Randomized Trial of Molnupiravir or Placebo in Patients Hospitalized with Covid-19
et al., NEJM Evidence, doi:10.1056/EVIDoa2100044, MOVe-IN, NCT04575584, Dec 2021
RCT 304 hospitalized patients, 218 treated with molnupiravir, showing no significant differences. MOVe-IN MK-4482-001. NCT04575584 (history).
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
|
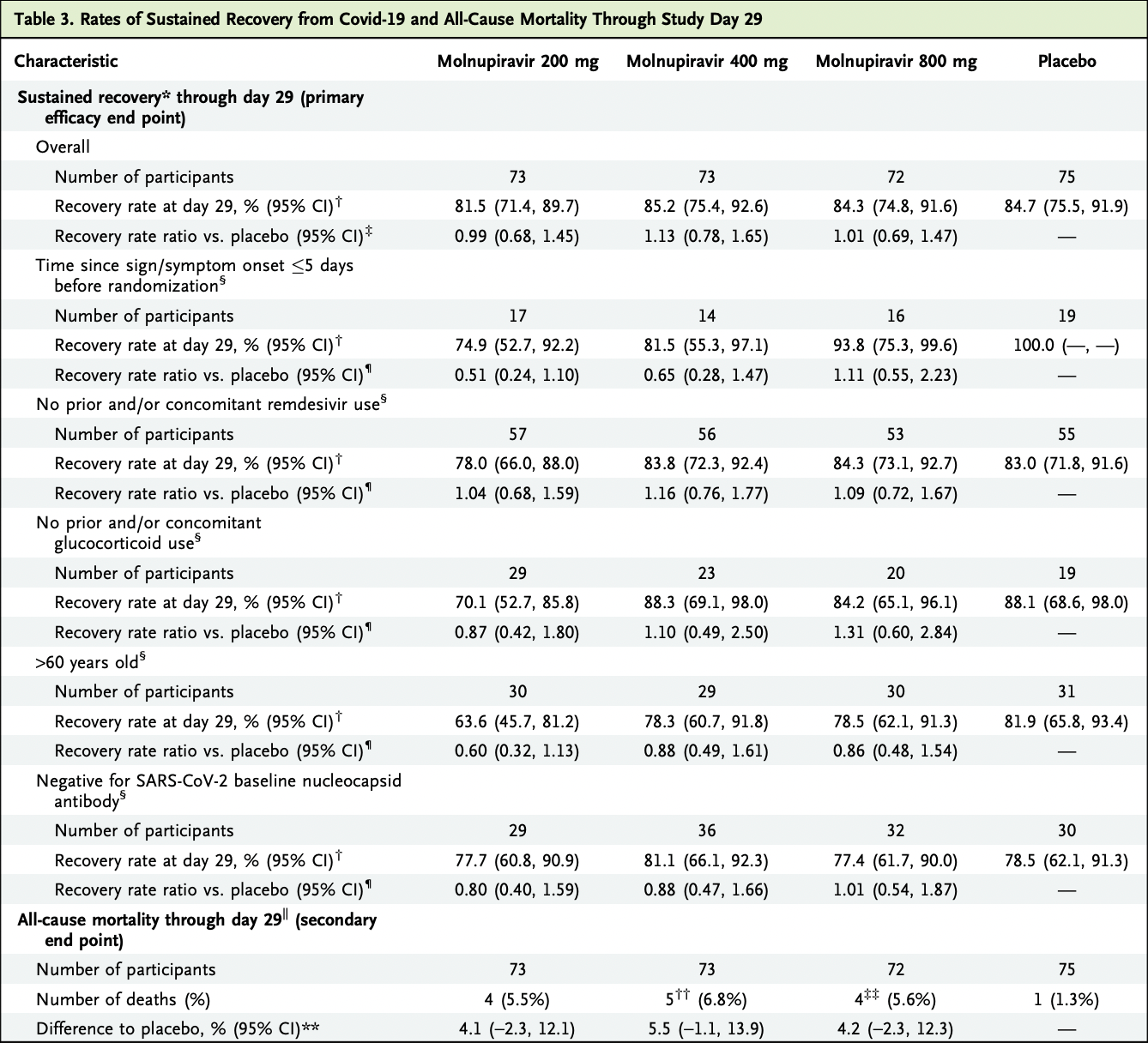
risk of death, 281.9% higher, RR 3.82, p = 0.31, treatment 11 of 216 (5.1%), control 1 of 75 (1.3%), combined, excluding imputed deaths.
|
|
risk of death, 216.9% higher, RR 3.17, p = 0.36, treatment 3 of 71 (4.2%), control 1 of 75 (1.3%), 800mg, excluding imputed death.
|
|
risk of death, 316.7% higher, RR 4.17, p = 0.20, treatment 4 of 72 (5.6%), control 1 of 75 (1.3%), 400mg, excluding imputed death.
|
|
risk of death, 311.0% higher, RR 4.11, p = 0.21, treatment 4 of 73 (5.5%), control 1 of 75 (1.3%), 200mg.
|
|
risk of no recovery, 1.0% lower, RR 0.99, p = 0.96, treatment 72, control 75, inverted to make RR<1 favor treatment, 800mg.
|
|
risk of no recovery, 11.5% lower, RR 0.88, p = 0.53, treatment 73, control 75, inverted to make RR<1 favor treatment, 400mg.
|
|
risk of no recovery, 1.0% higher, RR 1.01, p = 0.96, treatment 73, control 75, inverted to make RR<1 favor treatment, 200mg.
|
|
recovery time, no change, relative time 1.00, treatment 72, control 75, 800mg.
|
|
recovery time, no change, relative time 1.00, treatment 73, control 75, 400mg.
|
|
recovery time, no change, relative time 1.00, treatment 73, control 75, 200mg.
|
|
risk of no viral clearance, 11.8% lower, RR 0.88, p = 0.57, treatment 26 of 52 (50.0%), control 34 of 60 (56.7%), NNT 15, 800mg, Table S16, day 15 mid-recovery.
|
|
risk of no viral clearance, 2.4% higher, RR 1.02, p = 1.00, treatment 29 of 50 (58.0%), control 34 of 60 (56.7%), 400mg, Table S16, day 15 mid-recovery.
|
|
risk of no viral clearance, 20.6% lower, RR 0.79, p = 0.27, treatment 27 of 60 (45.0%), control 34 of 60 (56.7%), NNT 8.6, 200mg, Table S16, day 15 mid-recovery.
|
|
risk of no viral clearance, 8.3% lower, RR 0.92, p = 1.00, treatment 9 of 53 (17.0%), control 10 of 54 (18.5%), NNT 65, 800mg, Table S16, day 29.
|
|
risk of no viral clearance, 48.2% higher, RR 1.48, p = 0.35, treatment 14 of 51 (27.5%), control 10 of 54 (18.5%), 400mg, Table S16, day 29.
|
|
risk of no viral clearance, 21.5% lower, RR 0.79, p = 0.61, treatment 8 of 55 (14.5%), control 10 of 54 (18.5%), NNT 25, 200mg, Table S16, day 29.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Arribas et al., 16 Dec 2021, Double Blind Randomized Controlled Trial, multiple countries, peer-reviewed, 21 authors, study period 19 October, 2020 - 12 January, 2021, average treatment delay 7.1 days, trial NCT04575584 (history) (MOVe-IN).
Randomized Trial of Molnupiravir or Placebo in Patients Hospitalized with Covid-19
NEJM Evidence, doi:10.1056/evidoa2100044
BACKGROUND Molnupiravir is an oral prodrug of b-D-N4-hydroxycytidine, active against SARS-CoV-2 in vitro and in animal models. We report data from the phase 2 component of MOVe-IN, a clinical trial evaluating molnupiravir in patients hospitalized with Covid-19. METHODS We conducted a randomized, placebo-controlled, double-blind phase 2/3 trial in patients 18 years old and older requiring in-hospital treatment for laboratoryconfirmed Covid-19 with symptom onset 10 or fewer days before randomization. Participants were randomly assigned to placebo or molnupiravir 200 mg, 400 mg, or 800 mg (1:1:1:1 ratio), twice daily for 5 days. Primary end points were safety and sustained recovery (participant alive and either not hospitalized or medically ready for discharge) through day 29. RESULTS Of 304 randomly assigned participants, 218 received at least one dose of molnupiravir and 75 of placebo. At baseline, 74.0% had at least one risk factor for severe Covid-19. Adverse events were reported in 121 of 218 (55.5%) molnupiravir-treated and 46 of 75 (61.3%) placebo-treated participants, with no apparent dose effect on adverse event rates and no evidence of hematologic toxicity based on prespecified adverse events. Of 16 confirmed deaths, most were in participants with severe Covid-19 (75.0%), with underlying comorbidities (87.5%), older than 60 years of age (81.3%), and/or symptom duration longer than 5 days (75.0%) at randomization. Median time to sustained recovery was 9 days in all groups, with similar day 29 recovery rates ranging from 81.5% to 85.2%.
Author Affiliations 1 Infectious Diseases Unit, Hospital Universitario La Paz-IdiPAZ, Madrid 2 Department of Infectious Diseases, Royal Free Hospital, London
References
Abdelnabi, Foo, Jonghe, Maes, Weynand et al., Molnupiravir inhibits the replication of the emerging SARS-CoV-2 variants of concern (VoCs) in a hamster infection model, J Infect Dis, doi:10.1093/infdis/jiab361
Beigel, Tomashek, Dodd, ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19 -final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Caraco, Crofoot, Moncada, Phase 2/3 trial of molnupiravir for treatment of Covid-19 in non-hospitalized adults, NEJM Evid, doi:10.1056/EVIDoa2100043
Cevik, Tate, Lloyd, Maraolo, Schafers et al., SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis, Lancet Microbe, doi:10.1016/S2666-5247(20)30172-5
Chawla, Cao, Stone, Model-based dose selection for the phase 3 evaluation of molnupiravir (MOV) in the treatment of COVID-19 in adults, ESCMID eAcademy
Cox, Wolf, Plemper, Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets, Nat Microbiol, doi:10.1038/s41564-020-00835-2
Docherty, Harrison, Green, ISARIC4C investigators. Features of 20 133 UK patients in hospital with covid-19 using the ISA-RIC WHO Clinical Characterisation Protocol: prospective observational cohort study, BMJ, doi:10.1136/bmj.m1985
Fischer, Eron, Holman, Molnupiravir, an oral antiviral treatment for COVID-19, medRxiv, doi:10.1101/2021.06.17.21258639v1
Gottlieb, Nirula, Chen, Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.0202
Heflich, Dertinger, Dobrovolsky, The in vivo erythrocyte Pig-a gene mutation assay -Part 1 -detailed review paper and retrospective performance assessment
Horby, Lim, Emberson, RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Jones, Biele, M€ Uhlemann B, Estimating infectiousness throughout SARS-CoV-2 infection course, Science, doi:10.1126/science.abi5273
Kalil, Patterson, Mehta, ACTT-2 Study Group Members. Baricitinib plus remdesivir for hospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2031994
Klopfenstein, Gendrin, Gerazime, HNF Hospital tocilizumab multidisciplinary team. Systematic review and subgroup meta-analysis of randomized trials to determine tocilizumab's place in COVID-19 pneumonia, Infect Dis Ther, doi:10.1007/s40121-021-00488-6
Kyriazopoulou, Poulakou, Milionis, Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial, Nat Med, doi:10.1038/s41591-021-01499-z
Libster, Erez Marc, Wappner, Fundaci on INFANT-COVID-19 Group. Early high-titer plasma therapy to prevent severe COVID-19 in older adults, N Engl J Med, doi:10.1056/NEJMoa2033700
Ly-Cov555, Group; Lundgren, Grund, Barkauskas, A neutralizing monoclonal antibody for hospitalized patients with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2033130
Macedo, Gonc ¸alves, Febra, COVID-19 fatality rates in hospitalized patients: systematic review and meta-analysis, Ann Epidemiol, doi:10.1016/j.annepidem.2021.02.012
Marconi, Ramanan, De Bono, Efficacy and safety of baricitinib in patients with COVID-19 infection: results from the randomised, double-blind, placebo-controlled, parallel-group COV-BARRIER phase 3 trial, Lancet Respir Med, doi:10.1101/2021.04.30.21255934v2
Painter, Holman, Bush, Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2, Antimicrob Agents Chemother, doi:10.1128/AAC.02428-20
Pan, Peto, Henao-Restrepo, WHO Solidarity Trial Consortium. Repurposed antiviral drugs for Covid-19 -interim WHO solidarity trial results, N Engl J Med, doi:10.1056/NEJMoa2023184
Patel, Beishuizen, Ruiz, A randomized trial of otilimab in severe COVID-19 pneumonia (OSCAR), medRxiv, doi:10.1101/2021.04.14.21255475v1
Salama, Han, Yau, Tocilizumab in patients hospitalized with Covid-19 pneumonia, N Engl J Med, doi:10.1056/NEJMoa2030340
Sheahan, Sims, Zhou, An orally bioavailable broadspectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice, Sci Transl Med, doi:10.1126/scitranslmed.abb5883
Tenforde, Rose, Lindsell, CDC COVID-19 Response Team. Characteristics of adult outpatients and inpatients with COVID-19 -11 academic medical centers, United States, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm6926e3
Troth, Butterton, Deanda, Letter to the editor in response to Zhou et al, J Infect Dis, doi:10.1093/infdis/jiab362
Urakova, Kuznetsova, Crossman, b-d-N 4 -Hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome, J Virol, doi:10.1128/JVI.01965-17
Wahl, Gralinski, Johnson, SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801, Nature, doi:10.1038/s41586-021-03312-w
Weinreich, Sivapalasingam, Norton, Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
Yoon, Toots, Lee, Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses, Antimicrob Agents Chemother, doi:10.1128/AAC.00766-18
Zhou, Hill, Sarkar, b-d-N4-hydroxycytidine inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to mammalian cells, J Infect Dis, doi:10.1093/infdis/jiab247
DOI record:
{
"DOI": "10.1056/evidoa2100044",
"ISSN": [
"2766-5526"
],
"URL": "http://dx.doi.org/10.1056/evidoa2100044",
"abstract": "<jats:p>Molnupiravir is an oral agent a metabolite of which has activity against SARS-CoV-2. In a controlled trial in adults hospitalized for Covid-19 who had symptoms for 10 days or less prior to randomization, patients received placebo (n=75) or varying doses of molnupiravir (n=218) administered twice-daily for 5 days. There was no impact of treatment on death. Median time to sustained recovery was 9 days in all groups, with day 29 recovery rates ranging from 81.5%-85.2%. There were no dose-limiting side effects or adverse events.</jats:p>",
"author": [
{
"affiliation": [],
"family": "Arribas",
"given": "José R.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Bhagani",
"given": "Sanjay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lobo",
"given": "Suzana M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khaertynova",
"given": "Ilsiyar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mateu",
"given": "Lourdes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fishchuk",
"given": "Roman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Park",
"given": "William Y.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hussein",
"given": "Khetam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Sei Won",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghosn",
"given": "Jade",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown",
"given": "Michelle L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Ying",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gao",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Assaid",
"given": "Christopher",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grobler",
"given": "Jay A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Strizki",
"given": "Julie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vesnesky",
"given": "Mary",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Paschke",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Butterton",
"given": "Joan R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Anda",
"given": "Carisa",
"sequence": "additional"
},
{
"affiliation": [],
"name": "the MOVe-IN study group",
"sequence": "additional"
}
],
"container-title": [
"NEJM Evidence"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
22
]
],
"date-time": "2021-12-22T00:04:10Z",
"timestamp": 1640131450000
},
"deposited": {
"date-parts": [
[
2021,
12,
22
]
],
"date-time": "2021-12-22T00:04:11Z",
"timestamp": 1640131451000
},
"indexed": {
"date-parts": [
[
2021,
12,
22
]
],
"date-time": "2021-12-22T06:49:18Z",
"timestamp": 1640155758025
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "2766-5526"
}
],
"issued": {
"date-parts": [
[
2021,
12,
16
]
]
},
"member": "150",
"original-title": [],
"prefix": "10.1056",
"published": {
"date-parts": [
[
2021,
12,
16
]
]
},
"published-online": {
"date-parts": [
[
2021,
12,
16
]
]
},
"publisher": "Massachusetts Medical Society",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [
"NEJM evid"
],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": [
"Randomized Trial of Molnupiravir or Placebo in Patients Hospitalized with Covid-19"
],
"type": "journal-article"
}