume": "19",
"year": "2016"
},
{
"DOI": "10.1097/01.ASN.0000043900.39397.48",
"article-title": "Inhibition of prostasin secretion by serine protease inhibitors in the kidney",
"author": "Iwashita",
"doi-asserted-by": "crossref",
"first-page": "11",
"journal-title": "J. Am. Soc. Nephrol.",
"key": "ref_27",
"volume": "14",
"year": "2003"
},
{
"DOI": "10.1371/journal.pone.0141169",
"doi-asserted-by": "crossref",
"key": "ref_28",
"unstructured": "Nimishakavi, S., Raymond, W.W., Gruenert, D.C., and Caughey, G.H. (2015). Divergent Inhibitor Susceptibility among Airway Lumen-Accessible Tryptic Proteases. PLoS ONE, 10."
},
{
"DOI": "10.1016/0306-3623(95)00072-0",
"article-title": "Mechanisms of hyperkalemia caused by nafamostat mesilate",
"author": "Muto",
"doi-asserted-by": "crossref",
"first-page": "1627",
"journal-title": "Gen. Pharmacol.",
"key": "ref_29",
"volume": "26",
"year": "1995"
},
{
"DOI": "10.7554/eLife.57278",
"article-title": "Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients",
"author": "Rossi",
"doi-asserted-by": "crossref",
"first-page": "e57278",
"journal-title": "eLife",
"key": "ref_30",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.ebiom.2022.103856",
"doi-asserted-by": "crossref",
"key": "ref_31",
"unstructured": "Quinn, T.M., Gaughan, E.E., Bruce, A., Antonelli, J., O’Connor, R., Li, F., McNamara, S., Koch, O., MacKintosh, C., and Dockrell, D. (2022). Randomised controlled trial of intravenous nafamostat mesylate in COVID pneumonitis: Phase 1b/2a experimental study to investigate safety, Pharmacokinetics and Pharmacodynamics. EBio Med., 76."
},
{
"key": "ref_32",
"unstructured": "Dhawan, S. (2020). Sun Pharma Laboratories Limited, Mumbai, India, Unpublished work."
},
{
"key": "ref_33",
"unstructured": "Kim, D. (2020). Korea Cancer Center Hospital, Seoul, Republic of Korea, Unpublished work."
},
{
"DOI": "10.7883/yoken.JJID.2021.699",
"article-title": "Nafamostat Mesylate Monotherapy in Patients with Moderate COVID-19: A Single-Center, Retrospective Study",
"author": "Soma",
"doi-asserted-by": "crossref",
"first-page": "484",
"journal-title": "Jpn. J. Infect. Dis.",
"key": "ref_34",
"volume": "75",
"year": "2022"
},
{
"DOI": "10.3390/jcm11010116",
"doi-asserted-by": "crossref",
"key": "ref_35",
"unstructured": "Inokuchi, R., Kuno, T., Komiyama, J., Uda, K., Miyamoto, Y., Taniguchi, Y., Abe, T., Ishimaru, M., Adomi, M., and Tamiya, N. (2021). Association between Nafamostat Mesylate and In-Hospital Mortality in Patients with Coronavirus Disease 2019: A Multicenter Observational Study. J. Clin. Med., 11."
},
{
"DOI": "10.1128/AAC.00754-20",
"article-title": "Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "e00754-20",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_36",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1007/s00216-008-2054-4",
"article-title": "A method for quantifying the unstable and highly polar drug nafamostat mesilate in human plasma with optimized solid-phase extraction and ESI-MS detection: More accurate evaluation for pharmacokinetic study",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "1063",
"journal-title": "Anal. Bioanal. Chem.",
"key": "ref_37",
"volume": "391",
"year": "2008"
},
{
"DOI": "10.2133/dmpk.21.147",
"article-title": "Involvement of Human Blood Arylesterases and Liver Microsomal Carboxylesterases in Nafamostat Hydrolysis",
"author": "Yamaori",
"doi-asserted-by": "crossref",
"first-page": "147",
"journal-title": "Drug Metab. Pharmacokinet.",
"key": "ref_38",
"volume": "21",
"year": "2006"
},
{
"DOI": "10.1001/jama.2018.6360",
"article-title": "Randomization in clinical trials: Permuted blocks and stratification",
"author": "Broglio",
"doi-asserted-by": "crossref",
"first-page": "2223",
"journal-title": "JAMA",
"key": "ref_39",
"volume": "319",
"year": "2018"
},
{
"DOI": "10.1056/NEJMsr2005760",
"article-title": "Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19",
"author": "Vaduganathan",
"doi-asserted-by": "crossref",
"first-page": "1653",
"journal-title": "N. Engl. J. Med.",
"key": "ref_40",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1161/CIRCRESAHA.120.317134",
"article-title": "Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients with Hypertension Hospitalized With COVID-19",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "1671",
"journal-title": "Circ. Res.",
"key": "ref_41",
"volume": "126",
"year": "2020"
},
{
"key": "ref_42",
"unstructured": "World Health Organization (2020). WHO R&D Blueprint Informal Consultation on Prioritization of Candidate Therapeutic Agents for Use in Novel Coronavirus 2019 Infection."
},
{
"key": "ref_43",
"unstructured": "Lesaffre, E. (2014). Understanding Evidence-Based Rheumatology, Springer."
},
{
"DOI": "10.2307/2531201",
"article-title": "Evaluation of Sample Size and Power for Analyses of Survival with Allowance for Nonuniform Patient Entry, Losses to Follow-Up, Noncompliance, and Stratification",
"author": "Lachin",
"doi-asserted-by": "crossref",
"first-page": "507",
"journal-title": "Biometrics",
"key": "ref_44",
"volume": "42",
"year": "1986"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "ref_45",
"volume": "395",
"year": "2020"
},
{
"key": "ref_46",
"unstructured": "R_Core Team (2020, August 02). R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: 2020. Available online: https://www.r-project.org/."
},
{
"key": "ref_47",
"unstructured": "Keaven Anderson, K. (2020, August 02). gsDesign: Group Sequential Design. 30-1, Package Version; 2016. Available online: https://cranr-project.org/package=gsdesign."
}
],
"reference-count": 47,
"references-count": 47,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2077-0383/12/20/6618"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "RAndomized Clinical Trial Of NAfamostat Mesylate, A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia",
"type": "journal-article",
"volume": "12"
}
