Favipiravir, camostat, and ciclesonide combination therapy in patients with moderate COVID-19 pneumonia with/without oxygen therapy: An open-label, single-center phase 3 randomized clinical trial
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2022.101484, jRCTs031200196, Jun 2022
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
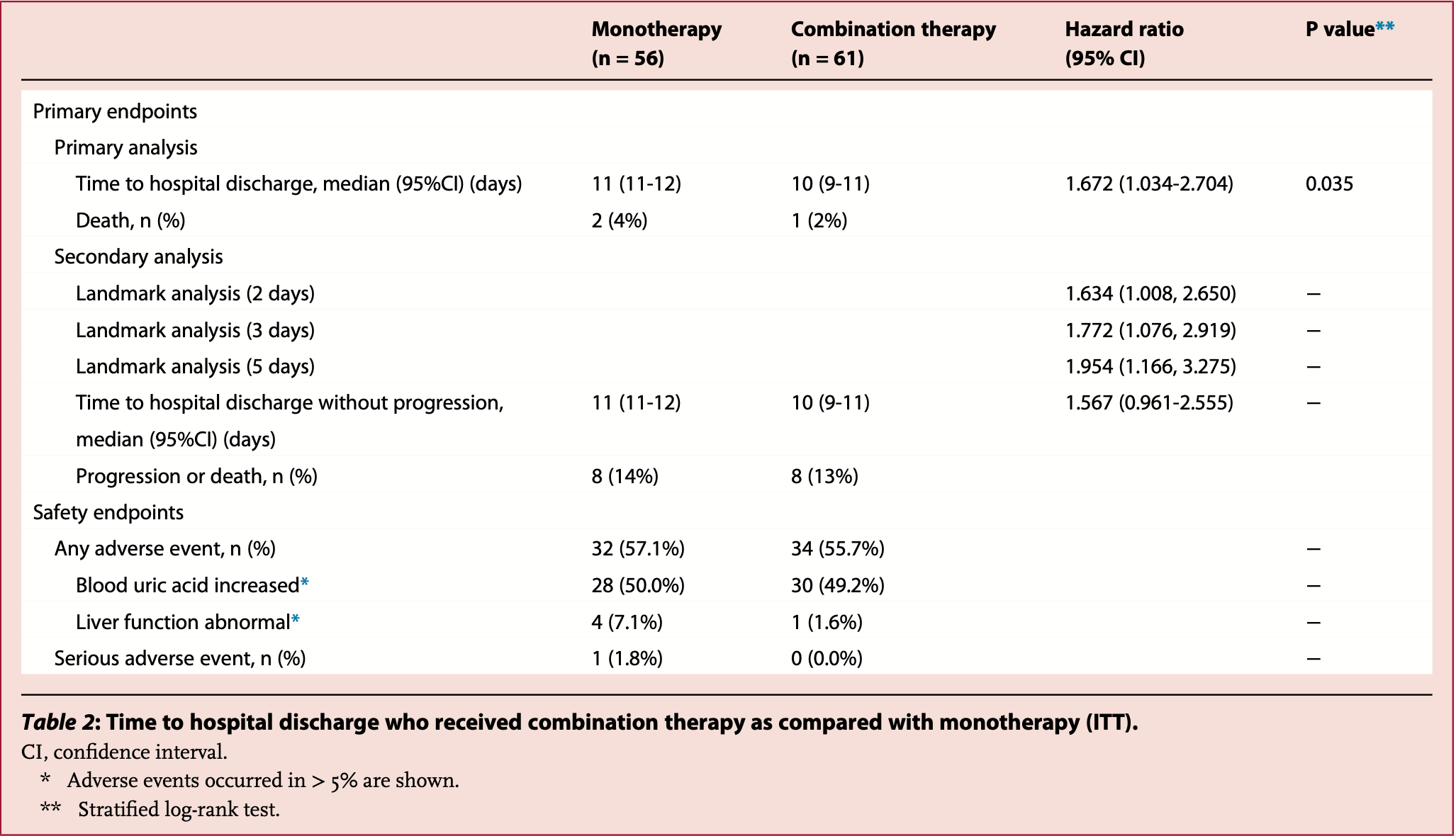
RCT 117 hospitalized patients with moderate COVID-19 pneumonia in Japan, showing a shorter time to discharge with favipiravir, camostat, and ciclesonide combination therapy compared to favipiravir monotherapy. Subgroup analysis showed greater benefit in patients ≤60 years old and those with less severe disease not requiring oxygen. There were no significant differences between groups in clinical findings, laboratory values, or adverse events. The mortality numbers in the main results table and the text are different, without explanation.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
Study covers TMPRSS2 inhibitors and camostat.
|
risk of death, 54.1% lower, RR 0.46, p = 0.61, treatment 1 of 61 (1.6%), control 2 of 56 (3.6%), NNT 52.
|
|
risk of progression, 8.2% lower, RR 0.92, p = 1.00, treatment 8 of 61 (13.1%), control 8 of 56 (14.3%), NNT 85.
|
|
risk of no hospital discharge, 40.2% lower, HR 0.60, p = 0.04, treatment 61, control 56, inverted to make HR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Terada et al., 3 Jun 2022, Randomized Controlled Trial, Japan, peer-reviewed, mean age 57.0, 11 authors, study period 11 November, 2020 - 31 May, 2021, average treatment delay 6.35 days, this trial uses multiple treatments in the treatment arm (combined with ciclesonide) - results of individual treatments may vary, trial jRCTs031200196.
Contact: jirotera@chiba-u.jp.
Favipiravir, camostat, and ciclesonide combination therapy in patients with moderate COVID-19 pneumonia with/without oxygen therapy: An open-label, single-center phase 3 randomized clinical trial
eClinicalMedicine, doi:10.1016/j.eclinm.2022.101484
Background The effectiveness of combination therapy for COVID-19 pneumonia remains unclear. We evaluated favipiravir, camostat, and ciclesonide combination therapy in patients with moderate COVID-19 pneumonia. Methods In this open-label phase 3 study, hospitalized adults who were positive for SARS-CoV-2 and had COVID-19 pneumonia were enrolled prior to official vaccination drive in Japan. Participants were randomly assigned to favipiravir monotherapy or favipiravir + camostat + ciclesonide combination therapy. The primary outcome was the length of hospitalization due to COVID-19 infection after study treatment. The hospitalization period was calculated from the time of admission to the time of patient discharge using the clinical management guide of COVID-19 for front-line healthcare workers developed by the Japanese Ministry of Health, Labour, and Welfare (Version 3). Cases were registered between November 11, 2020, and May 31, 2021. Japan Registry of Clinical Trials registration: jRCTs031200196. Findings Of 121 enrolled patients, 56 received monotherapy and 61 received combination therapy. Baseline characteristics were balanced between the groups. The median time of hospitalization was 10 days for the combination and 11 days for the monotherapy group. The median time to discharge was statistically significantly lower in the combination therapy vs monotherapy group (HR, 1¢67 (95% CI 1¢03−2¢7; P = 0¢035). The hospital discharge rate was statistically significantly higher in the combination therapy vs monotherapy group in patients with less severe COVID-19 infections and those who were ≤60 years. There were no significant differences in clinical findings between the groups at 4, 8, 11, 15, and 29 days. Adverse events were comparable between the groups. There were two deaths, with one in each group. Interpretation Combination oral favipiravir, camostat and, ciclesonide therapy could decrease the length of hospitalization stays without safety concerns in patients with moderate COVID-19 pneumonia. However, lack of hard clinical primary outcome is one of the major limitations of the study.
Supplementary materials Supplementary material associated with this article can be found in the online version at doi:10.1016/j. eclinm.2022.101484.
References
Arab-Zozani, Hassanipour, Ghoddoosi-Nejad, Favipiravir for treating patients with novel coronavirus (COVID-19): protocol for a systematic review and meta-analysis of randomised clinical trials, BMJ Open
Breining, Frølund, Højen, Camostat mesylate against SARS-CoV-2 and COVID-19-Rationale, dosing and safety, Basic Clin Pharmacol Toxicol
Brookmeyer, Crowley, A confidence interval for the median survival time, Biometrics
Cantini, Goletti, Petrone, Fard, Niccoli et al., Immune therapy, or antiviral therapy, or both for COVID-19: a systematic review, Drugs
Coppock, Baram, Chang, COVID-19 treatment combinations and associations with mortality in a large multi-site healthcare system, PLOS ONE
Deokar, Agarwal, Dutt, A review of Ciclesonide in COVID-19. Still a long way to go, Adv Respir Med
Doi, Hibino, Hase, A prospective, randomized, openlabel trial of early versus late favipiravir therapy in hospitalized patients with COVID-19, Antimicrob Agents Chemother
Finney, Glanville, Farne, Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon, J Allergy Clin Immunol
Fischer, Eron, Holman, Molnupiravir, an oral antiviral treatment for COVID-19, MedRxiv Prepr Serv Health Sci
Guan, Ni, Hu, Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Gunst, Staerke, Pahus, Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19-a double-blind randomized controlled trial, EClinicalMedicine
Hoffmann, Hofmann-Winkler, Smith, Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity, EBioMedicine, doi:10.1016/j.ebiom.2021.103255
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell
Hofmann-Winkler, Moerer, Alt-Epping, Camostat mesylate may reduce severity of coronavirus disease 2019 sepsis: a first observation, Crit Care Explor
Joshi, Parkar, Ansari, Role of favipiravir in the treatment of COVID-19, Int J Infect Dis
Mccaw, Tian, Vassy, How to Quantify and Interpret Treatment Effects in Comparative Clinical Studies of COVID-19, Ann Intern Med
Saber-Ayad, Saleh, Abu-Gharbieh, The rationale for potential pharmacotherapy of COVID-19, Pharm Basel Switz
Shinkai, Tsushima, Tanaka, Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial, Infect Dis Ther, doi:10.1007/s40121-021-00517-4
Shrestha, Budhathoki, Khadka, Shah, Pokharel et al., Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis, Virol J
Song, Yoon, Seo, Ciclesonide inhaler treatment for mild-to-moderate COVID-19: a randomized, open-label, phase 2 trial, J Clin Med
Terada-Hirashima, Suzuki, Efficacy and safety of inhaled ciclesonide in treating patients with asymptomatic or mild COVID-19 in the RACCO trial: protocol for a multicenter, open-label, randomized controlled trial, JMIR Res Protoc
DOI record:
{
"DOI": "10.1016/j.eclinm.2022.101484",
"ISSN": [
"2589-5370"
],
"URL": "http://dx.doi.org/10.1016/j.eclinm.2022.101484",
"alternative-id": [
"S2589537022002140"
],
"article-number": "101484",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-9019-4053",
"affiliation": [],
"authenticated-orcid": false,
"family": "Terada",
"given": "Jiro",
"sequence": "first"
},
{
"affiliation": [],
"family": "Fujita",
"given": "Retsu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kawahara",
"given": "Takuya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hirasawa",
"given": "Yasutaka",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kinoshita",
"given": "Taku",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Takeshita",
"given": "Yuichiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Isaka",
"given": "Yuri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kinouchi",
"given": "Toru",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tajima",
"given": "Hiroshi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tada",
"given": "Yuji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tsushima",
"given": "Kenji",
"sequence": "additional"
}
],
"container-title": "eClinicalMedicine",
"container-title-short": "eClinicalMedicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
6,
3
]
],
"date-time": "2022-06-03T17:29:44Z",
"timestamp": 1654277384000
},
"deposited": {
"date-parts": [
[
2022,
6,
3
]
],
"date-time": "2022-06-03T17:30:01Z",
"timestamp": 1654277401000
},
"indexed": {
"date-parts": [
[
2022,
6,
3
]
],
"date-time": "2022-06-03T18:15:22Z",
"timestamp": 1654280122544
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
7
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
1
]
],
"date-time": "2022-07-01T00:00:00Z",
"timestamp": 1656633600000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
5,
14
]
],
"date-time": "2022-05-14T00:00:00Z",
"timestamp": 1652486400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537022002140?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537022002140?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "101484",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
7
]
]
},
"published-print": {
"date-parts": [
[
2022,
7
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "The rationale for potential pharmacotherapy of COVID-19",
"author": "Saber-Ayad",
"first-page": "96",
"journal-title": "Pharm Basel Switz",
"key": "10.1016/j.eclinm.2022.101484_bib0001",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1007/s40265-020-01421-w",
"article-title": "Immune therapy, or antiviral therapy, or both for COVID-19: a systematic review",
"author": "Cantini",
"doi-asserted-by": "crossref",
"first-page": "1929",
"journal-title": "Drugs",
"key": "10.1016/j.eclinm.2022.101484_bib0002",
"volume": "80",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.10.069",
"article-title": "Role of favipiravir in the treatment of COVID-19",
"author": "Joshi",
"doi-asserted-by": "crossref",
"first-page": "501",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.eclinm.2022.101484_bib0003",
"volume": "102",
"year": "2021"
},
{
"DOI": "10.1186/s12985-020-01412-z",
"article-title": "Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis",
"author": "Shrestha",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Virol J",
"key": "10.1016/j.eclinm.2022.101484_bib0004",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.1007/s40121-021-00517-4",
"article-title": "Efficacy and safety of favipiravir in moderate COVID-19 pneumonia patients without oxygen therapy: a randomized, phase III clinical trial",
"author": "Shinkai",
"doi-asserted-by": "crossref",
"first-page": "2489",
"journal-title": "Infect Dis Ther",
"key": "10.1016/j.eclinm.2022.101484_bib0005",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2020-039730",
"article-title": "Favipiravir for treating patients with novel coronavirus (COVID-19): protocol for a systematic review and meta-analysis of randomised clinical trials",
"author": "Arab-Zozani",
"doi-asserted-by": "crossref",
"journal-title": "BMJ Open",
"key": "10.1016/j.eclinm.2022.101484_bib0006",
"volume": "10",
"year": "2020"
},
{
"key": "10.1016/j.eclinm.2022.101484_bib0007",
"unstructured": "COVID-19 treatment guidelines panel. Coronavirus disease 2019 (COVID-19) treatment guidelines [Internet]. National Institutes of Health. Available from: https://www.covid19treatmentguidelines.nih.gov/. Accessed on 1 December 2021."
},
{
"DOI": "10.5603/ARM.a2020.0173",
"article-title": "A review of Ciclesonide in COVID-19. Still a long way to go",
"author": "Deokar",
"doi-asserted-by": "crossref",
"first-page": "79",
"journal-title": "Adv Respir Med",
"key": "10.1016/j.eclinm.2022.101484_bib0008",
"volume": "89",
"year": "2021"
},
{
"DOI": "10.2196/23830",
"article-title": "Efficacy and safety of inhaled ciclesonide in treating patients with asymptomatic or mild COVID-19 in the RACCO trial: protocol for a multicenter, open-label, randomized controlled trial",
"author": "Terada-Hirashima",
"doi-asserted-by": "crossref",
"first-page": "e23830",
"journal-title": "JMIR Res Protoc",
"key": "10.1016/j.eclinm.2022.101484_bib0009",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.3390/jcm10163545",
"article-title": "Ciclesonide inhaler treatment for mild-to-moderate COVID-19: a randomized, open-label, phase 2 trial",
"author": "Song",
"doi-asserted-by": "crossref",
"first-page": "3545",
"journal-title": "J Clin Med",
"key": "10.1016/j.eclinm.2022.101484_bib0010",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/j.ebiom.2021.103255",
"article-title": "Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"journal-title": "EBioMedicine",
"key": "10.1016/j.eclinm.2022.101484_bib0011",
"volume": "65",
"year": "2021"
},
{
"DOI": "10.1097/CCE.0000000000000284",
"article-title": "Camostat mesylate may reduce severity of coronavirus disease 2019 sepsis: a first observation",
"author": "Hofmann-Winkler",
"doi-asserted-by": "crossref",
"first-page": "e0284",
"journal-title": "Crit Care Explor",
"key": "10.1016/j.eclinm.2022.101484_bib0012",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1111/bcpt.13533",
"article-title": "Camostat mesylate against SARS-CoV-2 and COVID-19—Rationale, dosing and safety",
"author": "Breining",
"doi-asserted-by": "crossref",
"first-page": "204",
"journal-title": "Basic Clin Pharmacol Toxicol",
"key": "10.1016/j.eclinm.2022.101484_bib0013",
"volume": "128",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "10.1016/j.eclinm.2022.101484_bib0014",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1016/j.jaci.2020.09.034",
"article-title": "Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon",
"author": "Finney",
"doi-asserted-by": "crossref",
"first-page": "510",
"journal-title": "J Allergy Clin Immunol",
"key": "10.1016/j.eclinm.2022.101484_bib0015",
"volume": "147",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.100849",
"article-title": "Efficacy of the TMPRSS2 inhibitor camostat mesilate in patients hospitalized with Covid-19-a double-blind randomized controlled trial",
"author": "Gunst",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "10.1016/j.eclinm.2022.101484_bib0016",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1001/jamainternmed.2021.6759",
"article-title": "Efficacy of Inhaled Ciclesonide for Outpatient Treatment of Adolescents and Adults With Symptomatic COVID-19: A Randomized Clinical Trial",
"author": "Clemency",
"doi-asserted-by": "crossref",
"first-page": "42",
"journal-title": "JAMA Intern Med",
"key": "10.1016/j.eclinm.2022.101484_bib0017",
"volume": "182",
"year": "2022"
},
{
"key": "10.1016/j.eclinm.2022.101484_bib0018",
"unstructured": "Clinical management of patients with COVID-19. A guide for front-line healthcare worker version 3.0 Issued by Ministry of Health, Labour and Welfare in Japan. (in Japanese. current latest version: version 6.0) [Internet]. 2021. Available from: https://www.mhlw.go.jp/content/000829136.pdf. Accessed on 1 December 2021"
},
{
"DOI": "10.2307/2530286",
"article-title": "A confidence interval for the median survival time",
"author": "Brookmeyer",
"doi-asserted-by": "crossref",
"first-page": "29",
"journal-title": "Biometrics",
"key": "10.1016/j.eclinm.2022.101484_bib0019",
"volume": "38",
"year": "1982"
},
{
"DOI": "10.7326/M20-4044",
"article-title": "How to Quantify and Interpret Treatment Effects in Comparative Clinical Studies of COVID-19",
"author": "McCaw",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.eclinm.2022.101484_bib0020",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0252591",
"article-title": "COVID-19 treatment combinations and associations with mortality in a large multi-site healthcare system",
"author": "Coppock",
"doi-asserted-by": "crossref",
"journal-title": "PLOS ONE",
"key": "10.1016/j.eclinm.2022.101484_bib0021",
"volume": "16",
"year": "2021"
},
{
"article-title": "Molnupiravir, an oral antiviral treatment for COVID-19",
"author": "Fischer",
"journal-title": "MedRxiv Prepr Serv Health Sci",
"key": "10.1016/j.eclinm.2022.101484_bib0022",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2022.101484_bib0023",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1128/AAC.01897-20",
"article-title": "A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19",
"author": "Doi",
"doi-asserted-by": "crossref",
"first-page": "e01897",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.eclinm.2022.101484_bib0024",
"volume": "64",
"year": "2020"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589537022002140"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Favipiravir, camostat, and ciclesonide combination therapy in patients with moderate COVID-19 pneumonia with/without oxygen therapy: An open-label, single-center phase 3 randomized clinical trial",
"type": "journal-article",
"volume": "49"
}
