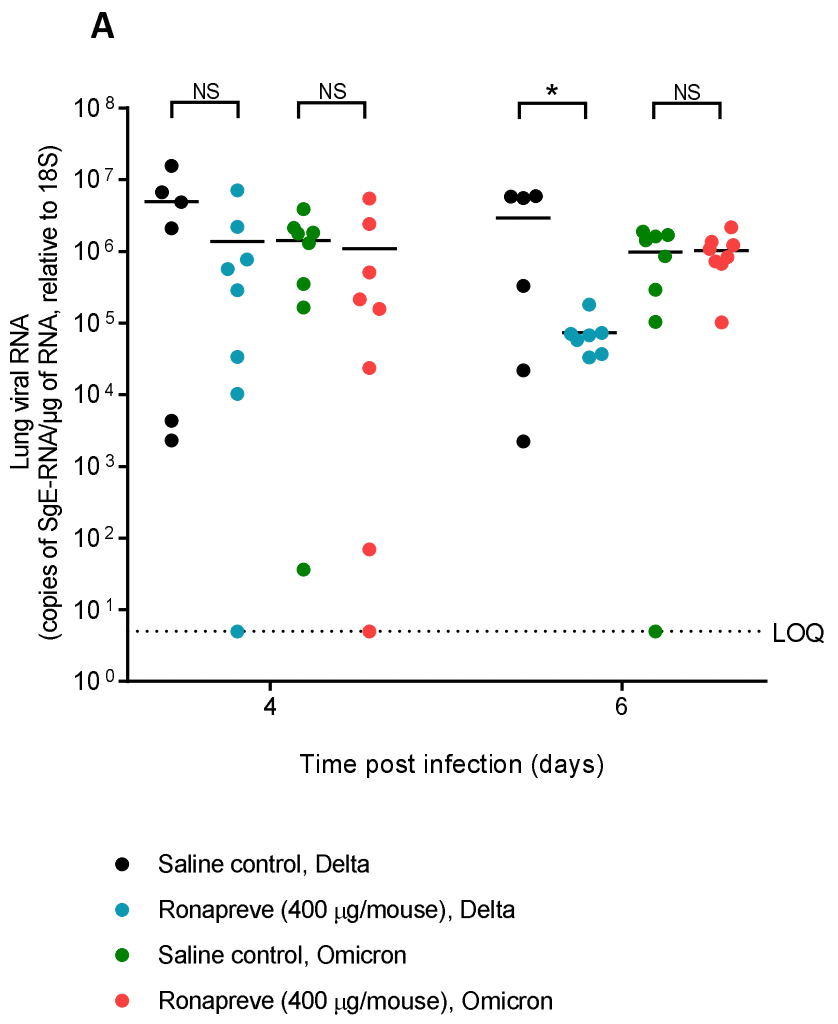
Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice
et al., bioRxiv, doi:10.1101/2022.01.23.477397, Jan 2022
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
K18-hACE2 mouse study showing that casirivimab/imdevimab was not effective for omicron at doses 2x higher than those effective for previous variants.
Tatham et al., 24 Jan 2022, preprint, 15 authors.
Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV-2 Omicron variant (B.1.1.529) in K18-hACE2 mice
doi:10.1101/2022.01.23.477397
AO and SR are Directors of Tandem Nano Ltd and co-inventors of patents relating to drug delivery. AO has received research funding from ViiV, Merck, Janssen and consultancy from Gilead, ViiV and Merck not related to the current paper. SR has received research funding from ViiV and AstraZeneca and consultancy from Gilead not related to the current paper. No other conflicts are declared by the authors.
References
Ashraf, Altered sirtuin expression is associated with node-positive breast cancer, British Journal of Cancer, doi:10.1038/sj.bjc.6603384
Bentley, SARS-CoV-2 Omicron-B.1.1.529 Variant leads to less severe disease than Pango B and Delta variants strains in a mouse model of severe COVID-19
Chen, In vivo monoclonal antibody efficacy against SARS-CoV-2 variant strains, Nature, doi:10.1038/s41586-021-03720-y
Choudhary, Emergence of SARS-CoV-2 Resistance with Monoclonal Antibody Therapy (Cold Spring Harbor Laboratory
Dejnirattisai, Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum, The Lancet, doi:10.1016/s0140-6736(21)02844-0
Dejnirattisai, leads to widespread escape from neutralizing antibody responses
Hiscox, Khoo, Stewart, Owen, Shutting the gate before the horse has bolted: is it time for a conversation about SARS-CoV-2 and antiviral drug resistance?, Journal of Antimicrobial Chemotherapy, doi:10.1093/jac/dkab189
Horby, Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial
Ikemura, Engineered ACE2 counteracts vaccine-evading SARS-CoV-2 Omicron variant (Cold Spring Harbor Laboratory
Keeton, SARS-CoV-2 spike T cell responses induced upon vaccination or infection remain robust against Omicron
Lamontagne, A living WHO guideline on drugs to prevent covid-19, BMJ, doi:10.1136/bmj.n526
Medigeshi, Sub-optimal Neutralisation of Omicron
Neary, Evaluation of intranasal nafamostat or camostat for SARS-CoV-2 chemoprophylaxis in Syrian golden hamsters (Cold Spring Harbor Laboratory
O'brien, Subcutaneous REGEN-COV Antibody Combination to Prevent Covid-19
Peter Horby, Hiscox, Chand, Breuer, Sherwood et al., Antiviral drug resistance and the use of directly acting antiviral drugs (DAAs) for COVID-19
Redd, Minimal cross-over between mutations associated with Omicron variant of SARS-CoV-2 and CD8+ T cell epitopes identified in COVID-19 convalescent individuals
Rothenberger, a novel trispecific DARPin candidate that protects against SARS-CoV-2 variants (Cold Spring Harbor Laboratory
Ryan, Dose-dependent response to infection with SARS-CoV-2 in the ferret model and evidence of protective immunity, Nature communications, doi:10.1038/s41467-020-20439-y
Salzer, Single-dose immunisation with a multimerised SARS-CoV-2 receptor binding domain (RBD) induces an enhanced and protective response in mice (Cold Spring Harbor Laboratory
Torjesen, Covid-19: Omicron may be more transmissible than other variants and partly resistant to existing vaccines, scientists fear, BMJ, doi:10.1136/bmj.n2943
Vanblargan, An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies (Cold Spring Harbor Laboratory
Weinreich, REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19, New England Journal of Medicine, doi:10.1056/nejmoa2108163
Who, None
Wölfel, Virological assessment of hospitalized patients with COVID-2019, Nature, doi:10.1038/s41586-020-2196-x
DOI record:
{
"DOI": "10.1101/2022.01.23.477397",
"URL": "http://dx.doi.org/10.1101/2022.01.23.477397",
"abstract": "<jats:p>The Omicron variant (B.1.1.529) of SARS-CoV-2 has placed enormous strain on global healthcare systems since it was first identified by South African researchers in late 2021. Omicron has >50 mutations which mainly occur in the surface spike protein and this has led to rapid assessment of monoclonal antibodies to assess the impact on virus neutralisation. Ronapreve has shown potential application in post-exposure prophylaxis, mild/moderate disease and in seronegative patients with severe COVID19, but several early reports of loss of in vitro neutralisation activity have been documented. Here, the virological efficacy of Ronapreve was assessed in K18-hACE2 mice to provide an in vivo outcome. Ronapreve reduced sub-genomic RNA in lung and nasal turbinate for the Delta variant but not the Omicron variant of SARS-CoV-2 at doses 2-fold higher than those shown to be active against previous variants of the virus. These data add to the growing evidence that the effectiveness of Ronapreve is compromised for the Omicron variant.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
1,
24
]
]
},
"author": [
{
"affiliation": [],
"family": "Tatham",
"given": "Lee",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sharp",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kijak",
"given": "Edyta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herriott",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Neary",
"given": "Megan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Box",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valentijn",
"given": "Anthony",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cox",
"given": "Helen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pertinez",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Curley",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arshad",
"given": "Usman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rajoli",
"given": "Rajith KR",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rannard",
"given": "Steve",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stewart",
"given": "James",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9819-7651",
"affiliation": [],
"authenticated-orcid": false,
"family": "Owen",
"given": "Andrew",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
1,
25
]
],
"date-time": "2022-01-25T02:55:18Z",
"timestamp": 1643079318000
},
"deposited": {
"date-parts": [
[
2022,
1,
25
]
],
"date-time": "2022-01-25T02:55:19Z",
"timestamp": 1643079319000
},
"group-title": "Microbiology",
"indexed": {
"date-parts": [
[
2022,
1,
25
]
],
"date-time": "2022-01-25T03:10:36Z",
"timestamp": 1643080236339
},
"institution": [
{
"name": "bioRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
1,
24
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.01.23.477397",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
1,
24
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
1,
24
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice"
],
"type": "posted-content"
}
