Molnupiravir Inhibits Replication of the Emerging SARS-CoV-2 Variants of Concern in a Hamster Infection Model
et al., The Journal of Infectious Diseases, doi:10.1093/infdis/jiab361, Jul 2021
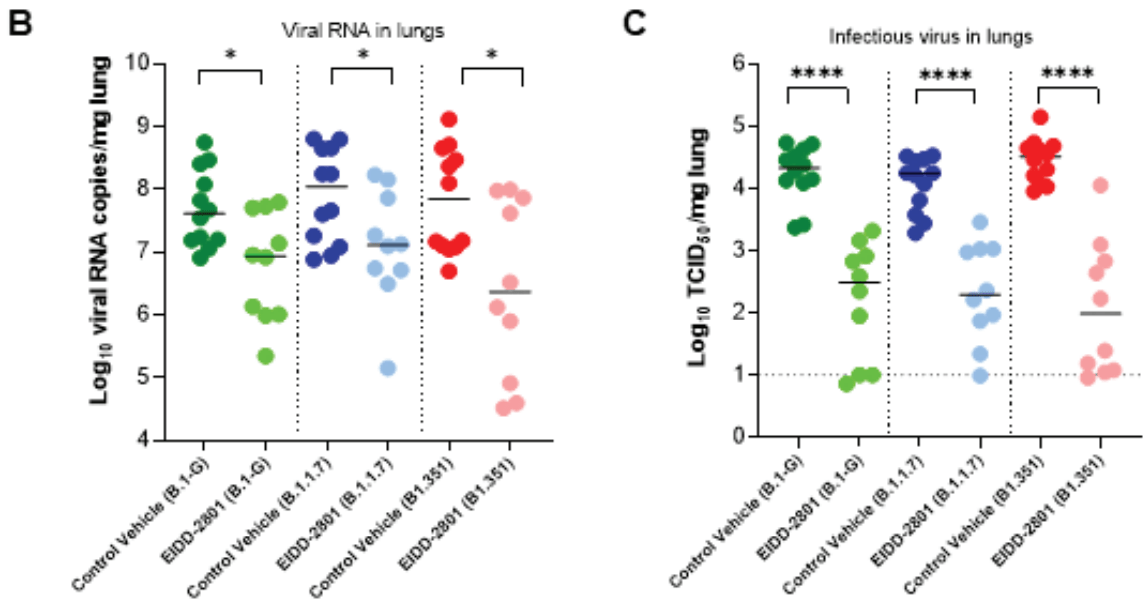
Hamster study showing molnupiravir effective against the original, B.1.1.7, and B.1.351 variants.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity1-15. Multiple analyses have identified variants potentially created by molnupiravir16-20. Studies show significantly increased risk of acute kidney injury21, cardiovascular toxocity22, and neurological symptoms21. Treatment may increase viral rebound23,24.
1.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
2.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
3.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
4.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
5.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
6.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
7.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
8.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
9.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
10.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
11.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
12.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
13.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
14.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
15.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
16.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
17.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
18.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
19.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
21.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
22.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Abdelnabi et al., 9 Jul 2021, peer-reviewed, 6 authors.
Abstract: The Journal of Infectious Diseases
Brief Report
Molnupiravir Inhibits Replication of
the Emerging SARS-CoV-2 Variants of
Concern in a Hamster Infection Model
Rana Abdelnabi,1 Caroline S. Foo,1 Steven De Jonghe,1 Piet Maes,2,3,
Birgit Weynand,4 and Johan Neyts1,5
The emergence of SARS-CoV-2 variants of concern (VoCs) has
exacerbated the COVID-19 pandemic. Currently available monoclonal antibodies and vaccines appear to have reduced efficacy
against some of these VoCs. Antivirals targeting conserved proteins of SARS-CoV-2 are unlikely to be affected by mutations
arising in VoCs and should therefore be effective against emerging
variants. We here investigate the efficacy of molnupiravir, currently
in phase 2 clinical trials, in hamsters infected with Wuhan strain or
B.1.1.7 and B.1.351 variants. Molnupiravir proved to be effective
against infections with each of the variants and therefore may have
potential combating current and future emerging VoCs.
Keywords. SARS-CoV-2; antivirals; molnupiravir; VoC,
hamsters, coronavirus; B.1.351.
Since its emergence in Wuhan, China in December 2019 [1],
the severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) has spread worldwide resulting in a global pandemic
with more than 148 million cases and approximately 3.1 million deaths reported up to 27 April 2021 (www.covid19.who.
int). Variants of SARS-CoV-2 are emerging in different parts
of the world, posing a new threat of increased virus spread and
potential to escape from both vaccine-induced and natural
infection-induced immunity. So far, 4 major circulating SARSCoV-2 variants of concern (VoC) have been identified: lineages
B.1.1.7 (UK), B.1.351 or 501Y.V2 (South Africa), B.1.1.28.1 or
P.1 (Brazil), and B.429 (California) [2]. These VoC have been
implicated in new, massive waves of infections and new spikes
Received 3 May 2021; editorial decision 25 June 2021; accepted 8 July 2021; published online
July 9, 2021.
Correspondence: Johan Neyts, PhD, Department of Microbiology and Immunology, Rega
Institute, Herestraat 49, Box 1043, 3000 Leuven, Belgium (johan.neyts@kuleuven.be).
The Journal of Infectious Diseases® 2021;224:749–53
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society
of America. This is an Open Access article distributed under the terms of the Creative Commons
Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted
reuse, distribution, and reproduction in any medium, provided the original work is properly cited.
DOI:10.1093/infdis/jiab361
METHODS
All virus-related work was conducted in the high-containment
biosafety level 3 facilities of the Katholieke Universiteit
Brief Report • jid 2021:224 (1 September) • 749
1
Laboratory of Virology and Chemotherapy, Department of Microbiology, Immunology and
Transplantation, Rega Institute for Medical Research, Katholieke Universiteit Leuven, Leuven,
Belgium, 2Laboratory of Clinical and Epidemiological Virology, Department of Microbiology,
Immunology and Transplantation, Rega Institute, Katholieke Universiteit Leuven, Leuven,
Belgium, 3Zoonotic Infectious Diseases Unit, KU Leuven, Leuven, Belgium, 4Department
of Imaging and Pathology, Division of Translational Cell and Tissue Research, Katholieke
Universiteit Leuven, Leuven, Belgium, and 5Global Virus Network, Baltimore, Maryland, USA
in excess mortality in..
DOI record:
{
"DOI": "10.1093/infdis/jiab361",
"ISSN": [
"0022-1899",
"1537-6613"
],
"URL": "http://dx.doi.org/10.1093/infdis/jiab361",
"abstract": "<jats:title>Abstract</jats:title><jats:p>The emergence of SARS-CoV-2 variants of concern (VoCs) has exacerbated the COVID-19 pandemic. Currently available monoclonal antibodies and vaccines appear to have reduced efficacy against some of these VoCs. Antivirals targeting conserved proteins of SARS-CoV-2 are unlikely to be affected by mutations arising in VoCs and should therefore be effective against emerging variants. We here investigate the efficacy of molnupiravir, currently in phase 2 clinical trials, in hamsters infected with Wuhan strain or B.1.1.7 and B.1.351 variants. Molnupiravir proved to be effective against infections with each of the variants and therefore may have potential combating current and future emerging VoCs.</jats:p>",
"author": [
{
"affiliation": [
{
"name": "Laboratory of Virology and Chemotherapy, Department of Microbiology, Immunology and Transplantation, Rega Institute for Medical Research, Katholieke Universiteit Leuven, Leuven, Belgium"
}
],
"family": "Abdelnabi",
"given": "Rana",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Laboratory of Virology and Chemotherapy, Department of Microbiology, Immunology and Transplantation, Rega Institute for Medical Research, Katholieke Universiteit Leuven, Leuven, Belgium"
}
],
"family": "Foo",
"given": "Caroline S",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Virology and Chemotherapy, Department of Microbiology, Immunology and Transplantation, Rega Institute for Medical Research, Katholieke Universiteit Leuven, Leuven, Belgium"
}
],
"family": "De Jonghe",
"given": "Steven",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9118-8608",
"affiliation": [
{
"name": "Laboratory of Clinical and Epidemiological Virology, Department of Microbiology, Immunology and Transplantation, Rega Institute, Katholieke Universiteit Leuven, Leuven, Belgium"
},
{
"name": "Zoonotic Infectious Diseases Unit, KU Leuven, Leuven, Belgium"
}
],
"authenticated-orcid": false,
"family": "Maes",
"given": "Piet",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Imaging and Pathology, Division of Translational Cell and Tissue Research, Katholieke Universiteit Leuven, Leuven, Belgium"
}
],
"family": "Weynand",
"given": "Birgit",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Laboratory of Virology and Chemotherapy, Department of Microbiology, Immunology and Transplantation, Rega Institute for Medical Research, Katholieke Universiteit Leuven, Leuven, Belgium"
},
{
"name": "Global Virus Network, Baltimore, Maryland, USA"
}
],
"family": "Neyts",
"given": "Johan",
"sequence": "additional"
}
],
"container-title": "The Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
7,
8
]
],
"date-time": "2021-07-08T19:13:00Z",
"timestamp": 1625771580000
},
"deposited": {
"date-parts": [
[
2023,
1,
3
]
],
"date-time": "2023-01-03T11:35:22Z",
"timestamp": 1672745722000
},
"funder": [
{
"DOI": "10.13039/501100004040",
"doi-asserted-by": "publisher",
"name": "Katholieke Universiteit Leuven"
},
{
"DOI": "10.13039/501100010648",
"doi-asserted-by": "crossref",
"name": "Universitair Ziekenhuis Leuven"
},
{
"DOI": "10.13039/501100003130",
"award": [
"G0G4820N"
],
"doi-asserted-by": "publisher",
"name": "Fonds Wetenschappelijk Onderzoek"
},
{
"DOI": "10.13039/501100000780",
"award": [
"101003627"
],
"doi-asserted-by": "publisher",
"name": "European Union"
},
{
"DOI": "10.13039/100000865",
"award": [
"INV-00636"
],
"doi-asserted-by": "publisher",
"name": "Bill and Melinda Gates Foundation"
}
],
"indexed": {
"date-parts": [
[
2024,
5,
12
]
],
"date-time": "2024-05-12T01:11:23Z",
"timestamp": 1715476283292
},
"is-referenced-by-count": 99,
"issue": "5",
"issued": {
"date-parts": [
[
2021,
7,
9
]
]
},
"journal-issue": {
"issue": "5",
"published-online": {
"date-parts": [
[
2021,
7,
9
]
]
},
"published-print": {
"date-parts": [
[
2021,
9,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
9
]
],
"date-time": "2021-07-09T00:00:00Z",
"timestamp": 1625788800000
}
}
],
"link": [
{
"URL": "http://academic.oup.com/jid/advance-article-pdf/doi/10.1093/infdis/jiab361/39721682/jiab361.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/jid/article-pdf/224/5/749/40166589/jiab361.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "http://academic.oup.com/jid/article-pdf/224/5/749/40166589/jiab361.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "749-753",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2021,
7,
9
]
]
},
"published-online": {
"date-parts": [
[
2021,
7,
9
]
]
},
"published-other": {
"date-parts": [
[
2021,
9,
1
]
]
},
"published-print": {
"date-parts": [
[
2021,
9,
1
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A novel coronavirus from patients with pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "N Engl J Med",
"key": "2021090109221259500_CIT0001",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/j.chom.2021.02.020",
"article-title": "The variant gambit: COVID-19’s next move",
"author": "Plante",
"doi-asserted-by": "crossref",
"first-page": "508",
"journal-title": "Cell Host Microbe",
"key": "2021090109221259500_CIT0002",
"volume": "29",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(21)00183-5",
"article-title": "Resurgence of COVID-19 in Manaus, Brazil, despite high seroprevalence",
"author": "Sabino",
"doi-asserted-by": "crossref",
"first-page": "452",
"journal-title": "Lancet",
"key": "2021090109221259500_CIT0003",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n359",
"article-title": "Covid-19: the E484K mutation and the risks it poses",
"author": "Wise",
"doi-asserted-by": "crossref",
"first-page": "n359",
"journal-title": "BMJ",
"key": "2021090109221259500_CIT0004",
"volume": "372",
"year": "2021"
},
{
"DOI": "10.1128/JVI.01965-17",
"article-title": "β-d-N4-hydroxycytidine is a potent anti-alphavirus compound that induces a high level of mutations in the viral genome",
"author": "Urakova",
"doi-asserted-by": "crossref",
"first-page": "e01965-17",
"journal-title": "J Virol",
"key": "2021090109221259500_CIT0005",
"volume": "92",
"year": "2017"
},
{
"DOI": "10.1126/scitranslmed.aax5866",
"article-title": "Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia",
"author": "Toots",
"doi-asserted-by": "crossref",
"first-page": "eaax5866",
"journal-title": "Sci Transl Med",
"key": "2021090109221259500_CIT0006",
"volume": "11",
"year": "2019"
},
{
"article-title": "The combined treatment of molnupiravir and favipiravir results in a marked potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model",
"author": "Abdelnabi",
"journal-title": "bioRxiv",
"key": "2021090109221259500_CIT0007",
"year": "10"
},
{
"DOI": "10.1038/s41586-021-03312-w",
"article-title": "SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801",
"author": "Wahl",
"doi-asserted-by": "crossref",
"journal-title": "Nature",
"key": "2021090109221259500_CIT0008",
"year": "2021"
},
{
"DOI": "10.1038/s41564-020-00835-2",
"article-title": "Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets",
"author": "Cox",
"doi-asserted-by": "crossref",
"first-page": "11",
"journal-title": "Nat Microbiol",
"key": "2021090109221259500_CIT0009",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1128/AAC.02428-20",
"article-title": "Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2",
"author": "Painter",
"doi-asserted-by": "crossref",
"first-page": "e02428-20",
"journal-title": "Antimicrob Agents Chemother",
"key": "2021090109221259500_CIT0010",
"volume": "65",
"year": "2021"
},
{
"article-title": "Reduction in infectious SARS-CoV-2 in treatment study of COVID-19 with molnupiravir",
"author": "Painter",
"key": "2021090109221259500_CIT0011"
},
{
"DOI": "10.1016/j.ebiom.2021.103403",
"article-title": "Comparing infectivity and virulence of emerging SARS-CoV-2 variants in Syrian hamsters",
"author": "Abdelnabi",
"doi-asserted-by": "crossref",
"first-page": "103403",
"journal-title": "EBioMedicine",
"key": "2021090109221259500_CIT0012",
"volume": "68",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03398-2",
"article-title": "Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "130",
"journal-title": "Nature",
"key": "2021090109221259500_CIT0013",
"volume": "593",
"year": "2021"
},
{
"DOI": "10.1126/science.371.6527.329",
"article-title": "New mutations raise specter of ‘immune escape’",
"author": "Kupferschmidt",
"doi-asserted-by": "crossref",
"first-page": "329",
"journal-title": "Science",
"key": "2021090109221259500_CIT0014",
"volume": "371",
"year": "2021"
},
{
"DOI": "10.1016/j.coviro.2021.03.010",
"article-title": "Structure and function of SARS-CoV-2 polymerase",
"author": "Hillen",
"doi-asserted-by": "crossref",
"first-page": "82",
"journal-title": "Curr Opin Virol",
"key": "2021090109221259500_CIT0015",
"volume": "48",
"year": "2021"
}
],
"reference-count": 15,
"references-count": 15,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jid/article/224/5/749/6318434"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Molnupiravir Inhibits Replication of the Emerging SARS-CoV-2 Variants of Concern in a Hamster Infection Model",
"type": "journal-article",
"volume": "224"
}