Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19
et al., NEJM, doi:10.1056/NEJMoa2109682 (press release 4/12/21), NCT04452318, Apr 2021
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Prophylaxis trial reporting lower hospitalization/ER and symptomatic cases, and faster recovery with 1,200mg subcutaneous casirivimab with imdevimab. The same trial has updated results available in1. NCT04452318 (history).
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants2-8.
|
risk of hospitalization/ER, 88.9% lower, RR 0.11, p = 0.06, treatment 0 of 753 (0.0%), control 4 of 752 (0.5%), NNT 188, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), day 29.
|
|
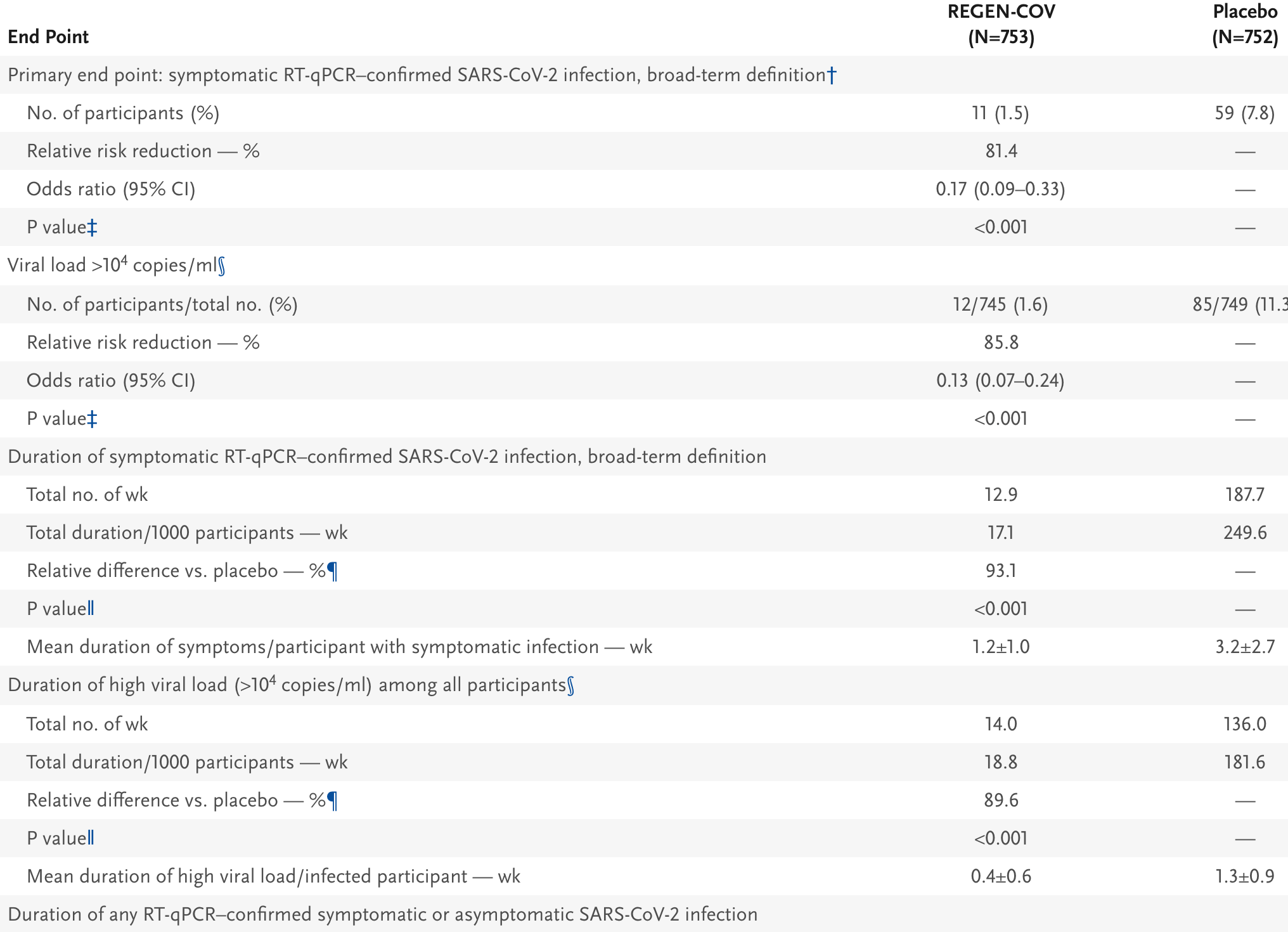
risk of symptomatic case, 81.4% lower, RR 0.19, p < 0.001, treatment 11 of 753 (1.5%), control 59 of 752 (7.8%), NNT 16, day 29.
|
|
recovery time, 62.5% lower, relative time 0.37, p < 0.001, treatment 753, control 752, relative time with symptoms.
|
|
time to viral-, 69.2% lower, relative time 0.31, p < 0.001, treatment 753, control 752, relative time with high viral load.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
2.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
3.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
4.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
5.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
6.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
O'Brien et al., 12 Apr 2021, Double Blind Randomized Controlled Trial, multiple countries, peer-reviewed, 36 authors, study period 13 July, 2020 - 28 January, 2021, trial NCT04452318 (history).
Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19
New England Journal of Medicine, doi:10.1056/nejmoa2109682
BACKGROUND REGEN-COV (previously known as REGN-COV2), a combination of the monoclonal antibodies casirivimab and imdevimab, has been shown to markedly reduce the risk of hospitalization or death among high-risk persons with coronavirus disease 2019 (Covid-19). Whether subcutaneous REGEN-COV prevents severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and subsequent Covid-19 in persons at high risk for infection because of household exposure to a person with SARS-CoV-2 infection is unknown.
METHODS We randomly assigned, in a 1:1 ratio, participants (≥12 years of age) who were enrolled within 96 hours after a household contact received a diagnosis of SARS-CoV-2 infection to receive a total dose of 1200 mg of REGEN-COV or matching placebo administered by means of subcutaneous injection. At the time of randomization, participants were stratified according to the results of the local diagnostic assay for SARS-CoV-2 and according to age. The primary efficacy end point was the development of symptomatic SARS-CoV-2 infection through day 28 in participants who did not have SARS-CoV-2 infection (as measured by reverse-transcriptasequantitative polymerase-chain-reaction assay) or previous immunity (seronegativity).
RESULTS Symptomatic SARS-CoV-2 infection developed in 11 of 753 participants in the REGEN-COV group (1.5%) and in 59 of 752 participants in the placebo group (7.8%) (relative risk reduction [1 minus the relative risk], 81.4%; P<0.001). In weeks 2 to 4, a total of 2 of 753 participants in the REGEN-COV group (0.3%) and 27 of 752 participants in the placebo group (3.6%) had symptomatic SARS-CoV-2 infection (relative risk reduction, 92.6%). REGEN-COV also prevented symptomatic and asymptomatic infections overall (relative risk reduction, 66.4%). Among symptomatic infected participants, the median time to resolution of symptoms was 2 weeks shorter with REGEN-COV than with placebo (1.2 weeks and 3.2 weeks, respectively), and the duration of a high viral load (>10 4 copies per milliliter) was shorter (0.4 weeks and 1.3 weeks, respectively). No dose-limiting toxic effects of REGEN-COV were noted.
CONCLUSIONS Subcutaneous REGEN-COV prevented symptomatic Covid-19 and asymptomatic SARS-CoV-2 infection in previously uninfected household contacts of infected persons. Among the participants who became infected, REGEN-COV reduced the duration of symptomatic disease and the duration of a high viral load. (Funded by Regeneron Pharmaceuticals and others; ClinicalTrials.gov number, NCT04452318.
Appendix The authors' full names and academic degrees are as follows: Meagan P. O'Brien, M.D., Eduardo Forleo-Neto, M.D., Bret J. Musser, Ph.D., Flonza Isa, M.D., Kuo-Chen Chan, Ph.D., Neena Sarkar, Ph.D., Katharine J. Bar
References
Baum, Fulton, Wloga, Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies, Science
Copin, Baum, Wloga, The monoclonal antibody combination REGEN-COV protects against SARS-CoV-2 mutational escape in preclinical and human studies, Cell
Madewell, Yang, Longini, Jr, Halloran et al., Household transmission of SARS-CoV-2: a systematic review and meta-analysis, JAMA Netw Open
Wang, Nair, Liu, Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7, Nature
Weinreich, Sivapalasingam, Norton, Fact sheet for health care providers: Emergency Use Authorization (EUA) of REGEN-COV (casirivimab and imdevimab)
Weinreich, Sivapalasingam, Norton, REGEN-COV antibody cocktail clinical outcomes study in Covid-19 outpatients, doi:10.1101/2021.05.19
Weinreich, Sivapalasingam, Norton, REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med
Wu, Zhao, Yu, A new coronavirus associated with human respiratory disease in China, Nature
Zhu, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, N Engl J Med
DOI record:
{
"DOI": "10.1056/nejmoa2109682",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/NEJMoa2109682",
"alternative-id": [
"10.1056/NEJMoa2109682"
],
"author": [
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "O’Brien",
"given": "Meagan P.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Forleo-Neto",
"given": "Eduardo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Musser",
"given": "Bret J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Isa",
"given": "Flonza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Chan",
"given": "Kuo-Chen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Sarkar",
"given": "Neena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Bar",
"given": "Katharine J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Barnabas",
"given": "Ruanne V.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Barouch",
"given": "Dan H.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Cohen",
"given": "Myron S.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Hurt",
"given": "Christopher B.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Burwen",
"given": "Dale R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Marovich",
"given": "Mary A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Hou",
"given": "Peijie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Heirman",
"given": "Ingeborg",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6282-1670",
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"authenticated-orcid": false,
"family": "Davis",
"given": "John D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Turner",
"given": "Kenneth C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Ramesh",
"given": "Divya",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Mahmood",
"given": "Adnan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Hooper",
"given": "Andrea T.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Hamilton",
"given": "Jennifer D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Kim",
"given": "Yunji",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Purcell",
"given": "Lisa A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Baum",
"given": "Alina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Kyratsous",
"given": "Christos A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Krainson",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Perez-Perez",
"given": "Richard",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Mohseni",
"given": "Rizwana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Kowal",
"given": "Bari",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "DiCioccio",
"given": "A. Thomas",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Stahl",
"given": "Neil",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Lipsich",
"given": "Leah",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Braunstein",
"given": "Ned",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Herman",
"given": "Gary",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Yancopoulos",
"given": "George D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "From Regeneron Pharmaceuticals, Tarrytown, NY (M.P.O., E.F.-N., B.J.M., F.I., K.-C.C., N. Sarkar, P.H., I.H., J.D.D., K.C.T., D.R., A.M., A.T.H., J.D.H., Y.K., L.A.P., A.B., C.A.K., B.K., A.T.D., N. Stahl, L.L., N.B., G.H., G.D.Y., D.M.W.); the Departments of Medicine and Microbiology, University of Pennsylvania, Philadelphia (K.J.B.); the Departments of Global Health and Epidemiology and the Division of Allergy and Infectious Diseases, University of Washington, and the Vaccine and Infectious Diseases..."
}
],
"family": "Weinreich",
"given": "David M.",
"sequence": "additional"
}
],
"container-title": "New England Journal of Medicine",
"container-title-short": "N Engl J Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
8,
4
]
],
"date-time": "2021-08-04T21:01:35Z",
"timestamp": 1628110895000
},
"deposited": {
"date-parts": [
[
2022,
10,
20
]
],
"date-time": "2022-10-20T18:06:28Z",
"timestamp": 1666289188000
},
"funder": [
{
"DOI": "10.13039/100009857",
"doi-asserted-by": "publisher",
"name": "Regeneron Pharmaceuticals"
},
{
"DOI": "10.13039/100007013",
"doi-asserted-by": "publisher",
"name": "F. Hoffmann–La Roche"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
18
]
],
"date-time": "2024-04-18T17:55:49Z",
"timestamp": 1713462949740
},
"is-referenced-by-count": 342,
"issue": "13",
"issued": {
"date-parts": [
[
2021,
9,
23
]
]
},
"journal-issue": {
"issue": "13",
"published-print": {
"date-parts": [
[
2021,
9,
23
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
23
]
],
"date-time": "2021-09-23T00:00:00Z",
"timestamp": 1632355200000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2109682",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"page": "1184-1195",
"prefix": "10.1056",
"published": {
"date-parts": [
[
2021,
9,
23
]
]
},
"published-print": {
"date-parts": [
[
2021,
9,
23
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "publisher",
"key": "r1"
},
{
"DOI": "10.1038/s41586-020-2008-3",
"doi-asserted-by": "publisher",
"key": "r3"
},
{
"DOI": "10.1126/science.abd0831",
"doi-asserted-by": "publisher",
"key": "r4"
},
{
"DOI": "10.1016/j.cell.2021.06.002",
"doi-asserted-by": "publisher",
"key": "r5"
},
{
"DOI": "10.1038/s41586-021-03398-2",
"doi-asserted-by": "publisher",
"key": "r6"
},
{
"DOI": "10.1056/NEJMoa2035002",
"doi-asserted-by": "publisher",
"key": "r7"
},
{
"DOI": "10.1001/jamanetworkopen.2020.31756",
"doi-asserted-by": "publisher",
"key": "r12"
}
],
"reference-count": 7,
"references-count": 7,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2021.06.14.21258567",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2109682"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19",
"type": "journal-article",
"volume": "385"
}
