Bromhexine Hydrochloride Tablets for the Treatment of Moderate COVID-19: An Open-Label Randomized Controlled Pilot Study
et al., Clinical and Translational Science, doi:10.1111/cts.12881, NCT04273763, Sep 2020
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
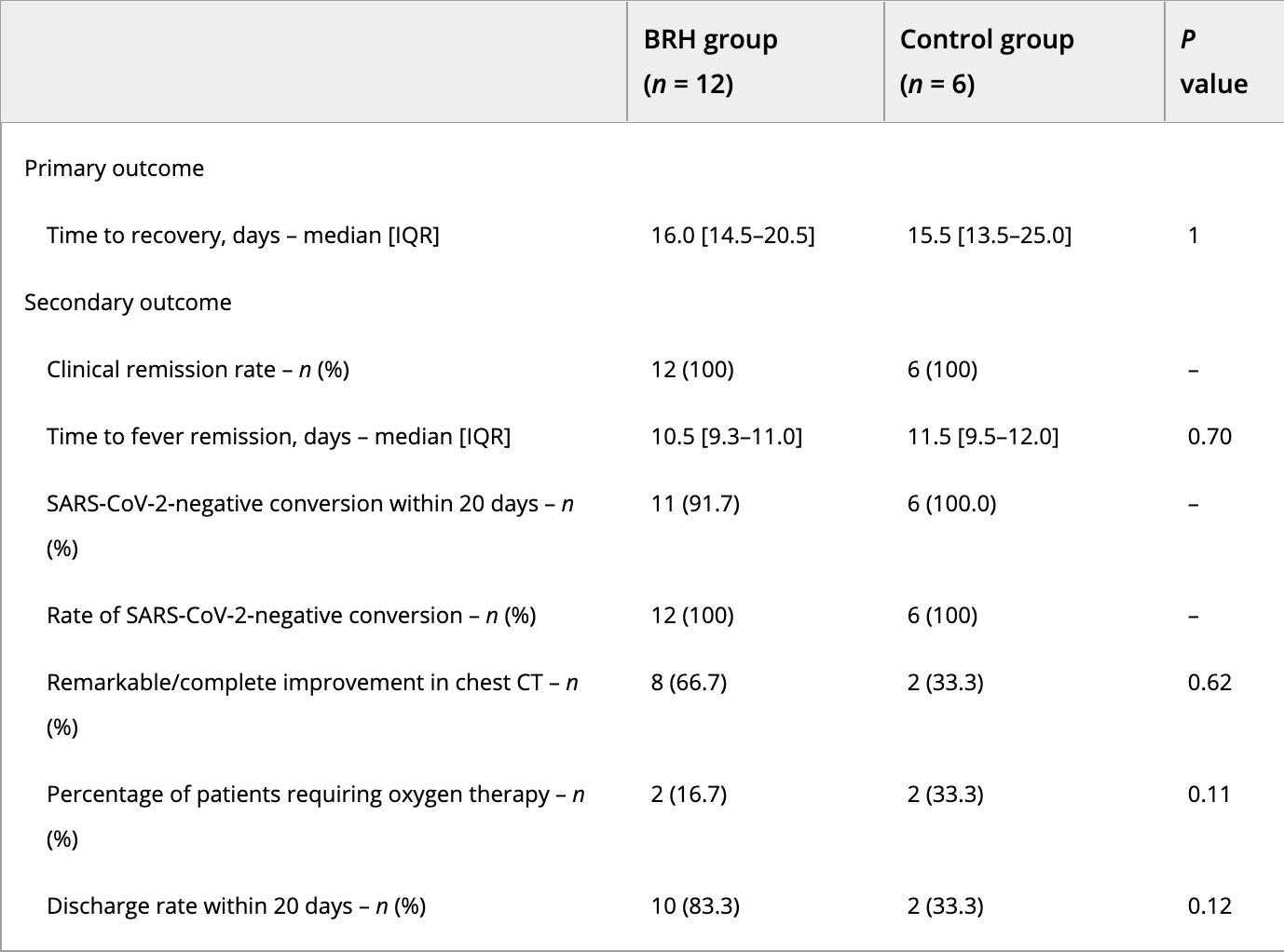
Tiny RCT with 12 bromhexine and 6 control patients showing non-statistically significant improvements in chest CT, need for oxygen therapy, and discharge rate within 20 days. Authors recommend a larger scale trial.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
Study covers TMPRSS2 inhibitors and bromhexine.
|
risk of no hospital discharge, 75.0% lower, RR 0.25, p = 0.11, treatment 2 of 12 (16.7%), control 4 of 6 (66.7%), NNT 2.0.
|
|
risk of oxygen therapy, 50.0% lower, RR 0.50, p = 0.57, treatment 2 of 12 (16.7%), control 2 of 6 (33.3%), NNT 6.0.
|
|
recovery time, 3.2% higher, relative time 1.03, treatment 12, control 6.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Li et al., 3 Sep 2020, Randomized Controlled Trial, China, peer-reviewed, 10 authors, study period 16 February, 2020 - 10 May, 2020, trial NCT04273763 (history).
Bromhexine Hydrochloride Tablets for the Treatment of Moderate COVID‐19: An Open‐Label Randomized Controlled Pilot Study
Clinical and Translational Science, doi:10.1111/cts.12881
This open-label randomized controlled pilot study aimed to test the study feasibility of bromhexine hydrochloride (BRH) tablets for the treatment of mild or moderate coronavirus disease 2019 (COVID-19) and to explore its clinical efficacy and safety. Patients with mild or moderate COVID-19 were randomly divided into the BRH group or the control group at a 2:1 ratio. Routine treatment according to China's Novel Coronavirus Pneumonia Diagnosis and Treatment Plan was performed in both groups, whereas patients in the BRH group were additionally given oral BRH (32 mg t.i.d.) for 14 consecutive days. The efficacy and safety of BRH were evaluated. A total of 18 patients with moderate COVID-19 were randomized into the BRH group (n = 12) or the control group (n = 6). There were suggestions of BRH advantage over placebo in improved chest computed tomography, need for oxygen therapy, and discharge rate within 20 days. However, none of these findings were statistically significant. BRH tablets may potentially have a beneficial effect in patients with COVID-19, especially for those with lung or hepatic injury. A further definitive large-scale clinical trial is feasible and necessary.
References
Chau, SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases, Hepatology
Chen, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet
Depfenhart, Markus, Marina, Dario, De Danielle, A SARS-CoV-2 prophylactic and treatment: a counter argument against the sole use of chloroquine, Am. J. Biomed. Sci. Res
Glowacka, Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response, J. Virol
Guan, Clinical characteristics of coronavirus disease 2019 in China, N. Engl. J. Med
Hatesuer, Tmprss2 is essential for influenza H1N1 virus pathogenesis in mice, PLoS Pathogens
Heurich, Hofmann-Winkler, Gierer, Liepold, Jahn et al., TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein, J. Virol
Hoffmann, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Iwata-Yoshikawa, Okamura, Shimizu, Hasegawa, Takeda et al., TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection, J. Virol
Lai, Shih, Ko, Tang, Hsueh, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges, Int. J. Antimicrob. Agents
Lee, The clinical and virological features of the first imported case causing MERS-CoV outbreak in South Korea, BMC Infect. Dis
Limburg, TMPRSS2 is the major activating protease of influenza A virus in primary human airway cells and influenza B virus in human type II pneumocytes, J. Virol
Lucas, The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis, Cancer Discov
Matsuyama, Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells, Proc. Natl. Acad. Sci
Pan, Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia, Radiology
Rodriguez-Morales, Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis, Travel Med. Infect. Dis
Sakai, The host protease TMPRSS2 plays a major role in in vivo replication of emerging H7N9 and seasonal influenza viruses, J. Virol
Tarnow, TMPRSS2 is a host factor that is essential for pneumotropism and pathogenicity of H7N9 influenza A virus in mice, J. Virol
Wang, Wang, Ye, Liu, A review of the 2019 novel coronavirus (COVID-19) based on current evidence, Int. J. Antimicrob. Agents
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and prevention, JAMA
Yang, Yuan, Tu, Determination of bromhexine in plasma by gas chromatography-electron capture detection and pharmacokinetic studies
Yang, Yuan, Tu, Determination of bromhexine in plasma by gas chromatography-electron capture detection and pharmacokinetic studies
Zhang, Shi, Wang, Liver injury in COVID-19: management and challenges, Lancet Gastroenterol. Hepatol
DOI record:
{
"DOI": "10.1111/cts.12881",
"ISSN": [
"1752-8054",
"1752-8062"
],
"URL": "http://dx.doi.org/10.1111/cts.12881",
"abstract": "<jats:p>This open‐label randomized controlled pilot study aimed to test the study feasibility of bromhexine hydrochloride (BRH) tablets for the treatment of mild or moderate coronavirus disease 2019 (COVID‐19) and to explore its clinical efficacy and safety. Patients with mild or moderate COVID‐19 were randomly divided into the BRH group or the control group at a 2:1 ratio. Routine treatment according to China’s Novel Coronavirus Pneumonia Diagnosis and Treatment Plan was performed in both groups, whereas patients in the BRH group were additionally given oral BRH (32 mg t.i.d.) for 14 consecutive days. The efficacy and safety of BRH were evaluated. A total of 18 patients with moderate COVID‐19 were randomized into the BRH group (<jats:italic>n</jats:italic> = 12) or the control group (<jats:italic>n</jats:italic> = 6). There were suggestions of BRH advantage over placebo in improved chest computed tomography, need for oxygen therapy, and discharge rate within 20 days. However, none of these findings were statistically significant. BRH tablets may potentially have a beneficial effect in patients with COVID‐19, especially for those with lung or hepatic injury. A further definitive large‐scale clinical trial is feasible and necessary.</jats:p>",
"alternative-id": [
"10.1111/cts.12881"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-05-27"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2020-08-17"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2020-10-10"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Anesthesia and Critical Care The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University Wenzhou Zhejiang China"
},
{
"name": "Clinical Research Unit The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University Wenzhou Zhejiang China"
}
],
"family": "Li",
"given": "Ting",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Emergency The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University Wenzhou Zhejiang China"
}
],
"family": "Sun",
"given": "Laifang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Critical Medicine The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University Wenzhou Zhejiang China"
}
],
"family": "Zhang",
"given": "Wenwu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Research Unit The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University Wenzhou Zhejiang China"
}
],
"family": "Zheng",
"given": "Chanfan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Research Unit The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University Wenzhou Zhejiang China"
}
],
"family": "Jiang",
"given": "Chenchen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Research Unit The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University Wenzhou Zhejiang China"
}
],
"family": "Chen",
"given": "Mingjing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Research Unit The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University Wenzhou Zhejiang China"
}
],
"family": "Chen",
"given": "Di",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Endocrinology The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University Wenzhou Zhejiang China"
}
],
"family": "Dai",
"given": "Zhijuan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacy The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University Wenzhou Zhejiang China"
}
],
"family": "Bao",
"given": "Shihui",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastrointestinal Surgery The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University Wenzhou Zhejiang China"
}
],
"family": "Shen",
"given": "Xian",
"sequence": "additional"
}
],
"container-title": "Clinical and Translational Science",
"container-title-short": "Clinical Translational Sci",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ascpt.onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2020,
9,
3
]
],
"date-time": "2020-09-03T15:07:01Z",
"timestamp": 1599145621000
},
"deposited": {
"date-parts": [
[
2023,
9,
4
]
],
"date-time": "2023-09-04T09:28:34Z",
"timestamp": 1693819714000
},
"indexed": {
"date-parts": [
[
2023,
9,
7
]
],
"date-time": "2023-09-07T20:46:35Z",
"timestamp": 1694119595311
},
"is-referenced-by-count": 31,
"issue": "6",
"issued": {
"date-parts": [
[
2020,
10,
10
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2020,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
10,
10
]
],
"date-time": "2020-10-10T00:00:00Z",
"timestamp": 1602288000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/cts.12881",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1111/cts.12881",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://ascpt.onlinelibrary.wiley.com/doi/pdf/10.1111/cts.12881",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "1096-1102",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2020,
10,
10
]
]
},
"published-online": {
"date-parts": [
[
2020,
10,
10
]
]
},
"published-print": {
"date-parts": [
[
2020,
11
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/j.ijantimicag.2020.105924",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_1_1"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105948",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_2_1"
},
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_3_1"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_4_1"
},
{
"DOI": "10.1128/JVI.02202-13",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_5_1"
},
{
"DOI": "10.1128/JVI.02232-10",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_6_1"
},
{
"DOI": "10.1128/JVI.00649-19",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_7_1"
},
{
"DOI": "10.34297/AJBSR.2020.08.001283",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_8_1"
},
{
"DOI": "10.1158/2159-8290.CD-13-1010",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_9_1"
},
{
"key": "e_1_2_11_10_1",
"unstructured": "Bisolvon Oral Solution Summary of Product Characteristics Health Products Regulatory Authority Ireland CRN 2173773 29/04/2016. <https://www.hpra.ie/img/uploaded/swedocuments/Licence_PA0540‐180‐001_19032020145920.pdf>."
},
{
"article-title": "Determination of bromhexine in plasma by gas chromatography‐electron capture detection and pharmacokinetic studies [in Chinese]",
"author": "Yang L.L.",
"first-page": "543",
"journal-title": "Se Pu",
"key": "e_1_2_11_11_1",
"volume": "18",
"year": "2000"
},
{
"DOI": "10.1148/radiol.2020200370",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_12_1"
},
{
"DOI": "10.1016/j.tmaid.2020.101623",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_13_1"
},
{
"DOI": "10.1073/pnas.2002589117",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_14_1"
},
{
"DOI": "10.1128/JVI.03799-13",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_15_1"
},
{
"DOI": "10.1128/JVI.03677-13",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_16_1"
},
{
"DOI": "10.1371/journal.ppat.1003774",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_17_1"
},
{
"DOI": "10.1128/JVI.01815-18",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_18_1"
},
{
"article-title": "Determination of bromhexine in plasma by gas chromatography‐electron capture detection and pharmacokinetic studies [in Chinese]",
"author": "Yang L.L.",
"first-page": "543",
"journal-title": "Se Pu",
"key": "e_1_2_11_19_1",
"volume": "18",
"year": "2000"
},
{
"DOI": "10.1056/NEJMoa2002032",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_20_1"
},
{
"DOI": "10.1016/S2468-1253(20)30057-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_21_1"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_22_1"
},
{
"DOI": "10.1002/hep.20111",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_23_1"
},
{
"DOI": "10.1186/s12879-017-2576-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_24_1"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://ascpt.onlinelibrary.wiley.com/doi/10.1111/cts.12881"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Pharmacology, Toxicology and Pharmaceutics",
"General Biochemistry, Genetics and Molecular Biology",
"General Medicine",
"General Neuroscience"
],
"subtitle": [],
"title": "Bromhexine Hydrochloride Tablets for the Treatment of Moderate COVID‐19: An Open‐Label Randomized Controlled Pilot Study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "13"
}
