A multicenter, double-blind, randomized, parallel-group, placebo-controlled study to evaluate the efficacy and safety of camostat mesilate in patients with COVID-19 (CANDLE study)
et al., BMC Medicine, doi:10.1186/s12916-022-02518-7, CANDLE, NCT04657497, Sep 2022
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 155 hospitalized patients showing no significant differences with camostat.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers TMPRSS2 inhibitors and camostat.
|
progression to non-invasive ventilation or high-flow oxygen, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 74 (0.0%), control 1 of 74 (1.4%), NNT 74, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), Figure 4.
|
|
oxygen at LE, 50.0% lower, RR 0.50, p = 0.37, treatment 4 of 74 (5.4%), control 8 of 74 (10.8%), NNT 18, Figure 4.
|
|
risk of no recovery, 1.5% higher, RR 1.01, p = 1.00, treatment 30 of 53 (56.6%), control 29 of 52 (55.8%).
|
|
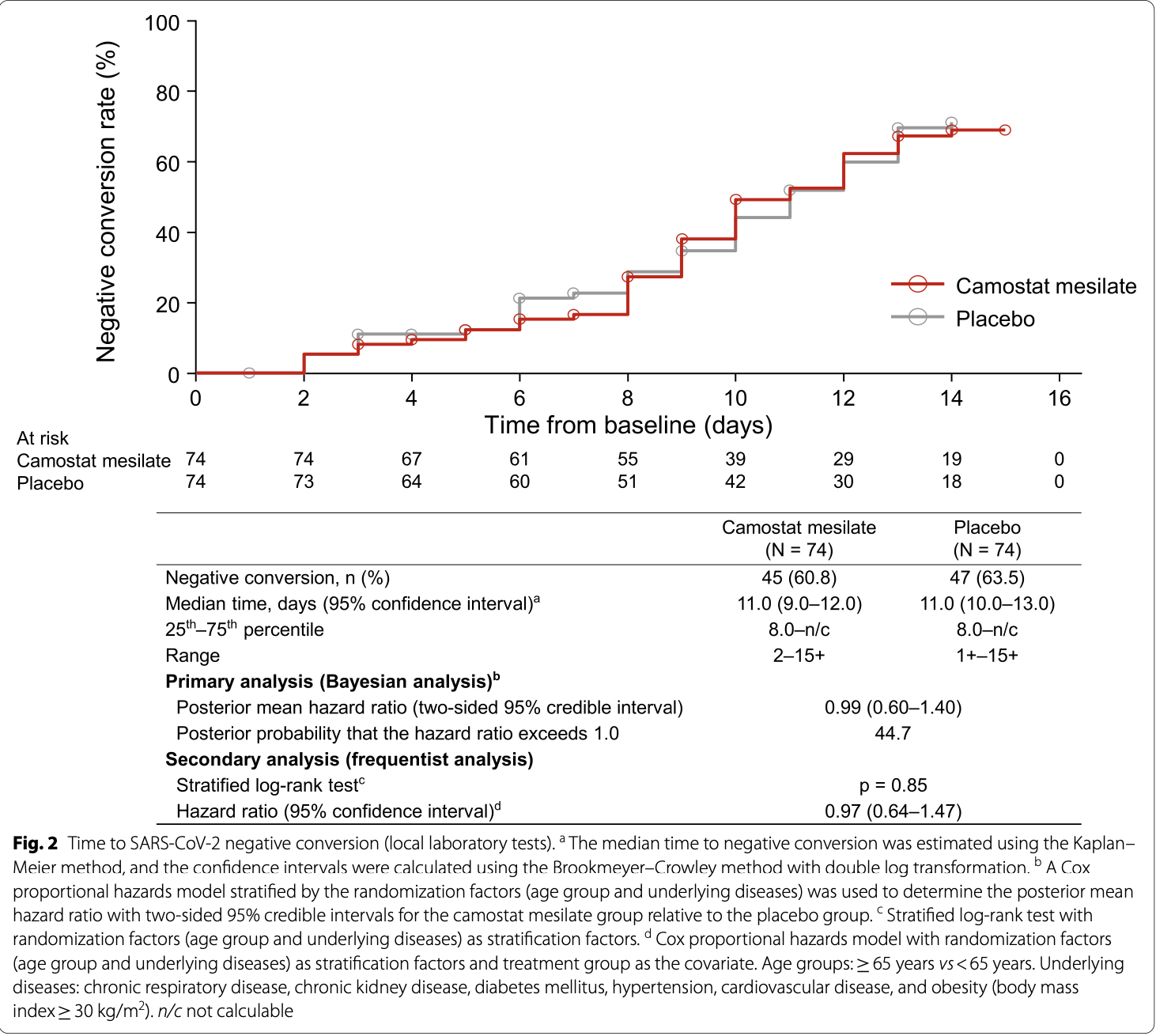
risk of no viral clearance, 1.0% higher, HR 1.01, p = 0.97, treatment 78, control 77, inverted to make HR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kinoshita et al., 27 Sep 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Japan, peer-reviewed, 14 authors, study period November 2020 - March 2021, trial NCT04657497 (history) (CANDLE).
Contact: uemura@oita-u.ac.jp (corresponding author).
A multicenter, double-blind, randomized, parallel-group, placebo-controlled study to evaluate the efficacy and safety of camostat mesilate in patients with COVID-19 (CANDLE study)
BMC Medicine, doi:10.1186/s12916-022-02518-7
Background: In vitro drug screening studies have indicated that camostat mesilate (FOY-305) may prevent SARS-CoV-2 infection into human airway epithelial cells. This study was conducted to investigate whether camostat mesilate is an effective treatment for SARS-CoV-2 infection . Methods: This was a multicenter, double-blind, randomized, parallel-group, placebo-controlled study. Patients were enrolled if they were admitted to a hospital within 5 days of onset of COVID-19 symptoms or within 5 days of a positive test for asymptomatic patients. Severe cases (e.g., those requiring oxygenation/ventilation) were excluded. Patients were enrolled, randomized, and allocated to each group using an interactive web response system. Randomization was performed using a minimization method with the factors medical institution, age, and underlying diseases (chronic respiratory disease, chronic kidney disease, diabetes mellitus, hypertension, cardiovascular diseases, and obesity). The patients, investigators/subinvestigators, study coordinators, and other study personnel were blinded throughout the study. Patients were administered camostat mesilate (600 mg qid; four to eight times higher than the clinical doses in Japan) or placebo for up to 14 days. The primary efficacy endpoint was the time to the first two consecutive negative tests for SARS-CoV-2. Results: One-hundred fifty-five patients were randomized to receive camostat mesilate (n = 78) or placebo (n = 77). The median time to the first test was 11.0 days (95% confidence interval [CI]: 9.0-12.0) in the camostat mesilate group and 11.0 days (95% CI: 10.0-13.0) in the placebo group. Conversion to negative viral status by day 14 was observed in 45 of 74 patients (60.8%) in the camostat mesilate group and 47 of 74 patients (63.5%) in the placebo group. The primary (Bayesian) and secondary (frequentist) analyses found no significant differences in the primary endpoint
Abbreviations ACE2: Angiotensin-converting enzyme II; BMI: Body mass index; CANDLE: Camostat could bring delight to people; EC 50 : Half maximal effective concentration; GBPA: 4-(4-Guanidinobenzoyloxy)phenylacetic acid; LAMP: Loopmediated isothermal amplification; NOAEL: No-observed adverse effect level; PD: Pharmacodynamic; PK: Pharmacokinetic; RT-PCR: Reverse transcriptasepolymerase chain reaction; TMPRSS2: Type II transmembrane serine protease.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s12916-022-02518-7.
Additional file 1: Study protocol. Additional file 2: IRB information. Sample size calculation. Table S1 . Discontinuation criteria. Table S2 . Ordinal scale of severity. Table S3 . Events and reasons for censoring patients for the analysis of time to SARS-CoV-2 negativity. Table S4 . Events and reasons for censoring patients in the sensitivity analysis of the time to SARS-CoV-2 negativity. Table S5 . Changes to the statistical analyses plan from the protocol. Table S6 . Patient disposition. Table S7 . Results of the sensitivity analysis of the primary endpoint. Table S8 . Time to negative SARS-CoV-2 status as measured by the central laboratory. Table S9 . Results of the subgroup analyses of time to negative SARS-CoV-2 status. Additional file 3: CONSORT Checklist.
Authors' contributions Conceptualization: NK and JK. Data curation: not applicable. Formal analysis: KY. Funding..
References
Beckh, Göke, Müller, Arnold, Elimination of the low-molecular weight proteinase inhibitor camostate (FOY 305) and its degradation products by the rat liver, Res Exp Med (Berl), doi:10.1007/BF01852177
Breining, Frølund, Højen, Gunst, Staerke et al., Camostat mesylate against SARS-CoV-2 and COVID-19-rationale, dosing and safety, Basic Clin Pharmacol Toxicol, doi:10.1111/bcpt.13533
Budinger, Misharin, Ridge, Singer, Wunderink, Distinctive features of severe SARS-CoV-2 pneumonia, J Clin Invest, doi:10.1172/JCI149412
Cadegiani, Repurposing existing drugs for COVID-19: an endocrinology perspective, BMC Endocr Disord, doi:10.1186/s12902-020-00626-0
Chupp, Spichler-Moffarah, Søgaard, Esserman, Dziura et al., A phase 2 randomized, double-blind, placebo-controlled trial of oral camostat mesylate for early treatment of COVID-19 outpatients showed shorter illness course and attenuation of loss of smell and taste, medRxiv, doi:10.1101/2022.01.28.22270035
Deng, Rasool, Russell, Natesan, Asangani, Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19, iScience, doi:10.1016/j.isci.2021.102254
Escalante, Ferguson, Structural modeling and analysis of the SARS-CoV-2 cell entry inhibitor camostat bound to the trypsin-like protease TMPRSS2, Med Chem Res, doi:10.1007/s00044-021-02708-7
Faheem, Kumar, Sekhar, Kunjiappan, Jamalis et al., Druggable targets of SARS-CoV-2 and treatment opportunities for COVID-19, Bioorg Chem, doi:10.1016/j.bioorg.2020.104269
Gunst, Staerke, Pahus, Kristensen, Bodilsen et al., Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with COVID-19-a double-blind randomized controlled trial, EClinical-Medicine, doi:10.1016/j.eclinm.2021.100849
Gyebi, Adegunloye, Ibrahim, Ogunyemi, Afolabi et al., Prevention of SARS-CoV-2 cell entry: insight from in silico interaction of drug-like alkaloids with spike glycoprotein, human ACE2, and TMPRSS2, J Biomol Struct Dyn, doi:10.1080/07391102.2020.1835726
Hale, Angrist, Hale, Kira, Majumdar et al., Government responses and COVID-19 deaths: global evidence across multiple pandemic waves, PLoS One, doi:10.1371/journal.pone.0253116
Hiraku, Muryobayashi, Ito, Inagawa, Tuboshima, Absorption and excretion of camostat (FOY-305) orally administered to male rabbit and healthy subject, Iyaku Kenkyu
Hoffmann, Hofmann-Winkler, Smith, Krüger, Arora et al., Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity, EBio-Medicine, doi:10.1016/j.ebiom.2021.103255
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hofmann-Winkler, Moerer, Alt-Epping, Bräuer, Büttner et al., Camostat mesylate may reduce severity of coronavirus disease 2019 sepsis: a first observation, Crit Care Explor, doi:10.1097/CCE.0000000000000284
Hörnich, Großkopf, Schlagowski, Tenbusch, Kleine-Weber et al., SARS-CoV-2 and SARS-CoV spike-mediated cell-cell fusion differ in their requirements for receptor expression and proteolytic activation, J Virol, doi:10.1128/JVI.00002-21
Kim, Ejima, Iwanami, Fujita, Ohashi et al., A quantitative model used to compare within-host SARS-CoV-2, MERS-CoV, and SARS-CoV dynamics provides insights into the pathogenesis and treatment of SARS-CoV-2, PLoS Biol, doi:10.1371/journal.pbio.3001128
Kishk, Kishk, Yassen, Nafie, Nemr et al., Molecular insights into human transmembrane protease serine-2 (TMPS2) inhibitors against SARS-CoV2: homology modelling, molecular dynamics, and docking studies, Molecules, doi:10.3390/molecules25215007
Kitagawa, Arai, Iida, Mukai, Furukawa et al., A phase I study of high dose camostat mesylate in healthy adults provides a rationale to repurpose the TMPRSS2 inhibitor for the treatment of COVID-19, Clin Transl Sci, doi:10.1111/cts.13052
Kupferschmidt, Cohen, Race to find COVID-19 treatments accelerates, Science, doi:10.1126/science.367.6485.1412
Matsuoka, Fujita, Matsuo, Toxicity study of FOY-305(I): acute toxicity study in mice and rats, subacute toxicity study in dogs, Gendai Iryo
Mehta, Fajgenbaum, Is severe COVID-19 a cytokine storm syndrome: a hyperinflammatory debate, Curr Opin Rheumatol, doi:10.1097/BOR.0000000000000822
Midgley, Hood, Proctor, Chasseaud, Irons et al., Metabolic fate of 14C-camostat mesylate in man, rat and dog after intravenous administration, Xenobiotica, doi:10.3109/00498259409043223
O'brien, Forleo-Neto, Musser, Chan, Sarkar, Subcutaneous REGEN-COV antibody combination to prevent COVID-19, N Engl J Med, doi:10.1056/NEJMoa2109682
Sakr, Bensasi, Taha, Bauer, Ismail, Camostat mesylate therapy in critically ill patients with COVID-19 pneumonia, Intensive Care Med, doi:10.1007/s00134-021-06395-1
Santos, Brierley, Gandhi, Cohen, Moschella et al., Repurposing therapeutics for potential treatment of SARS-CoV-2: a review, Viruses, doi:10.3390/v12070705
Uno, Camostat mesilate therapy for COVID-19, Intern Emerg Med, doi:10.1007/s11739-020-02345-9
Viana, Van Dorp, Nunes, Gomes, Van Boven et al., Controlling the pandemic during the SARS-CoV-2 vaccination rollout, Nat Commun, doi:10.1038/s41467-021-23938-8
Watson, Kissler, Day, Grad, White, Characterizing SARS-CoV-2 viral clearance kinetics to improve the design of antiviral pharmacometric studies, Antimicrob Agents Chemother, doi:10.1128/aac.00192-22
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.1186/s12916-022-02518-7",
"ISSN": [
"1741-7015"
],
"URL": "http://dx.doi.org/10.1186/s12916-022-02518-7",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>In vitro drug screening studies have indicated that camostat mesilate (FOY-305) may prevent SARS-CoV-2 infection into human airway epithelial cells. This study was conducted to investigate whether camostat mesilate is an effective treatment for SARS-CoV-2 infection (COVID-19).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This was a multicenter, double-blind, randomized, parallel-group, placebo-controlled study. Patients were enrolled if they were admitted to a hospital within 5 days of onset of COVID-19 symptoms or within 5 days of a positive test for asymptomatic patients. Severe cases (e.g., those requiring oxygenation/ventilation) were excluded. Patients were enrolled, randomized, and allocated to each group using an interactive web response system. Randomization was performed using a minimization method with the factors medical institution, age, and underlying diseases (chronic respiratory disease, chronic kidney disease, diabetes mellitus, hypertension, cardiovascular diseases, and obesity). The patients, investigators/subinvestigators, study coordinators, and other study personnel were blinded throughout the study. Patients were administered camostat mesilate (600 mg qid; four to eight times higher than the clinical doses in Japan) or placebo for up to 14 days. The primary efficacy endpoint was the time to the first two consecutive negative tests for SARS-CoV-2.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>One-hundred fifty-five patients were randomized to receive camostat mesilate (<jats:italic>n</jats:italic> = 78) or placebo (<jats:italic>n</jats:italic> = 77). The median time to the first test was 11.0 days (95% confidence interval [CI]: 9.0–12.0) in the camostat mesilate group and 11.0 days (95% CI: 10.0–13.0) in the placebo group. Conversion to negative viral status by day 14 was observed in 45 of 74 patients (60.8%) in the camostat mesilate group and 47 of 74 patients (63.5%) in the placebo group. The primary (Bayesian) and secondary (frequentist) analyses found no significant differences in the primary endpoint between the two groups. No additional safety concerns beyond those already known for camostat mesilate were identified.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Camostat mesilate did not substantially reduce the time to viral clearance, based on upper airway viral loads, compared with placebo for treating patients with mild to moderate SARS-CoV-2 infection with or without symptoms.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Trial registration</jats:title>\n <jats:p>ClinicalTrials.gov, NCT04657497. Japan Registry for Clinical Trials, jRCT2031200198.</jats:p>\n </jats:sec>",
"alternative-id": [
"2518"
],
"article-number": "342",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "28 April 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "5 August 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "27 September 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Date",
"name": "change_date",
"order": 4,
"value": "8 December 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Type",
"name": "change_type",
"order": 5,
"value": "Correction"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Details",
"name": "change_details",
"order": 6,
"value": "A Correction to this paper has been published:"
},
{
"URL": "https://doi.org/10.1186/s12916-022-02695-5",
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Change Details",
"name": "change_details",
"order": 7,
"value": "https://doi.org/10.1186/s12916-022-02695-5"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This study adhered to the Declaration of Helsinki, Good Clinical Practice, and relevant local/international guidelines. The protocol and patient consent forms were first reviewed and approved by the institutional review board at the leading study site (International University of Health and Welfare Narita Hospital, approval number FN-1-2005-075) and subsequently by the institutional review boards/ethics committees at all participating sites, which are listed in Additional file InternalRef removed: IRB information. All patients provided written informed consent to participate in this study."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The sponsor provided funding for the study and publication of the manuscript. Employees of the sponsor were involved in the study design and collection, analysis, and interpretation of the data and reviewed the manuscript. The corresponding author had full access to all the data and had final responsibility for the decision to submit the manuscript for publication.TK, MS1, KS, YH, and KT report institution research funding from Ono Pharmaceutical Co. Ltd. in relation to this work.YN reports institution research funding from Ono Pharmaceutical Co. Ltd. in relation to this work and institution research funding from ITO EN Co. Ltd., AstaReal Co. Ltd., Mizkan Holdings Co. Ltd., and Kanagawa Institute of Industrial Science and Technology unrelated to this work.YK1 reports institution research funding from Ono Pharmaceutical Co. Ltd. in relation to this work and institution research funding from Nobelpharma Co. Ltd., Chugai Pharmaceutical Co. Ltd., Japan Tobacco Inc., and Pfizer Japan Inc. unrelated to this work.MS2 reports institution research funding from Ono Pharmaceutical Co. Ltd. in relation to this work and institutional research funding from FUJIFILM Toyama Chemical Co. Ltd., AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd., Pfizer Japan Inc., and Genova Inc. unrelated to this work.NK and KY are employees of Ono Pharmaceutical Co. Ltd.YK2 reports consultancy fees from Ono Pharmaceutical Co. Ltd. in relation to this work; research funding from Abbott Medical Japan, LLC, and ROHTO Pharmaceutical Co. Ltd. unrelated to this work; lecture fees from Abbott Medical Japan, LLC, unrelated to this work; equity in Quantum Molecular Diagnostics Japan, LLC, unrelated to this work; and reagents for serological testing from Abbott Medical Japan, LLC, unrelated to this work.HK reports consultancy fees from Ono Pharmaceutical Co., Ltd. in relation to this work and lecture fees from MSD K.K., Pfizer Japan Inc., and Shionogi & Co. Ltd. unrelated to this work.NU reports consultancy fees from Ono Pharmaceutical Co. Ltd. in relation to this work and the following activities unrelated to this work: institution research grants from Regeneron Pharma Inc., Eli Lilly Japan K.K., Japan Agency for Medical Research and Development, ARTham Therapeutics Inc., EA Pharma Co. Ltd., and VLP Therapeutics, LLC; consultancy fees from Ono Pharmaceutical Co. Ltd., Mitsubishi Tanabe Pharma Corp., Sato Pharmaceutical, Co. Ltd., ARTham Therapeutics Inc., Otsuka Pharmaceutical Co. Ltd., EA Pharma Co. Ltd., and Maruho Co. Ltd., honoraria from Mochida Pharmaceutical Co. Ltd., fees for participating on advisory boards from Japan Research Foundation Clinical Pharmacology, the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour and Welfare; fees for leadership or fiduciary roles for American Society for Clinical Pharmacology and Therapeutics and GHIT Fund; unpaid board member for Japanese Society for Clinical Pharmacology and Therapeutics, Clinical Research Support Center Kyushu and Oita IAM; stock or stock options in ARTham Therapeutics Inc. and Oita IAM; and monetary gifts to the institution from Oita IAM.JK reports consultancy fees from Ono Pharmaceutical Co. Ltd. in relation to this work and consultancy fees from FUJIFILM Toyama Chemical Co. Ltd., KOBAYASHI Pharma Co. Ltd., and Kyorin Pharma Co. Ltd. unrelated to this work; institutional research funding from MSD Co. Ltd., Taisho Pharma Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Daiichi Sankyo Co. Ltd., Pfizer Japan Inc., Kyorin Pharma Co. Ltd., Astellas Pharma Inc., Chugai Pharmaceutical Co. Ltd., Shionogi & Co. Ltd., and Teijin Pharma Ltd. unrelated to this work; and lecture fees from Ono Pharmaceutical Co. Ltd., MSD Co. Ltd., AstraZeneca K.K., Nippon Boehringer Ingelheim Co. Ltd., Pfizer Japan Inc., Shionogi & Co. Ltd., Taisho Toyama Pharma Co. Ltd., Meiji Seika Pharma Co. Ltd., Sanofi K.K., Kyorin Pharma Co. Ltd., Astellas Pharma Inc., Sumitomo Dainippon Pharma Co. Ltd., Bristol-Myers Squibb Company, Daiichi Sankyo Co. Ltd., Chugai Pharmaceutical Co. Ltd., Novartis Pharma K.K., Taisho Pharma Co. Ltd., FUJIFILM Medical Co. Ltd., GlaxoSmithKline K.K., and DENKA SEIKEN Co. Ltd. unrelated to this work."
}
],
"author": [
{
"affiliation": [],
"family": "Kinoshita",
"given": "Taku",
"sequence": "first"
},
{
"affiliation": [],
"family": "Shinoda",
"given": "Masahiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nishizaki",
"given": "Yasuhiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shiraki",
"given": "Katsuya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hirai",
"given": "Yuji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kichikawa",
"given": "Yoshiko",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsushima",
"given": "Kenji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shinkai",
"given": "Masaharu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Komura",
"given": "Naoyuki",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yoshida",
"given": "Kazuo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kido",
"given": "Yasutoshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kakeya",
"given": "Hiroshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Uemura",
"given": "Naoto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kadota",
"given": "Junichi",
"sequence": "additional"
}
],
"container-title": "BMC Medicine",
"container-title-short": "BMC Med",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
9,
27
]
],
"date-time": "2022-09-27T00:02:33Z",
"timestamp": 1664236953000
},
"deposited": {
"date-parts": [
[
2022,
12,
9
]
],
"date-time": "2022-12-09T07:03:46Z",
"timestamp": 1670569426000
},
"funder": [
{
"DOI": "10.13039/501100013170",
"doi-asserted-by": "publisher",
"name": "Ono Pharmaceutical"
}
],
"indexed": {
"date-parts": [
[
2023,
10,
5
]
],
"date-time": "2023-10-05T17:40:34Z",
"timestamp": 1696527634315
},
"is-referenced-by-count": 9,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
9,
27
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
27
]
],
"date-time": "2022-09-27T00:00:00Z",
"timestamp": 1664236800000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
27
]
],
"date-time": "2022-09-27T00:00:00Z",
"timestamp": 1664236800000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12916-022-02518-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12916-022-02518-7/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12916-022-02518-7.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2022,
9,
27
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
27
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1371/journal.pone.0253116",
"doi-asserted-by": "publisher",
"key": "2518_CR1",
"unstructured": "Hale T, Angrist N, Hale AJ, Kira B, Majumdar S, Petherick A, et al. Government responses and COVID-19 deaths: global evidence across multiple pandemic waves. PLoS One. 2021;16:e0253116. https://doi.org/10.1371/journal.pone.0253116."
},
{
"DOI": "10.1038/s41467-021-23938-8",
"author": "J Viana",
"doi-asserted-by": "publisher",
"first-page": "3674",
"journal-title": "Nat Commun",
"key": "2518_CR2",
"unstructured": "Viana J, van Dorp CH, Nunes A, Gomes MC, van Boven M, Kretzschmar ME, et al. Controlling the pandemic during the SARS-CoV-2 vaccination rollout. Nat Commun. 2021;12:3674. https://doi.org/10.1038/s41467-021-23938-8.",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1097/BOR.0000000000000822",
"author": "P Mehta",
"doi-asserted-by": "publisher",
"first-page": "419",
"journal-title": "Curr Opin Rheumatol",
"key": "2518_CR3",
"unstructured": "Mehta P, Fajgenbaum DC. Is severe COVID-19 a cytokine storm syndrome: a hyperinflammatory debate. Curr Opin Rheumatol. 2021;33:419–30. https://doi.org/10.1097/BOR.0000000000000822.",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.1172/JCI149412",
"author": "GR Scott Budinger",
"doi-asserted-by": "publisher",
"first-page": "e149412",
"journal-title": "J Clin Invest",
"key": "2518_CR4",
"unstructured": "Scott Budinger GR, Misharin AV, Ridge KM, Singer BD, Wunderink RG. Distinctive features of severe SARS-CoV-2 pneumonia. J Clin Invest. 2021;131:e149412. https://doi.org/10.1172/JCI149412.",
"volume": "131",
"year": "2021"
},
{
"key": "2518_CR5",
"unstructured": "National Institutes of Health. COVID-19 treatment guidelines: antiviral therapy. Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/. Accessed: June 24, 2022."
},
{
"DOI": "10.1111/bcpt.13533",
"doi-asserted-by": "publisher",
"key": "2518_CR6",
"unstructured": "Breining P, Frølund AL, Højen JF, Gunst JD, Staerke NB, Saedder E, et al. Camostat mesylate against SARS-CoV-2 and COVID-19—rationale, dosing and safety. Basic Clin Pharmacol Toxicol. 2021;128:204–12. https://doi.org/10.1111/bcpt.13533."
},
{
"DOI": "10.1016/j.isci.2021.102254",
"author": "Q Deng",
"doi-asserted-by": "publisher",
"first-page": "102254",
"journal-title": "iScience",
"key": "2518_CR7",
"unstructured": "Deng Q, Rasool RU, Russell RM, Natesan R, Asangani IA. Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19. iScience. 2021;24:102254. https://doi.org/10.1016/j.isci.2021.102254.",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2021.103255",
"author": "M Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "103255",
"journal-title": "EBioMedicine",
"key": "2518_CR8",
"unstructured": "Hoffmann M, Hofmann-Winkler H, Smith JC, Krüger N, Arora P, Sørensen LK, et al. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine. 2021;65:103255. https://doi.org/10.1016/j.ebiom.2021.103255.",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "2518_CR9",
"unstructured": "Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80.e8. https://doi.org/10.1016/j.cell.2020.02.052."
},
{
"DOI": "10.1007/s00044-021-02708-7",
"author": "DE Escalante",
"doi-asserted-by": "publisher",
"first-page": "399",
"journal-title": "Med Chem Res",
"key": "2518_CR10",
"unstructured": "Escalante DE, Ferguson DM. Structural modeling and analysis of the SARS-CoV-2 cell entry inhibitor camostat bound to the trypsin-like protease TMPRSS2. Med Chem Res. 2021;30:399–409. https://doi.org/10.1007/s00044-021-02708-7.",
"volume": "30",
"year": "2021"
},
{
"DOI": "10.1016/j.bioorg.2020.104269",
"doi-asserted-by": "publisher",
"key": "2518_CR11",
"unstructured": "Faheem, Kumar BK, Sekhar KVGC, Kunjiappan S, Jamalis J, Balaña-Fouce R, et al. Druggable targets of SARS-CoV-2 and treatment opportunities for COVID-19. Bioorg Chem. 2020;104:104269. https://doi.org/10.1016/j.bioorg.2020.104269."
},
{
"DOI": "10.1080/07391102.2020.1835726",
"author": "GA Gyebi",
"doi-asserted-by": "publisher",
"first-page": "2121",
"journal-title": "J Biomol Struct Dyn",
"key": "2518_CR12",
"unstructured": "Gyebi GA, Adegunloye AP, Ibrahim IM, Ogunyemi OM, Afolabi SO, Ogunro OB. Prevention of SARS-CoV-2 cell entry: insight from in silico interaction of drug-like alkaloids with spike glycoprotein, human ACE2, and TMPRSS2. J Biomol Struct Dyn. 2022;40:2121–45. https://doi.org/10.1080/07391102.2020.1835726.",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.1128/JVI.00002-21",
"author": "BF Hörnich",
"doi-asserted-by": "publisher",
"first-page": "e00002",
"journal-title": "J Virol",
"key": "2518_CR13",
"unstructured": "Hörnich BF, Großkopf AK, Schlagowski S, Tenbusch M, Kleine-Weber H, Neipel F, et al. SARS-CoV-2 and SARS-CoV spike-mediated cell-cell fusion differ in their requirements for receptor expression and proteolytic activation. J Virol. 2021;95:e00002-21. https://doi.org/10.1128/JVI.00002-21.",
"volume": "95",
"year": "2021"
},
{
"DOI": "10.3390/molecules25215007",
"author": "SM Kishk",
"doi-asserted-by": "publisher",
"first-page": "5007",
"journal-title": "Molecules",
"key": "2518_CR14",
"unstructured": "Kishk SM, Kishk RM, Yassen ASA, Nafie MS, Nemr NA, ElMasry G, et al. Molecular insights into human transmembrane protease serine-2 (TMPS2) inhibitors against SARS-CoV2: homology modelling, molecular dynamics, and docking studies. Molecules. 2020;25:5007. https://doi.org/10.3390/molecules25215007.",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1186/s12902-020-00626-0",
"author": "FA Cadegiani",
"doi-asserted-by": "publisher",
"first-page": "149",
"journal-title": "BMC Endocr Disord",
"key": "2518_CR15",
"unstructured": "Cadegiani FA. Repurposing existing drugs for COVID-19: an endocrinology perspective. BMC Endocr Disord. 2020;20:149. https://doi.org/10.1186/s12902-020-00626-0.",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.3390/v12070705",
"author": "J Santos",
"doi-asserted-by": "publisher",
"first-page": "705",
"journal-title": "Viruses",
"key": "2518_CR16",
"unstructured": "Santos J, Brierley S, Gandhi MJ, Cohen MA, Moschella PC, Declan ABL. Repurposing therapeutics for potential treatment of SARS-CoV-2: a review. Viruses. 2020;12:705. https://doi.org/10.3390/v12070705.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1007/s11739-020-02345-9",
"author": "Y Uno",
"doi-asserted-by": "publisher",
"first-page": "1577",
"journal-title": "Intern Emerg Med",
"key": "2518_CR17",
"unstructured": "Uno Y. Camostat mesilate therapy for COVID-19. Intern Emerg Med. 2020;15:1577–8. https://doi.org/10.1007/s11739-020-02345-9.",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1097/CCE.0000000000000284",
"author": "H Hofmann-Winkler",
"doi-asserted-by": "publisher",
"first-page": "e0284",
"journal-title": "Crit Care Explor",
"key": "2518_CR18",
"unstructured": "Hofmann-Winkler H, Moerer O, Alt-Epping S, Bräuer A, Büttner B, Müller M, et al. Camostat mesylate may reduce severity of coronavirus disease 2019 sepsis: a first observation. Crit Care Explor. 2020;2:e0284. https://doi.org/10.1097/CCE.0000000000000284.",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"journal-title": "Lancet",
"key": "2518_CR19",
"unstructured": "Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. https://doi.org/10.1016/S0140-6736(20)30566-3.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1111/cts.13052",
"author": "J Kitagawa",
"doi-asserted-by": "publisher",
"first-page": "1967",
"journal-title": "Clin Transl Sci",
"key": "2518_CR20",
"unstructured": "Kitagawa J, Arai H, Iida H, Mukai J, Furukawa K, Ohtsu S, et al. A phase I study of high dose camostat mesylate in healthy adults provides a rationale to repurpose the TMPRSS2 inhibitor for the treatment of COVID-19. Clin Transl Sci. 2021;14:1967–76. https://doi.org/10.1111/cts.13052.",
"volume": "14",
"year": "2021"
},
{
"key": "2518_CR21",
"unstructured": "World Health Organization R&D Blue Print Team. WHO R&D Blueprint: novel coronavirus - COVID-19 therapeutic trial synopsis. Available at: https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis. Accessed: July 22, 2021."
},
{
"DOI": "10.1007/s00134-021-06395-1",
"author": "Y Sakr",
"doi-asserted-by": "publisher",
"first-page": "707",
"journal-title": "Intensive Care Med",
"key": "2518_CR22",
"unstructured": "Sakr Y, Bensasi H, Taha A, Bauer M, Ismail K. Camostat mesylate therapy in critically ill patients with COVID-19 pneumonia. Intensive Care Med. 2021;47:707–9. https://doi.org/10.1007/s00134-021-06395-1.",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.100849",
"doi-asserted-by": "publisher",
"key": "2518_CR23",
"unstructured": "Gunst JD, Staerke NB, Pahus MH, Kristensen LH, Bodilsen J, Lohse N, et al. Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with COVID-19—a double-blind randomized controlled trial. EClinicalMedicine. 2021;35:100849. https://doi.org/10.1016/j.eclinm.2021.100849."
},
{
"DOI": "10.1101/2022.01.28.22270035",
"doi-asserted-by": "publisher",
"key": "2518_CR24",
"unstructured": "Chupp G, Spichler-Moffarah A, Søgaard OS, Esserman D, Dziura J, Danzig L, et al. A phase 2 randomized, double-blind, placebo-controlled trial of oral camostat mesylate for early treatment of COVID-19 outpatients showed shorter illness course and attenuation of loss of smell and taste. medRxiv. 2022.01.28.22270035. https://doi.org/10.1101/2022.01.28.22270035."
},
{
"DOI": "10.1126/science.367.6485.1412",
"author": "K Kupferschmidt",
"doi-asserted-by": "publisher",
"first-page": "1412",
"journal-title": "Science",
"key": "2518_CR25",
"unstructured": "Kupferschmidt K, Cohen J. Race to find COVID-19 treatments accelerates. Science. 2020;367:1412–3. https://doi.org/10.1126/science.367.6485.1412.",
"volume": "367",
"year": "2020"
},
{
"DOI": "10.1371/journal.pbio.3001128",
"author": "KS Kim",
"doi-asserted-by": "publisher",
"first-page": "e3001128",
"journal-title": "PLoS Biol",
"key": "2518_CR26",
"unstructured": "Kim KS, Ejima K, Iwanami S, Fujita Y, Ohashi H, Koizumi Y, et al. A quantitative model used to compare within-host SARS-CoV-2, MERS-CoV, and SARS-CoV dynamics provides insights into the pathogenesis and treatment of SARS-CoV-2. PLoS Biol. 2021;19:e3001128. https://doi.org/10.1371/journal.pbio.3001128.",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2109682",
"author": "MP O’Brien",
"doi-asserted-by": "publisher",
"first-page": "1184",
"journal-title": "N Engl J Med",
"key": "2518_CR27",
"unstructured": "O’Brien MP, Forleo-Neto E, Musser BJ, Isa F, Chan KC, Sarkar N, et al. Subcutaneous REGEN-COV antibody combination to prevent COVID-19. N Engl J Med. 2021;385:1184–95. https://doi.org/10.1056/NEJMoa2109682.",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1007/BF01852177",
"author": "K Beckh",
"doi-asserted-by": "publisher",
"first-page": "401",
"journal-title": "Res Exp Med (Berl)",
"key": "2518_CR28",
"unstructured": "Beckh K, Göke B, Müller R, Arnold R. Elimination of the low-molecular weight proteinase inhibitor camostate (FOY 305) and its degradation products by the rat liver. Res Exp Med (Berl). 1987;187:401–6. https://doi.org/10.1007/BF01852177.",
"volume": "187",
"year": "1987"
},
{
"DOI": "10.3109/00498259409043223",
"author": "I Midgley",
"doi-asserted-by": "publisher",
"first-page": "79",
"journal-title": "Xenobiotica",
"key": "2518_CR29",
"unstructured": "Midgley I, Hood AJ, Proctor P, Chasseaud LF, Irons SR, Cheng KN, et al. Metabolic fate of 14C-camostat mesylate in man, rat and dog after intravenous administration. Xenobiotica. 1994;24:79–92. https://doi.org/10.3109/00498259409043223.",
"volume": "24",
"year": "1994"
},
{
"author": "S Hiraku",
"first-page": "756",
"journal-title": "Iyaku Kenkyu",
"key": "2518_CR30",
"unstructured": "Hiraku S, Muryobayashi K, Ito H, Inagawa T, Tuboshima M. Absorption and excretion of camostat (FOY-305) orally administered to male rabbit and healthy subject. Iyaku Kenkyu. 1982;13:756–65 [In Japanese with English abstract].",
"volume": "13",
"year": "1982"
},
{
"key": "2518_CR31",
"unstructured": "Matsuoka Y, Fujita T, Matsuo S, et al. Toxicity study of FOY-305(I): acute toxicity study in mice and rats, subacute toxicity study in dogs. Gendai Iryo. 1980;12:153–78 [In Japanese]."
},
{
"key": "2518_CR32",
"unstructured": "U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers, July 2005. Available at: https://www.fda.gov/media/72309/download. Accessed 9 Dec 2020."
},
{
"DOI": "10.1128/aac.00192-22",
"doi-asserted-by": "publisher",
"key": "2518_CR33",
"unstructured": "Watson JA, Kissler SM, Day NPJ, Grad YH, White NJ. Characterizing SARS-CoV-2 viral clearance kinetics to improve the design of antiviral pharmacometric studies. Antimicrob Agents Chemother. 2022;66(7):e0019222. https://doi.org/10.1128/aac.00192-22. "
}
],
"reference-count": 33,
"references-count": 33,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-022-02518-7"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "A multicenter, double-blind, randomized, parallel-group, placebo-controlled study to evaluate the efficacy and safety of camostat mesilate in patients with COVID-19 (CANDLE study)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "20"
}
