A Phase 2 Randomized, Double-Blind, Placebo-controlled Trial of Oral Camostat Mesylate for Early Treatment of COVID-19 Outpatients Showed Shorter Illness Course and Attenuation of Loss of Smell and Taste
et al., medRxiv, doi:10.1101/2022.01.28.22270035, NCT04353284, Jan 2022
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
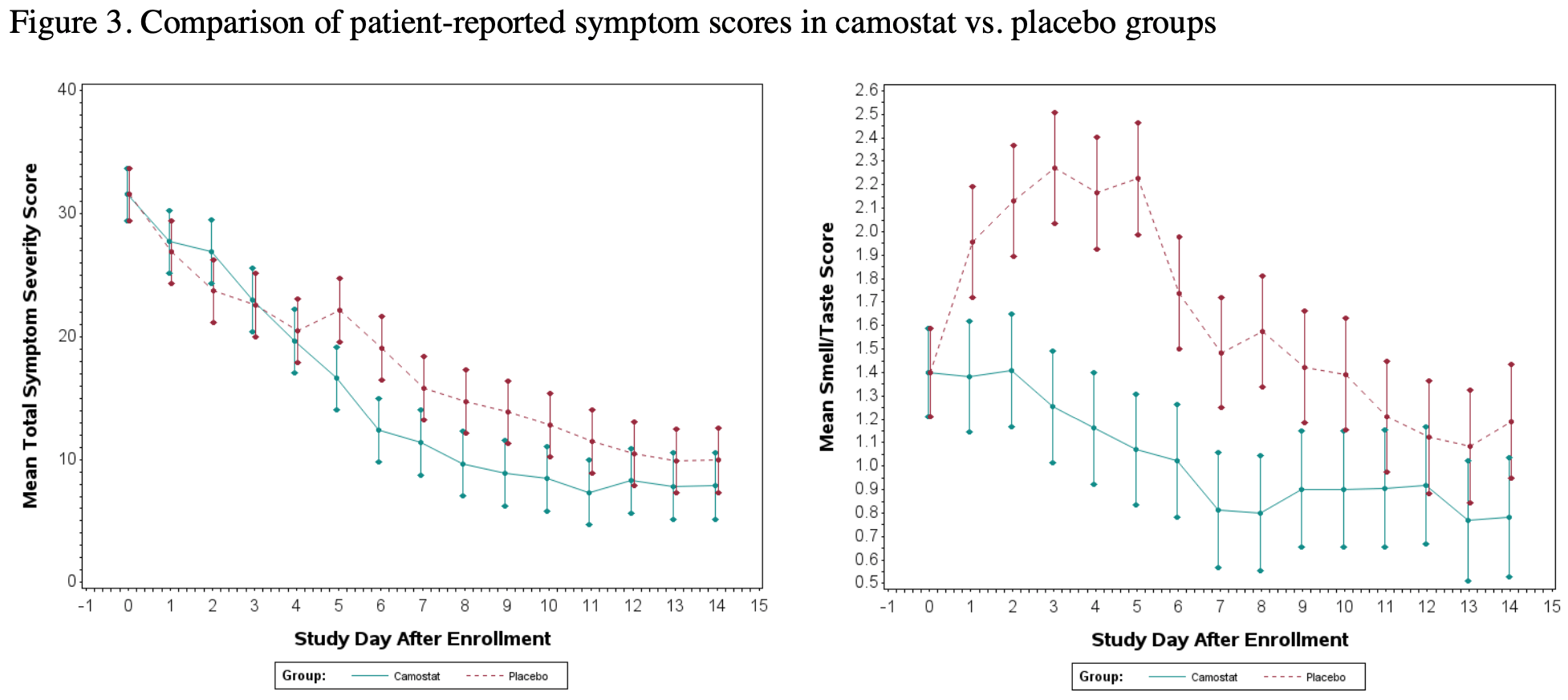
RCT 70 outpatients showing significantly lower symptom scores at day 6, faster recovery, and improved taste/smell, and fatigue with camostat treatment. There was no significant difference for viral load. The recovery result is from1.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments2.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers TMPRSS2 inhibitors and camostat.
|
risk of hospitalization, no change, RR 1.00, p = 1.00, treatment 1 of 35 (2.9%), control 1 of 35 (2.9%).
|
|
risk of no recovery, 36.8% lower, RR 0.63, p = 0.15, treatment 12 of 35 (34.3%), control 19 of 35 (54.3%), NNT 5.0.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Chupp et al., 31 Jan 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, preprint, 24 authors, study period June 2020 - April 2021, trial NCT04353284 (history).
Contact: joseph.vinetz@yale.edu.
A Phase 2 Randomized, Double-Blind, Placebo-controlled Trial of Oral Camostat Mesylate for Early Treatment of COVID-19 Outpatients Showed Shorter Illness Course and Attenuation of Loss of Smell and Taste
doi:10.1101/2022.01.28.22270035
Question: Will early treatment of COVID-19 with a repurposed medication, camostat mesylate, improve clinical outcomes? Findings: In this phase 2 randomized, double-blind placebo-controlled clinical trial that included 70 adults with early COVID-19, the oral administration of camostat mesylate treatment within 3 days of diagnosis prevented the loss of smell/taste and reduced the duration of illness. Meaning: In the current COVID-19 pandemic, phase III testing of an inexpensive, repurposed drug for early COVID-19 is warranted. .
References
Bardsley-Elliot, Noble, None, Oseltamivir, doi:10.2165/00003495-199958050-00007
Boscolo-Rizzo, Borsetto, Fabbris, Evolution of Altered Sense of Smell or Taste in Patients With Mildly Symptomatic COVID-19, JAMA Otolaryngol Head Neck Surg, doi:10.1001/jamaoto.2020.1379
Fitzmaurice, Applied Longitudinal Analysis
Han, Poon, Powers, Leidy, Yu et al., Using the Influenza Patient-reported Outcome (FLU-PRO) diary to evaluate symptoms of influenza viral infection in a healthy human challenge model, BMC Infect Dis, doi:10.1186/s12879-018-3220-8
Hayden, Treanor, Fritz, Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment, JAMA, doi:10.1001/jama.282.13.1240
Hoffmann, Hofmann-Winkler, Smith, Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity, EBioMedicine, doi:10.1016/j.ebiom.2021.103255
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hoffmann, Schroeder, Kleine-Weber, Muller, Drosten et al., Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19, Antimicrob Agents Chemother, doi:10.1128/AAC.00754-20
Kono, Takahashi, Sugai, Oral trypsin inhibitor can improve reflux esophagitis after distal gastrectomy concomitant with decreased trypsin activity, Am J Surg, doi:10.1016/j.amjsurg.2005.05.044
Li, Meyerholz, Bartlett, Mccray, The TMPRSS2 Inhibitor Nafamostat Reduces SARS-CoV-2 Pulmonary Infection in Mouse Models of COVID-19, mBio, doi:10.1128/mBio.00970-21
Motoo, Antiproteases in the treatment of chronic pancreatitis, JOP
Sell, Prough, Weisz, Widen, Hellmich, Leveraging publicly available coronavirus data to identify new therapeutic targets for COVID-19, PLoS One, doi:10.1371/journal.pone.0257965
Sun, Zhang, Cummings, Targeting Enteropeptidase with Reversible Covalent Inhibitors To Achieve Metabolic Benefits, J Pharmacol Exp Ther, doi:10.1124/jpet.120.000219
Watanabe, Chang, Kim, Chu, Ohashi et al., Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: A double-blind, randomized, noninferiority clinical trial, Clin Infect Dis, doi:10.1086/656802
Weinreich, Sivapalasingam, Norton, REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
Zhou, Vedantham, Lu, Protease inhibitors targeting coronavirus and filovirus entry, Antiviral Res, doi:10.1016/j.antiviral.2015.01.011
DOI record:
{
"DOI": "10.1101/2022.01.28.22270035",
"URL": "http://dx.doi.org/10.1101/2022.01.28.22270035",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Importance</jats:title><jats:p>Early treatment of mild SARS-CoV-2 infection might lower the risk of clinical deterioration in COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Objective</jats:title><jats:p>To determine whether oral camostat mesylate would reduce upper respiratory SARS-CoV-2 viral load in newly diagnosed outpatients with mild COVID-19, and would lead to improvement in COVID-19 symptoms.</jats:p></jats:sec><jats:sec><jats:title>Design</jats:title><jats:p>From June, 2020 to April, 2021, we conducted a randomized, double-blind, placebo-controlled phase 2 trial.</jats:p></jats:sec><jats:sec><jats:title>Setting</jats:title><jats:p>Single site, academic medical center, outpatient setting in Connecticut, USA.</jats:p></jats:sec><jats:sec><jats:title>Participants</jats:title><jats:p>Of 568 COVID-19 positive potential adult participants diagnosed within 3 days of study entry and assessed for eligibility, 70 were randomized and 498 were excluded (198 did not meet eligibility criteria, 37 were not interested, 265 were excluded for unknown or other reasons). The primary inclusion criteria were a positive SARS-CoV-2 nucleic acid amplification result in adults within 3 days of screening regardless of COVID-19 symptoms.</jats:p></jats:sec><jats:sec><jats:title>Intervention</jats:title><jats:p>Treatment was 7 days of oral camostat mesylate, 200 mg po four times a day, or placebo.</jats:p></jats:sec><jats:sec><jats:title>Main Outcomes and Measures</jats:title><jats:p>The primary outcome was reduction of 4-day log<jats:sub>10</jats:sub> nasopharyngeal swab viral load by 0.5 log<jats:sub>10</jats:sub> compared to placebo. The main prespecified secondary outcome was reduction in symptom scores as measured by a quantitative Likert scale instrument, Flu-PRO-Plus modified to measure changes in smell/taste measured using FLU-PRO-Plus.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Participants receiving camostat had statistically significant lower quantitative symptom scores (FLU-Pro-Plus) at day 6, accelerated overall symptom resolution and notably improved taste/smell, and fatigue beginning at onset of intervention in the camostat mesylate group compared to placebo. Intention-to-treat analysis demonstrated that camostat mesylate was not associated with a reduction in 4-day log<jats:sub>10</jats:sub> NP viral load compared to placebo.</jats:p></jats:sec><jats:sec><jats:title>Conclusions and relevance</jats:title><jats:p>The camostat group had more rapid resolution of COVID-19 symptoms and amelioration of the loss of taste and smell. Camostat compared to placebo was not associated with reduction in nasopharyngeal SARS-COV-2 viral load. Additional clinical trials are warranted to validate the role of camostat mesylate on SARS-CoV-2 infection in the treatment of mild COVID-19.</jats:p></jats:sec><jats:sec><jats:title>Trial registration: Clinicaltrials.gov, <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"clintrialgov\" xlink:href=\"NCT04353284\">NCT04353284</jats:ext-link> (04/20/20)</jats:title><jats:p><jats:bold>(<jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://clinicaltrials.gov/ct2/show/NCT04353284?term=camostat+%2C+yale&draw=2&rank=1\">https://clinicaltrials.gov/ct2/show/NCT04353284?term=camostat+%2C+yale&draw=2&rank=1</jats:ext-link>)</jats:bold></jats:p></jats:sec><jats:sec><jats:title>Key Points</jats:title><jats:sec><jats:title>Question</jats:title><jats:p>Will early treatment of COVID-19 with a repurposed medication, camostat mesylate, improve clinical outcomes?</jats:p></jats:sec><jats:sec><jats:title>Findings</jats:title><jats:p>In this phase 2 randomized, double-blind placebo-controlled clinical trial that included 70 adults with early COVID-19, the oral administration of camostat mesylate treatment within 3 days of diagnosis prevented the loss of smell/taste and reduced the duration of illness.</jats:p></jats:sec><jats:sec><jats:title>Meaning</jats:title><jats:p>In the current COVID-19 pandemic, phase III testing of an inexpensive, repurposed drug for early COVID-19 is warranted.</jats:p></jats:sec></jats:sec>",
"accepted": {
"date-parts": [
[
2022,
1,
31
]
]
},
"author": [
{
"affiliation": [],
"family": "Chupp",
"given": "Geoffrey",
"sequence": "first"
},
{
"affiliation": [],
"family": "Spichler-Moffarah",
"given": "Anne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Søgaard",
"given": "Ole S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Esserman",
"given": "Denise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dziura",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Danzig",
"given": "Lisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chaurasia",
"given": "Reetika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patra",
"given": "Kailash P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salovey",
"given": "Aryeh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nunez",
"given": "Angela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "May",
"given": "Jeanine",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Astorino",
"given": "Lauren",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel",
"given": "Amisha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Halene",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Jianhui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hui",
"given": "Pei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patel.",
"given": "Prashant",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Jing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Fangyong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gan",
"given": "Geliang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Parziale",
"given": "Stephen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Katsovich",
"given": "Lily",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Desir",
"given": "Gary V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vinetz",
"given": "Joseph M.",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
2,
1
]
],
"date-time": "2022-02-01T02:20:11Z",
"timestamp": 1643682011000
},
"deposited": {
"date-parts": [
[
2022,
2,
2
]
],
"date-time": "2022-02-02T19:25:42Z",
"timestamp": 1643829942000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2023,
8,
31
]
],
"date-time": "2023-08-31T14:02:58Z",
"timestamp": 1693490578885
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 12,
"issued": {
"date-parts": [
[
2022,
1,
31
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2022.01.28.22270035",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2022,
1,
31
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2022,
1,
31
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"DOI": "10.1056/NEJMoa2035002",
"doi-asserted-by": "publisher",
"key": "2022020211250964000_2022.01.28.22270035v1.1"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "2022020211250964000_2022.01.28.22270035v1.2"
},
{
"DOI": "10.1016/j.ebiom.2021.103255",
"doi-asserted-by": "publisher",
"key": "2022020211250964000_2022.01.28.22270035v1.3"
},
{
"DOI": "10.1128/AAC.00754-20",
"doi-asserted-by": "publisher",
"key": "2022020211250964000_2022.01.28.22270035v1.4"
},
{
"DOI": "10.1124/jpet.120.000219",
"doi-asserted-by": "publisher",
"key": "2022020211250964000_2022.01.28.22270035v1.5"
},
{
"DOI": "10.1016/j.antiviral.2015.01.011",
"doi-asserted-by": "publisher",
"key": "2022020211250964000_2022.01.28.22270035v1.6"
},
{
"DOI": "10.1128/mBio.00970-21",
"doi-asserted-by": "publisher",
"key": "2022020211250964000_2022.01.28.22270035v1.7"
},
{
"key": "2022020211250964000_2022.01.28.22270035v1.8",
"unstructured": "Fitzmaurice GM . Applied Longitudinal Analysis. Wiley Series in Probability and Statistics. Wiley-Interscience; 2004."
},
{
"DOI": "10.1186/s12879-018-3220-8",
"doi-asserted-by": "publisher",
"key": "2022020211250964000_2022.01.28.22270035v1.9"
},
{
"DOI": "10.1371/journal.pone.0257965",
"doi-asserted-by": "publisher",
"key": "2022020211250964000_2022.01.28.22270035v1.10"
},
{
"DOI": "10.1001/jamaoto.2020.1379",
"doi-asserted-by": "publisher",
"key": "2022020211250964000_2022.01.28.22270035v1.11"
},
{
"DOI": "10.1086/656802",
"doi-asserted-by": "publisher",
"key": "2022020211250964000_2022.01.28.22270035v1.12"
},
{
"DOI": "10.1001/jama.282.13.1240",
"doi-asserted-by": "publisher",
"key": "2022020211250964000_2022.01.28.22270035v1.13"
},
{
"DOI": "10.2165/00003495-199958050-00007",
"doi-asserted-by": "publisher",
"key": "2022020211250964000_2022.01.28.22270035v1.14"
},
{
"article-title": "Antiproteases in the treatment of chronic pancreatitis",
"first-page": "533",
"issue": "4 Suppl",
"journal-title": "JOP",
"key": "2022020211250964000_2022.01.28.22270035v1.15",
"volume": "8",
"year": "2007"
},
{
"DOI": "10.1016/j.amjsurg.2005.05.044",
"doi-asserted-by": "publisher",
"key": "2022020211250964000_2022.01.28.22270035v1.16"
}
],
"reference-count": 16,
"references-count": 16,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2022.01.28.22270035"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "A Phase 2 Randomized, Double-Blind, Placebo-controlled Trial of Oral Camostat Mesylate for Early Treatment of COVID-19 Outpatients Showed Shorter Illness Course and Attenuation of Loss of Smell and Taste",
"type": "posted-content"
}
