Camostat Mesylate Versus Lopinavir/Ritonavir in Hospitalized Patients With COVID-19—Results From a Randomized, Controlled, Open Label, Platform Trial (ACOVACT)
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2022.870493, ACOVACT, NCT04351724, Jul 2022
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
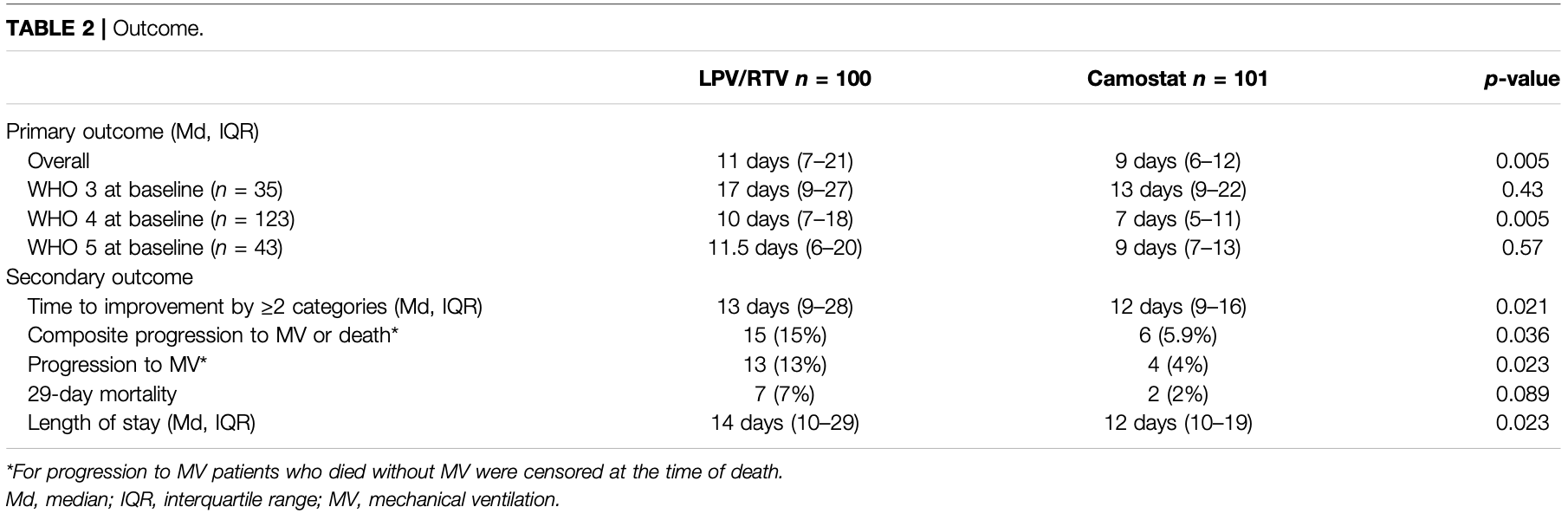
RCT 201 hospitalized COVID-19 patients showing faster clinical improvement, less progression to mechanical ventilation or death, and shorter hospital stay with camostat mesylate compared to lopinavir/ritonavir. There was also a trend towards lower 29-day mortality with camostat. Authors note that the lopinavir/ritonavir dose likely did not reach effective levels, so it may be considered similar to a placebo group.
Study covers TMPRSS2 inhibitors and camostat.
|
risk of death, 71.7% lower, RR 0.28, p = 0.10, treatment 2 of 101 (2.0%), control 7 of 100 (7.0%), NNT 20.
|
|
risk of mechanical ventilation, 69.5% lower, RR 0.30, p = 0.02, treatment 4 of 101 (4.0%), control 13 of 100 (13.0%), NNT 11.
|
|
risk of death/intubation, 60.4% lower, RR 0.40, p = 0.04, treatment 6 of 101 (5.9%), control 15 of 100 (15.0%), NNT 11.
|
|
relative time to sustained clinical improvement, 18.2% lower, relative time 0.82, p = 0.005, treatment 101, control 100, primary outcome.
|
|
relative time to improvement ≥2 categories, 7.7% lower, relative time 0.92, p = 0.02, treatment 101, control 100.
|
|
hospitalization time, 14.3% lower, relative time 0.86, p = 0.02, treatment 101, control 100.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Karolyi et al., 22 Jul 2022, Randomized Controlled Trial, Austria, peer-reviewed, mean age 58.6, 21 authors, study period 20 April, 2020 - 14 May, 2021, this trial compares with another treatment - results may be better when compared to placebo, trial NCT04351724 (history) (ACOVACT).
Contact: mario.karolyi@gesundheitsverbund.at.
Camostat Mesylate Versus Lopinavir/Ritonavir in Hospitalized Patients With COVID-19—Results From a Randomized, Controlled, Open Label, Platform Trial (ACOVACT)
Frontiers in Pharmacology, doi:10.3389/fphar.2022.870493
Background: To date, no oral antiviral drug has proven to be beneficial in hospitalized patients with COVID-19. Methods: In this randomized, controlled, open-label, platform trial, we randomly assigned patients ≥18 years hospitalized with COVID-19 pneumonia to receive either camostat mesylate (CM) (considered standard-of-care) or lopinavir/ritonavir (LPV/RTV). The primary endpoint was time to sustained clinical improvement (≥48 h) of at least one point on the 7category WHO scale. Secondary endpoints included length of stay (LOS), need for mechanical ventilation (MV) or death, and 29-day mortality. Results: 201 patients were included in the study (101 CM and 100 LPV/RTV) between 20 April 2020 and 14 May 2021. Mean age was 58.7 years, and 67% were male. The median time from symptom onset to randomization was 7 days (IQR 5-9). Patients in the CM group had a significantly shorter time to sustained clinical improvement (HR = 0.67, 95%-CI 0.49-0.90; 9 vs. 11 days, p = 0.008) and demonstrated less progression to MV or death [6/101 (5.9%) vs. 15/100 (15%), p = 0.036] and a shorter LOS (12 vs. 14 days, p = 0.023). A statistically nonsignificant trend toward a lower 29-day mortality in the CM group than the LPV/RTV group [2/101 (2%) vs. 7/100 (7%), p = 0.089] was observed.
Conclusion: In patients hospitalized for COVID-19, the use of CM was associated with shorter time to clinical improvement, reduced need for MV or death, and shorter LOS than the use of LPV/RTV. Furthermore, research is needed to confirm the efficacy of CM in larger placebo-controlled trials.
ETHICS STATEMENT The studies involving human participants were reviewed and approved and all amendments during the study period were approved by the ethics committee of the Medical University of Vienna and Vienna ethics committee. All methods were carried out in accordance with the ethical principles of the Declaration of Helsinki. All patients included in the trial signed an informed consent form. The patients/participants provided their written informed consent to participate in this study.
AUTHOR CONTRIBUTIONS MK and EP wrote the manuscript. MK, EP, SO, AK, HL, VK, BR, TS, VR, MM, and AgA recruited patients for the study and collected the data. FK and MK were responsible for statistical analysis. AZ, CW, BJ, MH, and MZ conceptualized the idea and designed the study. AZ, BJ, CS, and MH wrote the study protocol and ethics application. AlA, WH, SO, and MT proofread the manuscript and helped interpret the data. AZ, CW, and BJ supervised the study. MZ was responsible for all regulatory and legal aspects of the study.
SUPPLEMENTARY MATERIAL The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.870493/ full#supplementary-material Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Publisher's Note: All claims expressed in this article are solely those of the authors..
References
Amani, Khanijahani, Amani, Hydroxychloroquine Plus Standard of Care Compared with Standard of Care Alone in COVID-19: a Meta-Analysis of Randomized Controlled Trials, Sci. Rep, doi:10.1038/s41598-021-91089-3
Arribas, Bhagani, Lobo, Khaertynova, Mateu et al., Randomized Trial of Molnupiravir or Placebo in Patients Hospitalized with Covid-19, NEJM Evid, doi:10.1056/EVIDoa2100044
Baldelli, Corbellino, Clementi, Cattaneo, Gervasoni, Lopinavir/ritonavir in COVID-19 Patients: Maybe yes, but at what Dose?, J. Antimicrob. Chemother, doi:10.1093/jac/dkaa190
Bernal, Gomes Da Silva, Musungaie, Kovalchuk, Gonzalez et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, N. Engl. J. Med, doi:10.1056/NEJMoa2116044
Breining, Frølund, Højen, Gunst, Staerke et al., Camostat Mesylate against SARS-CoV-2 and COVID-19-Rationale, Dosing and Safety, Basic Clin. Pharmacol. Toxicol, doi:10.1111/bcpt.13533
Caly, Druce, Catton, Jans, Wagstaff, The FDA-Approved Drug Ivermectin Inhibits the Replication of SARS-CoV-2 In Vitro, Antivir. Res, doi:10.1016/j.antiviral.2020.104787
Cao, Wang, Wen, Liu, Wang et al., A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2001282
Caraco, Crofoot, Moncada, Galustyan, Musungaie et al., Phase 2/3 Trial of Molnupiravir for Treatment of Covid-19 in Nonhospitalized Adults, NEJM Evid, doi:10.1056/EVIDoa2100043
Choy, Wong, Kaewpreedee, Sia, Chen et al., Remdesivir, Lopinavir, Emetine, and Homoharringtonine Inhibit SARS-CoV-2 Replication In Vitro, Antivir. Res, doi:10.1016/j.antiviral.2020.104786
Gregoire, Le Turnier, Gaborit, Veyrac, Lecomte et al., Lopinavir Pharmacokinetics in COVID-19 Patients, J. Antimicrob. Chemother, doi:10.1093/jac/dkaa195
Gunst, Staerke, Pahus, Kristensen, Bodilsen et al., Efficacy of the TMPRSS2 Inhibitor Camostat Mesilate in Patients Hospitalized with Covid-19-A Double-Blind Randomized Controlled Trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100849
Hammond, Leister-Tebbe, Gardner, Abreu, Bao et al., Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N. Engl. J. Med, doi:10.1056/NEJMoa2118542
Hikmet, Méar, Edvinsson, Micke, Uhlén et al., The Protein Expression Profile of ACE2 in Human Tissues, Mol. Syst. Biol, doi:10.15252/msb.20209610
Hoffmann, Hofmann-Winkler, Smith, Krüger, Arora et al., Camostat Mesylate Inhibits SARS-CoV-2 Activation by TMPRSS2-Related Proteases and its Metabolite GBPA Exerts Antiviral Activity, EBioMedicine, doi:10.1016/j.ebiom.2021.103255
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hofmann-Winkler, Moerer, Alt-Epping, Bräuer, Büttner et al., Camostat Mesylate May Reduce Severity of Coronavirus Disease 2019 Sepsis: A First Observation, Crit. Care Explor, doi:10.1097/CCE.0000000000000284
Karolyi, Omid, Pawelka, Jilma, Stimpfl et al., High Dose Lopinavir/Ritonavir Does Not Lead to Sufficient Plasma Levels to Inhibit SARS-CoV-2 in Hospitalized Patients with COVID-19, Front. Pharmacol, doi:10.3389/fphar.2021.704767
Kitagawa, Arai, Iida, Mukai, Furukawa et al., A Phase I Study of High Dose Camostat Mesylate in Healthy Adults Provides a Rationale to Repurpose the TMPRSS2 Inhibitor for the Treatment of COVID-19, Clin. Transl. Sci, doi:10.1111/cts.13052
Liu, Cao, Xu, Wang, Zhang et al., Hydroxychloroquine, a Less Toxic Derivative of Chloroquine, Is Effective in Inhibiting SARS-CoV-2 Infection In Vitro, Cell Discov, doi:10.1038/s41421-020-0156-0
López-Medina, López, Hurtado, Dávalos, Ramirez et al., Effect of Ivermectin on Time to Resolution of Symptoms Among Adults with Mild COVID-19, JAMA, doi:10.1001/jama.2021.3071
Meng, Abdullahi, Ferreira, Goonawardane, Saito et al., Altered TMPRSS2 Usage by SARS-CoV-2 Omicron Impacts Infectivity and Fusogenicity, Nature, doi:10.1038/s41586-022-04474-x
Popp, Stegemann, Metzendorf, Kranke, Meybohm et al., Ivermectin for Preventing and Treating COVID-19, Cochrane Database Syst. Rev, doi:10.1002/14651858.cd015017
Sakr, Bensasi, Taha, Bauer, Ismail et al., Camostat Mesylate Therapy in Critically Ill Patients with COVID-19 Pneumonia, Intensive Care Med, doi:10.1007/s00134-021-06395-1
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19), JAMA, doi:10.1001/jama.2020.6019
Schoergenhofer, Jilma, Stimpfl, Karolyi, Zoufaly, Pharmacokinetics of Lopinavir and Ritonavir in Patients Hospitalized with Coronavirus Disease 2019 (COVID-19), Ann. Intern Med, doi:10.7326/M20-1550
Scudellari, How the Coronavirus Infects Cells -and Why Delta Is So Dangerous, Nature, doi:10.1038/d41586-021-02039-y
Talukdar, Saikia, Singal, Tandon, Chronic Pancreatitis: Evolving Paradigms, Pancreatology, doi:10.1159/000094561
Who Solidarity Trial Consortiumpan, Peto, Henao-Restrepo, Preziosi, Sathiyamoorthy et al., Repurposed Antiviral Drugs for Covid-19 -Interim WHO Solidarity Trial Results, N. Engl. J. Med, doi:10.1056/NEJMoa2023184
Yao, Ye, Zhang, Cui, Huang et al., In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin. Infect. Dis, doi:10.1093/cid/ciaa237
DOI record:
{
"DOI": "10.3389/fphar.2022.870493",
"ISSN": [
"1663-9812"
],
"URL": "http://dx.doi.org/10.3389/fphar.2022.870493",
"abstract": "<jats:p><jats:bold>Background:</jats:bold> To date, no oral antiviral drug has proven to be beneficial in hospitalized patients with COVID-19.</jats:p><jats:p><jats:bold>Methods:</jats:bold> In this randomized, controlled, open-label, platform trial, we randomly assigned patients ≥18 years hospitalized with COVID-19 pneumonia to receive either camostat mesylate (CM) (considered standard-of-care) or lopinavir/ritonavir (LPV/RTV). The primary endpoint was time to sustained clinical improvement (≥48 h) of at least one point on the 7-category WHO scale. Secondary endpoints included length of stay (LOS), need for mechanical ventilation (MV) or death, and 29-day mortality.</jats:p><jats:p><jats:bold>Results:</jats:bold> 201 patients were included in the study (101 CM and 100 LPV/RTV) between 20 April 2020 and 14 May 2021. Mean age was 58.7 years, and 67% were male. The median time from symptom onset to randomization was 7 days (IQR 5–9). Patients in the CM group had a significantly shorter time to sustained clinical improvement (HR = 0.67, 95%-CI 0.49–0.90; 9 vs. 11 days, <jats:italic>p</jats:italic> = 0.008) and demonstrated less progression to MV or death [6/101 (5.9%) vs. 15/100 (15%), <jats:italic>p</jats:italic> = 0.036] and a shorter LOS (12 vs. 14 days, <jats:italic>p</jats:italic> = 0.023). A statistically nonsignificant trend toward a lower 29-day mortality in the CM group than the LPV/RTV group [2/101 (2%) vs. 7/100 (7%), <jats:italic>p</jats:italic> = 0.089] was observed.</jats:p><jats:p><jats:bold>Conclusion:</jats:bold> In patients hospitalized for COVID-19, the use of CM was associated with shorter time to clinical improvement, reduced need for MV or death, and shorter LOS than the use of LPV/RTV. Furthermore, research is needed to confirm the efficacy of CM in larger placebo-controlled trials.</jats:p><jats:p><jats:bold>Systematic Review Registration</jats:bold>: [<jats:ext-link>https://clinicaltrials.gov/ct2/show/NCT04351724</jats:ext-link>, <jats:ext-link>https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-001302-30/AT</jats:ext-link>], identifier [NCT04351724, EUDRACT-NR: 2020–001302-30].</jats:p>",
"alternative-id": [
"10.3389/fphar.2022.870493"
],
"author": [
{
"affiliation": [],
"family": "Karolyi",
"given": "M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Pawelka",
"given": "E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Omid",
"given": "S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Koenig",
"given": "F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kauer",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rumpf",
"given": "B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoepler",
"given": "W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kuran",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Laferl",
"given": "H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seitz",
"given": "T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Traugott",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rathkolb",
"given": "V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mueller",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abrahamowicz",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schoergenhofer",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hecking",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Assinger",
"given": "A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wenisch",
"given": "C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zeitlinger",
"given": "M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jilma",
"given": "B.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zoufaly",
"given": "A.",
"sequence": "additional"
}
],
"container-title": "Frontiers in Pharmacology",
"container-title-short": "Front. Pharmacol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2022,
7,
22
]
],
"date-time": "2022-07-22T11:18:34Z",
"timestamp": 1658488714000
},
"deposited": {
"date-parts": [
[
2022,
7,
22
]
],
"date-time": "2022-07-22T11:18:37Z",
"timestamp": 1658488717000
},
"funder": [
{
"DOI": "10.13039/501100013699",
"doi-asserted-by": "publisher",
"name": "Bundesministerium für Bildung, Wissenschaft und Forschung"
},
{
"DOI": "10.13039/100006483",
"doi-asserted-by": "publisher",
"name": "AbbVie"
}
],
"indexed": {
"date-parts": [
[
2023,
8,
31
]
],
"date-time": "2023-08-31T14:15:48Z",
"timestamp": 1693491348202
},
"is-referenced-by-count": 3,
"issued": {
"date-parts": [
[
2022,
7,
22
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
22
]
],
"date-time": "2022-07-22T00:00:00Z",
"timestamp": 1658448000000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2022.870493/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2022,
7,
22
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
22
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1038/s41598-021-91089-3",
"article-title": "Hydroxychloroquine Plus Standard of Care Compared with Standard of Care Alone in COVID-19: a Meta-Analysis of Randomized Controlled Trials",
"author": "Amani",
"doi-asserted-by": "publisher",
"first-page": "11974",
"journal-title": "Sci. Rep.",
"key": "B1",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1056/EVIDoa2100044",
"article-title": "Randomized Trial of Molnupiravir or Placebo in Patients Hospitalized with Covid-19",
"author": "Arribas",
"doi-asserted-by": "publisher",
"journal-title": "NEJM Evid.",
"key": "B2",
"volume": "1",
"year": "2022"
},
{
"DOI": "10.1093/jac/dkaa190",
"article-title": "Lopinavir/ritonavir in COVID-19 Patients: Maybe yes, but at what Dose?",
"author": "Baldelli",
"doi-asserted-by": "publisher",
"first-page": "2704",
"journal-title": "J. Antimicrob. Chemother.",
"key": "B3",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1111/bcpt.13533",
"article-title": "Camostat Mesylate against SARS-CoV-2 and COVID-19-Rationale, Dosing and Safety",
"author": "Breining",
"doi-asserted-by": "publisher",
"first-page": "204",
"journal-title": "Basic Clin. Pharmacol. Toxicol.",
"key": "B4",
"volume": "128",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2020.104787",
"article-title": "The FDA-Approved Drug Ivermectin Inhibits the Replication of SARS-CoV-2 In Vitro",
"author": "Caly",
"doi-asserted-by": "publisher",
"first-page": "104787",
"journal-title": "Antivir. Res.",
"key": "B5",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19",
"author": "Cao",
"doi-asserted-by": "publisher",
"first-page": "1787",
"journal-title": "N. Engl. J. Med.",
"key": "B6",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/EVIDoa2100043",
"article-title": "Phase 2/3 Trial of Molnupiravir for Treatment of Covid-19 in Nonhospitalized Adults",
"author": "Caraco",
"doi-asserted-by": "publisher",
"journal-title": "NEJM Evid.",
"key": "B7",
"volume": "1",
"year": "2022"
},
{
"DOI": "10.1016/j.antiviral.2020.104786",
"article-title": "Remdesivir, Lopinavir, Emetine, and Homoharringtonine Inhibit SARS-CoV-2 Replication In Vitro",
"author": "Choy",
"doi-asserted-by": "publisher",
"first-page": "104786",
"journal-title": "Antivir. Res.",
"key": "B8",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1093/jac/dkaa195",
"article-title": "Lopinavir Pharmacokinetics in COVID-19 Patients",
"author": "Gregoire",
"doi-asserted-by": "publisher",
"first-page": "2702",
"journal-title": "J. Antimicrob. Chemother.",
"key": "B9",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2021.100849",
"article-title": "Efficacy of the TMPRSS2 Inhibitor Camostat Mesilate in Patients Hospitalized with Covid-19-A Double-Blind Randomized Controlled Trial",
"author": "Gunst",
"doi-asserted-by": "publisher",
"first-page": "100849",
"journal-title": "EClinicalMedicine",
"key": "B10",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "N. Engl. J. Med.",
"key": "B11",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.15252/msb.20209610",
"article-title": "The Protein Expression Profile of ACE2 in Human Tissues",
"author": "Hikmet",
"doi-asserted-by": "publisher",
"first-page": "e9610",
"journal-title": "Mol. Syst. Biol.",
"key": "B12",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"journal-title": "Cell",
"key": "B13",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.ebiom.2021.103255",
"article-title": "Camostat Mesylate Inhibits SARS-CoV-2 Activation by TMPRSS2-Related Proteases and its Metabolite GBPA Exerts Antiviral Activity",
"author": "Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "103255",
"journal-title": "EBioMedicine",
"key": "B14",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1097/CCE.0000000000000284",
"article-title": "Camostat Mesylate May Reduce Severity of Coronavirus Disease 2019 Sepsis: A First Observation",
"author": "Hofmann-Winkler",
"doi-asserted-by": "publisher",
"first-page": "e0284",
"journal-title": "Crit. Care Explor",
"key": "B15",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients",
"author": "Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"journal-title": "N. Engl. J. Med.",
"key": "B16",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2021.704767",
"article-title": "High Dose Lopinavir/Ritonavir Does Not Lead to Sufficient Plasma Levels to Inhibit SARS-CoV-2 in Hospitalized Patients with COVID-19",
"author": "Karolyi",
"doi-asserted-by": "publisher",
"first-page": "704767",
"journal-title": "Front. Pharmacol.",
"key": "B17",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1111/cts.13052",
"article-title": "A Phase I Study of High Dose Camostat Mesylate in Healthy Adults Provides a Rationale to Repurpose the TMPRSS2 Inhibitor for the Treatment of COVID-19",
"author": "Kitagawa",
"doi-asserted-by": "publisher",
"first-page": "1967",
"journal-title": "Clin. Transl. Sci.",
"key": "B18",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"article-title": "Hydroxychloroquine, a Less Toxic Derivative of Chloroquine, Is Effective in Inhibiting SARS-CoV-2 Infection In Vitro",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "16",
"journal-title": "Cell Discov.",
"key": "B19",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1001/jama.2021.3071",
"article-title": "Effect of Ivermectin on Time to Resolution of Symptoms Among Adults with Mild COVID-19",
"author": "López-Medina",
"doi-asserted-by": "publisher",
"first-page": "1426",
"journal-title": "JAMA",
"key": "B20",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1038/s41586-022-04474-x",
"article-title": "Altered TMPRSS2 Usage by SARS-CoV-2 Omicron Impacts Infectivity and Fusogenicity",
"author": "Meng",
"doi-asserted-by": "publisher",
"first-page": "706",
"journal-title": "Nature",
"key": "B21",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2023184",
"article-title": "Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results",
"author": "Pan",
"doi-asserted-by": "publisher",
"first-page": "497",
"journal-title": "N. Engl. J. Med.",
"key": "B22",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1002/14651858.cd015017",
"article-title": "Ivermectin for Preventing and Treating COVID-19",
"author": "Popp",
"doi-asserted-by": "publisher",
"first-page": "CD015017",
"journal-title": "Cochrane Database Syst. Rev.",
"key": "B23",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)32013-4",
"article-title": "Lopinavir-ritonavir in Patients Admitted to Hospital with COVID-19 (RECOVERY): a Randomised, Controlled, Open-Label, Platform Trial",
"doi-asserted-by": "publisher",
"first-page": "1345",
"journal-title": "Lancet",
"key": "B24",
"volume": "396",
"year": "2020"
},
{
"DOI": "10.1007/s00134-021-06395-1",
"article-title": "Camostat Mesylate Therapy in Critically Ill Patients with COVID-19 Pneumonia",
"author": "Sakr",
"doi-asserted-by": "publisher",
"first-page": "707",
"journal-title": "Intensive Care Med.",
"key": "B25",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.6019",
"article-title": "Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19)",
"author": "Sanders",
"doi-asserted-by": "publisher",
"first-page": "2020",
"journal-title": "JAMA",
"key": "B26",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.7326/M20-1550",
"article-title": "Pharmacokinetics of Lopinavir and Ritonavir in Patients Hospitalized with Coronavirus Disease 2019 (COVID-19)",
"author": "Schoergenhofer",
"doi-asserted-by": "publisher",
"first-page": "670",
"journal-title": "Ann. Intern Med.",
"key": "B27",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1038/d41586-021-02039-y",
"article-title": "How the Coronavirus Infects Cells - and Why Delta Is So Dangerous",
"author": "Scudellari",
"doi-asserted-by": "publisher",
"first-page": "640",
"journal-title": "Nature",
"key": "B28",
"volume": "595",
"year": "2021"
},
{
"DOI": "10.1159/000094561",
"article-title": "Chronic Pancreatitis: Evolving Paradigms",
"author": "Talukdar",
"doi-asserted-by": "publisher",
"first-page": "440",
"journal-title": "Pancreatology",
"key": "B29",
"volume": "6",
"year": "2006"
},
{
"DOI": "10.1093/cid/ciaa237",
"article-title": "In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)",
"author": "Yao",
"doi-asserted-by": "publisher",
"first-page": "732",
"journal-title": "Clin. Infect. Dis.",
"key": "B30",
"volume": "71",
"year": "2020"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2022.870493/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": "Camostat Mesylate Versus Lopinavir/Ritonavir in Hospitalized Patients With COVID-19—Results From a Randomized, Controlled, Open Label, Platform Trial (ACOVACT)",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "13"
}
