A Double-Blind, Randomized, Placebo-Controlled, Phase II Clinical Study To Evaluate the Efficacy and Safety of Camostat Mesylate (DWJ1248) in Adult Patients with Mild to Moderate COVID-19
et al., Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.00452-22, NCT04521296, Jan 2023
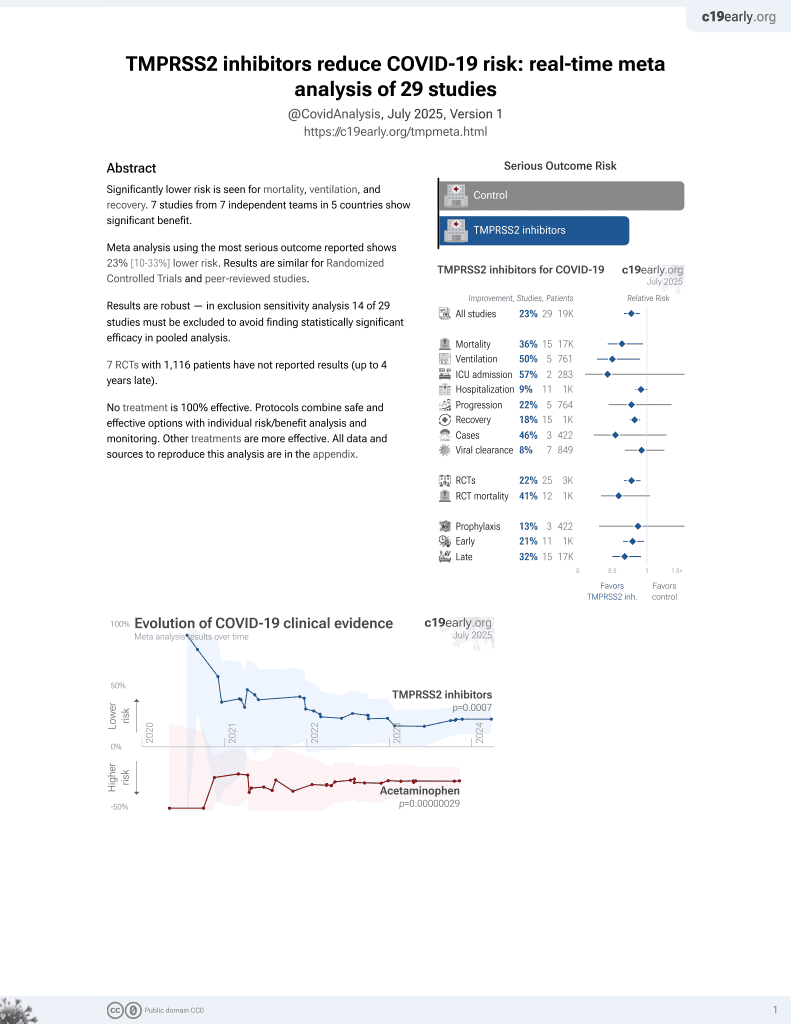
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
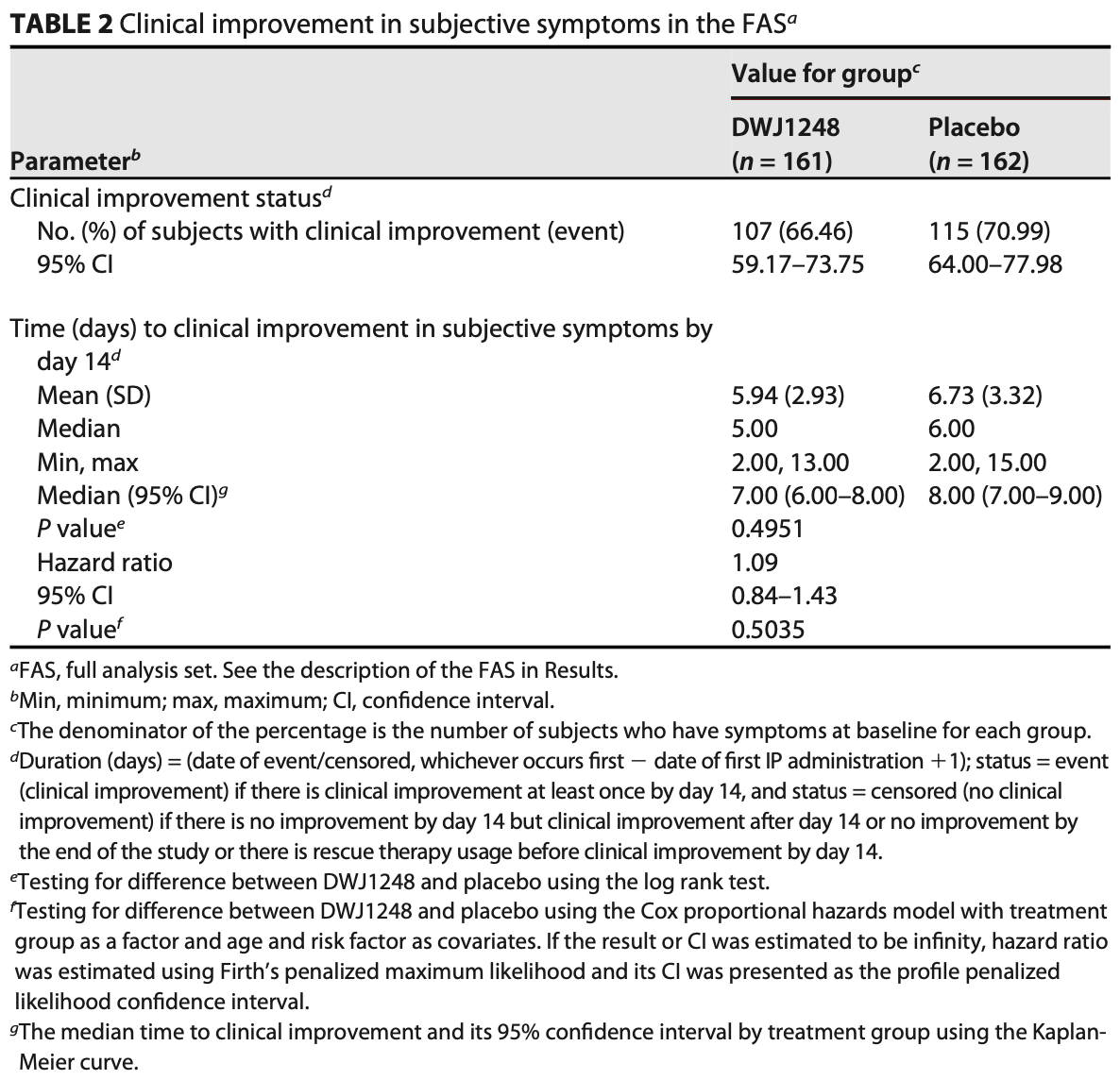
Double-blind RCT with 342 mild to moderate COVID-19 outpatients in South Korea, showing no significant difference in time to clinical improvement with camostat mesylate. In a post-hoc subgroup analysis of high-risk patients, there were non-statistically significant trends towards faster improvement in ordinal scale scores and subjective symptom scores at day 7 with treatment. Viral cultures suggested faster viral clearance with treatment, without statistical significance.
Study covers TMPRSS2 inhibitors and camostat.
|
time to clinical improvement, 8.3% lower, HR 0.92, p = 0.54, treatment 161, control 162, inverted to make HR<1 favor treatment, primary outcome.
|
|
improvement, 24.8% lower, HR 0.75, p = 0.31, treatment 109, control 104, inverted to make HR<1 favor treatment, high-risk subgroup, day 7.
|
|
improvement, 46.2% lower, HR 0.54, p = 0.06, treatment 77, control 78, inverted to make HR<1 favor treatment, high-risk subgroup, mFAS, day 7.
|
|
ordinal scale improvement, 40.5% lower, HR 0.60, p = 0.21, treatment 109, control 104, inverted to make HR<1 favor treatment, high-risk subgroup, day 7.
|
|
ordinal scale improvement, 58.2% lower, HR 0.42, p = 0.06, treatment 77, control 78, inverted to make HR<1 favor treatment, high-risk subgroup, mFAS, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kim et al., 24 Jan 2023, Double Blind Randomized Controlled Trial, placebo-controlled, South Korea, peer-reviewed, median age 53.0, mean age 51.4, 34 authors, study period February 2021 - May 2021, trial NCT04521296 (history).
Contact: mdohmd@snu.ac.kr, jhkim302@daewoong.co.kr.
A Double-Blind, Randomized, Placebo-Controlled, Phase II Clinical Study To Evaluate the Efficacy and Safety of Camostat Mesylate (DWJ1248) in Adult Patients with Mild to Moderate COVID-19
Antimicrobial Agents and Chemotherapy, doi:10.1128/aac.00452-22
Although several antiviral agents have become available for coronavirus disease 2019 (COVID-19) treatment, oral drugs are still limited. Camostat mesylate, an orally bioavailable serine protease inhibitor, has been used to treat chronic pancreatitis in South Korea, and it has an in vitro inhibitory potential against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This study was a doubleblind, randomized, placebo-controlled, multicenter, phase 2 clinical trial in mild to moderate COVID-19 patients. We randomly assigned patients to receive either camostat mesylate (DWJ1248) or placebo orally for 14 days. The primary endpoint was time to clinical improvement of subject symptoms within 14 days, measured using a subjective 4-point Likert scale. Three hundred forty-two patients were randomized. The primary endpoint was nonsignificant, where the median times to clinical improvement were 7 and 8 days in the camostat mesylate group and the placebo group,
References
Beigel, Tomashek, Dodd, Remdesivir for the treatment of COVID-19-preliminary report, Reply. N Engl J Med, doi:10.1056/NEJMc2022236
Chen, Nirula, Heller, Gottlieb, Boscia et al., SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2029849
Depfenhart, De Villiers, Lemperle, Meyer, Somma, Potential new treatment strategies for COVID-19: is there a role for bromhexine as add-on therapy?, Intern Emerg Med, doi:10.1007/s11739-020-02383-3
Gibo, Ito, Kawabe, Hisano, Inoue et al., Camostat mesilate attenuates pancreatic fibrosis via inhibition of monocytes and pancreatic stellate cells activity, Lab Invest, doi:10.1038/labinvest.3700203
Gottlieb, Nirula, Chen, Boscia, Heller et al., Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.0202
Gunst, Staerke, Pahus, Kristensen, Bodilsen et al., Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with COVID-19-a doubleblind randomized controlled trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100849
Hoffmann, Hofmann-Winkler, Smith, Krüger, Sørensen et al., Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity, bioRxiv, doi:10.1101/2020.08.05.237651
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hoffmann, Schroeder, Kleine-Weber, Muller, Drosten et al., Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19, Antimicrob Agents Chemother, doi:10.1128/AAC.00754-20
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in hospitalized patients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Iwata-Yoshikawa, Okamura, Shimizu, Hasegawa, Takeda et al., TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection, J Virol, doi:10.1128/JVI.01815-18
Kalil, Patterson, Mehta, Tomashek, Wolfe et al., Baricitinib plus remdesivir for hospitalized adults with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2031994
Keitel, Jensen, Feldt, Fischer, Bode et al., Reconvalescent plasma/camostat mesylate in early SARS-CoV-2 Q-PCR positive high-risk individuals (RES-Q-HR): a structured summary of a study protocol for a randomized controlled trial, Trials, doi:10.1186/s13063-021-05181-0
Kim, Cui, Shin, Bae, Kweon et al., Duration of culturable SARS-CoV-2 in hospitalized patients with COVID-19, N Engl J Med, doi:10.1056/NEJMc2027040
Laporte, Naesens, Airway proteases: an emerging drug target for influenza and other respiratory virus infections, Curr Opin Virol, doi:10.1016/j.coviro.2017.03.018
Libster, Marc, Wappner, Coviello, Bianchi et al., Early high-titer plasma therapy to prevent severe COVID-19 in older adults, N Engl J Med, doi:10.1056/NEJMoa2033700
Nicholson, Aoki, Osterhaus, Trottier, Carewicz et al., Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial, Lancet, doi:10.1016/S0140-6736(00)02288-1
Tamura, Hirado, Okamura, Minato, Fujii, Synthetic inhibitors of trypsin, plasmin, kallikrein, thrombin, C1r-, and C1 esterase, Biochim Biophys Acta, doi:10.1016/0005-2744(77)90097-3
Uno, Camostat mesilate therapy for COVID-19, Intern Emerg Med, doi:10.1007/s11739-020-02345-9
Yamaya, Shimotai, Hatachi, Kalonji, Tando et al., The serine protease inhibitor camostat inhibits influenza virus replication and cytokine production in primary cultures of human tracheal epithelial cells, Pulm Pharmacol Ther, doi:10.1016/j.pupt.2015.07.001
DOI record:
{
"DOI": "10.1128/aac.00452-22",
"ISSN": [
"0066-4804",
"1098-6596"
],
"URL": "http://dx.doi.org/10.1128/aac.00452-22",
"abstract": "<jats:p>\n Although several antiviral agents have become available for coronavirus disease 2019 (COVID-19) treatment, oral drugs are still limited. Camostat mesylate, an orally bioavailable serine protease inhibitor, has been used to treat chronic pancreatitis in South Korea, and it has an\n <jats:italic>in vitro</jats:italic>\n inhibitory potential against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).\n </jats:p>",
"alternative-id": [
"10.1128/aac.00452-22"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2022-03-28"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-10-27"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-12-14"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Internal Medicine, Chungnam National University School of Medicine, Daejeon, South Korea"
}
],
"family": "Kim",
"given": "Yeon-Sook",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea"
}
],
"family": "Jeon",
"given": "Seng-Ho",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Neurosurgery, Seoul Medical Center, Seoul, South Korea"
}
],
"family": "Kim",
"given": "Junghee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Seonam Hospital, Seoul, South Korea"
}
],
"family": "Koh",
"given": "Jong Hoon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, South Korea"
}
],
"family": "Ra",
"given": "Seung Won",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul National University College of Medicine, Seoul, South Korea"
}
],
"family": "Kim",
"given": "Ji Won",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Department of Internal Medicine, National Medical Center, Seoul, South Korea"
}
],
"family": "Kim",
"given": "Yeonjae",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Veterans Health Service Medical Center, Seoul, South Korea"
}
],
"family": "Kim",
"given": "Choon Kwan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Anesthesiology and Pain Medicine, Gyeonggi Provincial Medical Center Pocheon Hospital, Pocheon, South Korea"
}
],
"family": "Shin",
"given": "Yun Chul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Gyeonggi Provincial Medical Center Ansung Hospital, Ansung, South Korea"
}
],
"family": "Kang",
"given": "Beo Deul",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Chonmam National University Medical School, Gwangju, South Korea"
}
],
"family": "Kang",
"given": "Seung ji",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Gyeonggi Provincial Medical Center Icheon Hospital, Icheon, South Korea"
}
],
"family": "Park",
"given": "Chul Hee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Gyeonggi Provincial Medical Center Uljeongbu Hospital, Uljeongbu, South Korea"
}
],
"family": "Lee",
"given": "Boyoung",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Keimyung University Dongsan Hospital, Daegu, South Korea"
}
],
"family": "Lee",
"given": "Ji Yeon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Obstetrics and Gynecology, Gyeonggi Provincial Medical Center Suwon Hospital, Suwon, South Korea"
}
],
"family": "Lee",
"given": "Chung Hoon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Seoul Medical Center, Seoul, South Korea"
}
],
"family": "Choi",
"given": "Jae-phil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Incheon Medical Center, Incheon, South Korea"
}
],
"family": "Kim",
"given": "Jin Yong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Soonchunhyang University Cheonan Hospital, Cheonan, South Korea"
}
],
"family": "Yu",
"given": "Shi Nae",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Infectious Diseases, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea"
}
],
"family": "Peck",
"given": "Kyong Ran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine, Asan Medical Center, Seoul, South Korea"
}
],
"family": "Kim",
"given": "Sung-Han",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases, Ajou University School of Medicine, Suwon, South Korea"
}
],
"family": "Heo",
"given": "Jung Yeon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases, Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, South Korea"
}
],
"family": "Kim",
"given": "Hyun ah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Development Center, Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea"
}
],
"family": "Park",
"given": "Hyun-jin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Development Center, Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea"
}
],
"family": "Choi",
"given": "Jongwon",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Development Center, Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea"
}
],
"family": "Han",
"given": "Jumi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Development Center, Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea"
}
],
"family": "Kim",
"given": "JooHyun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Development Center, Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea"
}
],
"family": "Kim",
"given": "Hyoung jun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Development Center, Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea"
}
],
"family": "Han",
"given": "Se Hee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Development Center, Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea"
}
],
"family": "Yoon",
"given": "Aeri",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Development Center, Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea"
}
],
"family": "Park",
"given": "MiHee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Development Center, Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea"
}
],
"family": "Park",
"given": "SuJung",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Development Center, Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea"
}
],
"family": "Kim",
"given": "YuKyung",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8533-7873",
"affiliation": [
{
"name": "Clinical Development Center, Daewoong Pharmaceutical Co., Ltd., Seoul, South Korea"
}
],
"authenticated-orcid": true,
"family": "Jung",
"given": "Minji",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2344-7695",
"affiliation": [
{
"name": "Department of Internal Medicine, Seoul National University College of Medicine, Seoul, South Korea"
}
],
"authenticated-orcid": false,
"family": "Oh",
"given": "Myoung-don",
"sequence": "additional"
}
],
"container-title": "Antimicrobial Agents and Chemotherapy",
"container-title-short": "Antimicrob Agents Chemother",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.asm.org"
]
},
"created": {
"date-parts": [
[
2022,
12,
14
]
],
"date-time": "2022-12-14T14:00:56Z",
"timestamp": 1671026456000
},
"deposited": {
"date-parts": [
[
2023,
1,
24
]
],
"date-time": "2023-01-24T14:02:34Z",
"timestamp": 1674568954000
},
"indexed": {
"date-parts": [
[
2024,
1,
22
]
],
"date-time": "2024-01-22T22:05:09Z",
"timestamp": 1705961109871
},
"is-referenced-by-count": 10,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
1,
24
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2023,
1,
24
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
24
]
],
"date-time": "2023-01-24T00:00:00Z",
"timestamp": 1674518400000
}
},
{
"URL": "https://journals.asm.org/non-commercial-tdm-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
24
]
],
"date-time": "2023-01-24T00:00:00Z",
"timestamp": 1674518400000
}
}
],
"link": [
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/aac.00452-22",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.asm.org/doi/pdf/10.1128/aac.00452-22",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "235",
"original-title": [],
"prefix": "10.1128",
"published": {
"date-parts": [
[
2023,
1,
24
]
]
},
"published-print": {
"date-parts": [
[
2023,
1,
24
]
]
},
"publisher": "American Society for Microbiology",
"reference": [
{
"key": "e_1_3_4_2_2",
"unstructured": "World Health Organization. 2022. Weekly epidemiological update on COVID-19. WHO Geneva Switzerland. https://www.who.int/publications/m/item/weekly-epidemiological-update---2-march-2021. Accessed 8 February 2022."
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_3_2"
},
{
"DOI": "10.1056/NEJMc2022236",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_4_2"
},
{
"DOI": "10.1056/NEJMoa2031994",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_5_2"
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_6_2"
},
{
"DOI": "10.1001/jama.2021.0202",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_7_2"
},
{
"DOI": "10.1056/NEJMoa2033700",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_8_2"
},
{
"DOI": "10.1016/0005-2744(77)90097-3",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_9_2"
},
{
"DOI": "10.1038/labinvest.3700203",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_10_2"
},
{
"DOI": "10.1007/s11739-020-02345-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_11_2"
},
{
"DOI": "10.1016/j.coviro.2017.03.018",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_12_2"
},
{
"DOI": "10.1016/j.pupt.2015.07.001",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_13_2"
},
{
"DOI": "10.1101/2020.08.05.237651",
"doi-asserted-by": "crossref",
"key": "e_1_3_4_14_2",
"unstructured": "Hoffmann M Hofmann-Winkler H Smith JC Krüger N Sørensen LK Søgaard OS Hasselstrøm JB Winkler M Hempel T Raich L Olsson S Yamazoe T Yamatsuta K Mizuno H Ludwig S Noé F Sheltzer JM Kjolby M Pöhlmann S. 2020. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. bioRxiv. 10.1101/2020.08.05.237651."
},
{
"DOI": "10.1128/AAC.00754-20",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_15_2"
},
{
"DOI": "10.1128/JVI.01815-18",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_16_2"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_17_2"
},
{
"DOI": "10.1056/NEJMc2027040",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_18_2"
},
{
"DOI": "10.1016/j.eclinm.2021.100849",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_19_2"
},
{
"DOI": "10.1186/s13063-021-05181-0",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_20_2"
},
{
"DOI": "10.1007/s11739-020-02383-3",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_21_2"
},
{
"DOI": "10.1016/S0140-6736(00)02288-1",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_22_2"
},
{
"key": "e_1_3_4_23_2",
"unstructured": "National Cancer Institute. 2017. National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v5.0. National Cancer Institute Bethesda MD. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 25 October 2022."
}
],
"reference-count": 22,
"references-count": 22,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.asm.org/doi/10.1128/aac.00452-22"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "A Double-Blind, Randomized, Placebo-Controlled, Phase II Clinical Study To Evaluate the Efficacy and Safety of Camostat Mesylate (DWJ1248) in Adult Patients with Mild to Moderate COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1128/asmj-crossmark-policy-page",
"volume": "67"
}