Efficacy of Bromhexine versus Standard of Care in Reducing Viral Load in Patients with Mild-to-Moderate COVID-19 Disease Attended in Primary Care: A Randomized Open-Label Trial
et al., Journal of Clinical Medicine, doi:10.3390/jcm12010142, EudraCT2021-001227-41, Dec 2022
22nd treatment shown to reduce risk in
April 2021, now with p = 0.00063 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 191 low risk (no mortality) outpatients in Spain, showing no significant differences with bromhexine. Authors note that "statistical differences between the study groups were observed in the percentage of patients treated with bronchodilators (p = 0.033) and receiving symptomatic treatment (p = 0.034), which were higher in the SOC alone group", but do not provide details or perform adjustments. There were more moderate/severe cases in the treatment group (9 vs. 5).
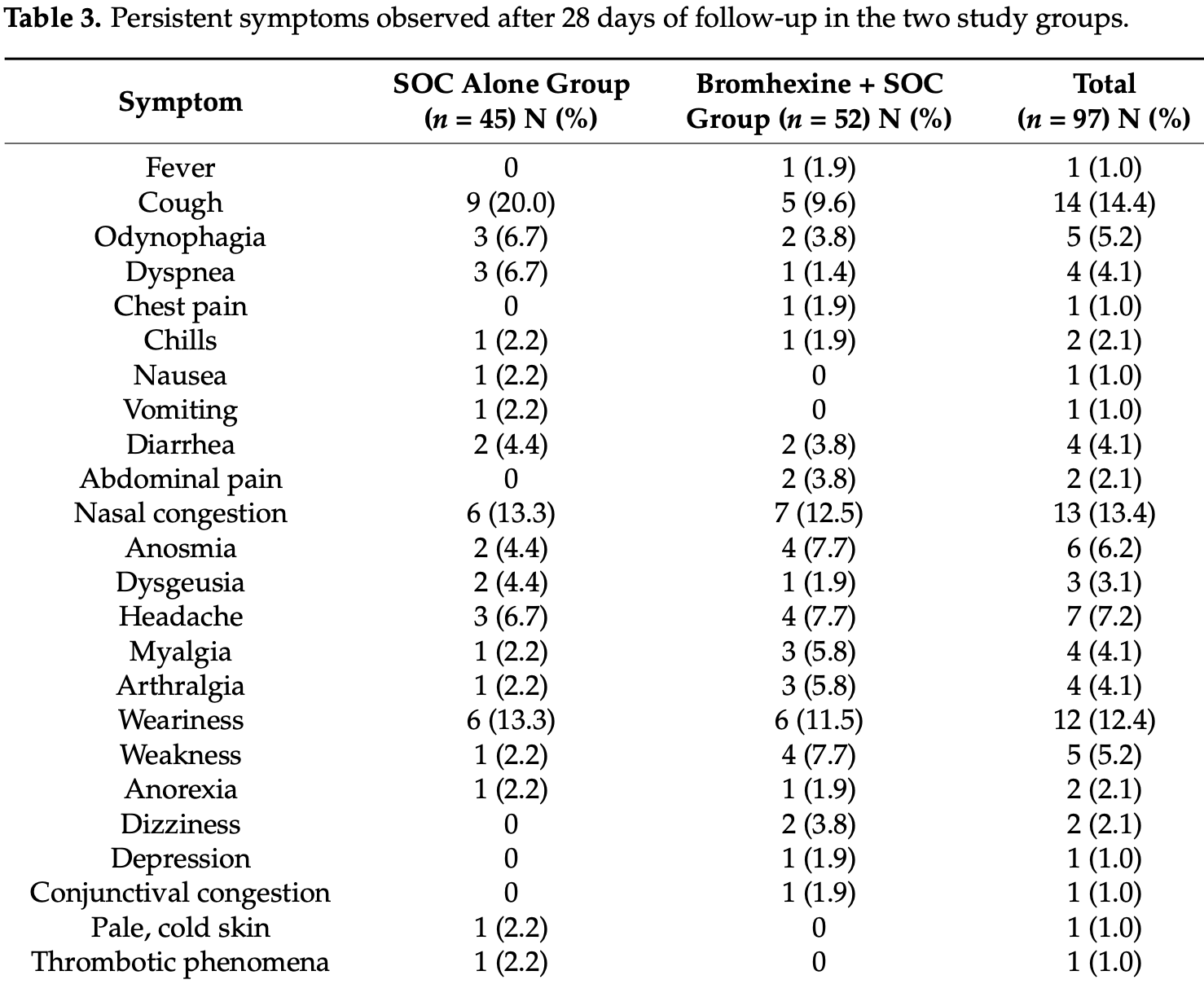
Many results appear to be missing including: reduction in the severity of each symptom (0-10 NRS score) at days 4, 7, 14, and 28 as compared with baseline; proportion of patients with clinical improvement and time to clinical improvement; proportion of patients with disappearance of each symptom at days 4, 7, 14, and 28, and time to disappearance; proportion of asymptomatic patients at days 4, 7, 14, and 28.
Bromhexine 48 mg/day for seven days. SOC included acetaminophen.
Study covers TMPRSS2 inhibitors and bromhexine.
|
risk of oxygen therapy, 67.3% lower, RR 0.33, p = 0.49, treatment 0 of 98 (0.0%), control 1 of 93 (1.1%), NNT 93, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 67.3% lower, RR 0.33, p = 0.49, treatment 0 of 98 (0.0%), control 1 of 93 (1.1%), NNT 93, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of no recovery, 71.2% lower, RR 0.29, p = 0.33, treatment 1 of 52 (1.9%), control 3 of 45 (6.7%), NNT 21, dyspnea.
|
|
risk of no recovery, 186.5% higher, RR 2.87, p = 1.00, treatment 1 of 52 (1.9%), control 0 of 45 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), fever.
|
|
viral load, 6.6% higher, relative load 1.07, p = 0.82, treatment mean 13.54 (±26.02) n=98, control mean 14.43 (±26.94) n=93, relative change in ORF1ab Ct value, day 4, primary outcome.
|
|
viral load, 17.4% lower, relative load 0.83, p = 0.60, treatment mean 6.36 (±17.05) n=98, control mean 7.7 (±18.47) n=93, relative change in N Ct value, day 4, primary outcome.
|
|
viral load, 41.5% higher, relative load 1.41, p = 0.32, treatment mean 9.74 (±29.54) n=98, control mean 13.78 (±26.81) n=93, relative change in S Ct value, day 4, primary outcome.
|
|
risk of no viral clearance, 13.4% lower, RR 0.87, p = 0.31, treatment 52 of 98 (53.1%), control 57 of 93 (61.3%), NNT 12, day 14.
|
|
risk of no viral clearance, 13.6% higher, RR 1.14, p = 0.21, treatment 73 of 98 (74.5%), control 61 of 93 (65.6%), day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Vila Méndez et al., 24 Dec 2022, Randomized Controlled Trial, Spain, peer-reviewed, 38 authors, study period 24 February, 2022 - 28 July, 2022, trial EudraCT2021-001227-41.
Contact: ana.martinez@csic.es (corresponding author), bsoler@ecbio.net.
Efficacy of Bromhexine versus Standard of Care in Reducing Viral Load in Patients with Mild-to-Moderate COVID-19 Disease Attended in Primary Care: A Randomized Open-Label Trial
Journal of Clinical Medicine, doi:10.3390/jcm12010142
A 28-day randomized open-label multicenter study was conducted to assess the efficacy of bromhexine plus standard of care (SOC) (n = 98) vs. SOC alone (n = 93) in 191 outpatients with mildto-moderate COVID-19 in the primary health care setting. Bromhexine three daily doses of 10 mL (48 mg/day) were administered for seven days. The primary efficacy endpoint was the reduction of viral load estimated as the cycle thresholds (Ct) to detect ORF1ab, N Protein, and S Protein genes by RT-qPCR in saliva samples on day 4 as compared with baseline. Ct values of the three genes increased from baseline throughout days 4 to 14 (p < 0.001) but significant differences between the study groups were not found. Differences in the percentages of patients with low, medium, and high viral loads at 4, 7, and 14 days were not found either. In summary, treatment with bromhexine plus SCO was associated with a viral load reduction of ORF1ab, N Protein, and S Protein genes at day 4, which was not significantly different than similar viral load reductions observed with SOC alone. The present findings do not seem to favor the use of bromhexine as an antiviral in patients with COVID-19.
Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest: A. Martínez and C. Gil are employees of the sponsor Agencia Estatal Consejo Superior de Investigaciones Científicas, M.P. (CSIC); B. Soler López was contracted to carry out the design, monitoring, statistical analysis and management of the publications derived from the study. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Al-Kuraishy, ; A-Niemi, Hussain, Al-Gaareb, Lugnier, The potential role of bromhexine in the management of COVID-19: Decipher and a real game-changer, Curr. Med. Drug Res, doi:10.53517/CMDR.2581-5008.512021212
Alegria-Arcos, Barbosa, Sepúlveda, Combariza, González et al., Network pharmacology reveals mechanism of action of drugs to be repurposed for COVID-19, Front. Pharmacol, doi:10.3389/fphar.2022.952192
Anderson, Heesterbeek, Klinkenberg, Hollingsworth, How will country-based mitigation measures influence the course of the COVID-19 epidemic?, Lancet, doi:10.1016/S0140-6736(20)30567-5
Ansarin, Tolouian, Ardalan, Taghizadieh, Varshochi et al., Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: A randomized clinical trial, Bioimpacts, doi:10.34172/bi.2020.27
Ashour, Abo Elmaaty, Sarhan, Elkaeed, Moussa et al., A systematic Review of the global intervention for SARS-CoV-2 combating: From drugs repurposing to molnupiravir approval, Drug Des. Dev. Ther, doi:10.2147/DDDT.S354841
Bahadoram, Keikhaei, Bahadoram, Mahmoudian-Sani, Hassanzadeh et al., Bromhexine is a potential drug for COVID-19; From hypothesis to clinical trials, Probl. Virol, doi:10.36233/0507-4088-106
Depfenhart, De Villiers, Lemperle, Meyer, Di Somma, Potential new treatment strategies for COVID-19: Is there a role for bromhexine as add-on therapy?, Intern. Emerg. Med, doi:10.1007/s11739-020-02383-3
Fu, Zheng, Zhou, Tang, Chen et al., Re-recognizing bromhexine hydrochloride: Pharmaceutical properties and its possible role in treating pediatric COVID-19, Eur. J. Clin. Pharmacol, doi:10.1007/s00228-020-02971-4
Gil, Ginex, Maestro, Nozal, Barrado-Gil et al., COVID-19: Drug Targets and Potential Treatments, J. Med. Chem, doi:10.1021/acs.jmedchem.0c00606
Ginex, Garaigorta, Ramírez, Castro, Nozal et al., Host-directed FDA-approved drugs with antiviral activity against SARS-CoV-2 identified by hierarchical in silico/in vitro screening methods, Pharmaceuticals, doi:10.3390/ph14040332
Glowacka, Bertram, Müller, Allen, Soilleux et al., Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response, J. Virol, doi:10.1128/JVI.02232-10
Habtemariam, Nabavi, Ghavami, Cismaru, Berindan-Neagoe et al., Possible use of the mucolytic drug, bromhexine hydrochloride, as a prophylactic agent against SARS-CoV-2 infection based on its action on the Transmembrane Serine Protease 2, Pharmacol. Res, doi:10.1016/j.phrs.2020.104853
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hörnich, Großkopf, Schlagowski, Tenbusch, Kleine-Weber et al., SARS-CoV-2 and SARS-CoV spike-mediated cell-cell fusion differ in their requirements for receptor expression and proteolytic activation, J. Virol, doi:10.1128/JVI.00002-21
Maggio, Corsini, Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection, Pharmacol. Res, doi:10.1016/j.phrs.2020.104837
Matsuyama, Nao, Shirato, Kawase, Saito et al., Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells, doi:10.1073/pnas.2002589117
Mikhaylov, Lyubimtseva, Vakhrushev, Stepanov, Lebedev et al., Bromhexine hydrochloride prophylaxis of COVID-19 for medical personnel: A randomized open-label study, Interdiscip. Perspect. Infect. Dis, doi:10.1155/2022/4693121
Shen, Mao, Wu, Tanaka, Zhang, TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections, Biochimie, doi:10.1016/j.biochi.2017.07.016
Tolouian, Mulla, Jamaati, Babamahmoodi, Marjani et al., Effect of bromhexine in hospitalized patients with COVID-19, J. Investig. Med, doi:10.1136/jim-2020-001747
Wang, Zhang, Chen, Xue, Zhang et al., Evaluating the efficacy and safety of bromhexine hydrochloride tablets in treating pediatric COVID-19: A protocol for meta-analysis and systematic review, Medicine, doi:10.1097/MD.0000000000022114
DOI record:
{
"DOI": "10.3390/jcm12010142",
"ISSN": [
"2077-0383"
],
"URL": "http://dx.doi.org/10.3390/jcm12010142",
"abstract": "<jats:p>A 28-day randomized open-label multicenter study was conducted to assess the efficacy of bromhexine plus standard of care (SOC) (n = 98) vs. SOC alone (n = 93) in 191 outpatients with mild-to-moderate COVID-19 in the primary health care setting. Bromhexine three daily doses of 10 mL (48 mg/day) were administered for seven days. The primary efficacy endpoint was the reduction of viral load estimated as the cycle thresholds (Ct) to detect ORF1ab, N Protein, and S Protein genes by RT-qPCR in saliva samples on day 4 as compared with baseline. Ct values of the three genes increased from baseline throughout days 4 to 14 (p < 0.001) but significant differences between the study groups were not found. Differences in the percentages of patients with low, medium, and high viral loads at 4, 7, and 14 days were not found either. In summary, treatment with bromhexine plus SCO was associated with a viral load reduction of ORF1ab, N Protein, and S Protein genes at day 4, which was not significantly different than similar viral load reductions observed with SOC alone. The present findings do not seem to favor the use of bromhexine as an antiviral in patients with COVID-19.</jats:p>",
"alternative-id": [
"jcm12010142"
],
"author": [
{
"affiliation": [],
"family": "Vila Méndez",
"given": "María Luz",
"sequence": "first"
},
{
"affiliation": [],
"family": "Antón Sanz",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cárdenas García",
"given": "Alicia del Rocío",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bravo Malo",
"given": "Amparo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torres Martínez",
"given": "Francisco Javier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Martín Moros",
"given": "José María",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Real Torrijos",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vendrell Covisa",
"given": "José Francisco Javier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guzmán Sierra",
"given": "Olga",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Molina Barcena",
"given": "Verónica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Viejo Pinero",
"given": "Nuria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernández Díaz",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arroyo Burguillo",
"given": "Purificación",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blanco Gallego",
"given": "Ana María",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guirao Sánchez",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montilla Bernabé",
"given": "Aránzazu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Villanueva Morán",
"given": "María del Pilar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Juárez Antón",
"given": "Salvador",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fernández Rodríguez",
"given": "Ángela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Somoza Calvo",
"given": "María Ángeles",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cerrada",
"given": "Ernesto Cerrada",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pérez Mañas",
"given": "Gemma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sánchez Calso",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vallejo Somohano",
"given": "Frida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cauqui Díaz",
"given": "Carmen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Viñas Fernández",
"given": "Gloria",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4716-3152",
"affiliation": [],
"authenticated-orcid": false,
"family": "Molina París",
"given": "Jesús",
"sequence": "additional"
},
{
"affiliation": [],
"family": "González Godoy",
"given": "Marina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lumbreras García",
"given": "Gonzalo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rosado Martín",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rodríguez Hernández",
"given": "Aida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "López Antúñez",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vázquez Perfecto",
"given": "Gabriel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marcello Andrés",
"given": "María Concepción",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Puente García",
"given": "Nieves Marina",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3882-6081",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gil",
"given": "Carmen",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2707-8110",
"affiliation": [],
"authenticated-orcid": false,
"family": "Martínez",
"given": "Ana",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5853-2307",
"affiliation": [],
"authenticated-orcid": false,
"family": "Soler López",
"given": "Begoña",
"sequence": "additional"
}
],
"container-title": "Journal of Clinical Medicine",
"container-title-short": "JCM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
12,
27
]
],
"date-time": "2022-12-27T07:53:11Z",
"timestamp": 1672127591000
},
"deposited": {
"date-parts": [
[
2022,
12,
29
]
],
"date-time": "2022-12-29T09:11:50Z",
"timestamp": 1672305110000
},
"funder": [
{
"award": [
"PIE 201980E024"
],
"name": "CSIC"
},
{
"award": [
"EU 2020/2094"
],
"name": "European Commission: NextGeneration EU"
}
],
"indexed": {
"date-parts": [
[
2022,
12,
30
]
],
"date-time": "2022-12-30T06:09:20Z",
"timestamp": 1672380560206
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
12,
24
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
24
]
],
"date-time": "2022-12-24T00:00:00Z",
"timestamp": 1671840000000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2077-0383/12/1/142/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "142",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
12,
24
]
]
},
"published-online": {
"date-parts": [
[
2022,
12,
24
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.2147/DDDT.S354841",
"article-title": "A systematic Review of the global intervention for SARS-CoV-2 combating: From drugs repurposing to molnupiravir approval",
"author": "Ashour",
"doi-asserted-by": "crossref",
"first-page": "685",
"journal-title": "Drug Des. Dev. Ther.",
"key": "ref_1",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "ref_2",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1128/JVI.02232-10",
"article-title": "Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response",
"author": "Glowacka",
"doi-asserted-by": "crossref",
"first-page": "4122",
"journal-title": "J. Virol.",
"key": "ref_3",
"volume": "85",
"year": "2011"
},
{
"DOI": "10.1073/pnas.2002589117",
"article-title": "Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells",
"author": "Matsuyama",
"doi-asserted-by": "crossref",
"first-page": "7001",
"journal-title": "Proc. Natl. Acad. Sci. USA",
"key": "ref_4",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1016/j.biochi.2017.07.016",
"article-title": "TMPRSS2: A potential target for treatment of influenza virus and coronavirus infections",
"author": "Shen",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Biochimie",
"key": "ref_5",
"volume": "142",
"year": "2017"
},
{
"DOI": "10.1128/JVI.00002-21",
"article-title": "SARS-CoV-2 and SARS-CoV spike-mediated cell-cell fusion differ in their requirements for receptor expression and proteolytic activation",
"author": "Schlagowski",
"doi-asserted-by": "crossref",
"first-page": "e00002-21",
"journal-title": "J. Virol.",
"key": "ref_6",
"volume": "95",
"year": "2021"
},
{
"DOI": "10.1021/acs.jmedchem.0c00606",
"article-title": "COVID-19: Drug Targets and Potential Treatments",
"author": "Gil",
"doi-asserted-by": "crossref",
"first-page": "12359",
"journal-title": "J. Med. Chem.",
"key": "ref_7",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.3390/ph14040332",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Ginex, T., Garaigorta, U., Ramírez, D., Castro, V., Nozal, V., Maestro, I., García-Cárceles, J., Campillo, N.E., Martinez, A., and Gastaminza, P. (2021). Host-directed FDA-approved drugs with antiviral activity against SARS-CoV-2 identified by hierarchical in silico/in vitro screening methods. Pharmaceuticals, 14."
},
{
"DOI": "10.3389/fphar.2022.952192",
"article-title": "Network pharmacology reveals mechanism of action of drugs to be repurposed for COVID-19",
"author": "Barbosa",
"doi-asserted-by": "crossref",
"first-page": "952192",
"journal-title": "Front. Pharmacol.",
"key": "ref_9",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1007/s11739-020-02383-3",
"article-title": "Potential new treatment strategies for COVID-19: Is there a role for bromhexine as add-on therapy?",
"author": "Depfenhart",
"doi-asserted-by": "crossref",
"first-page": "801",
"journal-title": "Intern. Emerg. Med.",
"key": "ref_10",
"volume": "15",
"year": "2020"
},
{
"article-title": "The potential role of bromhexine in the management of COVID-19: Decipher and a real game-changer",
"author": "Hussain",
"first-page": "212",
"journal-title": "Curr. Med. Drug Res.",
"key": "ref_11",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1136/jim-2020-001747",
"doi-asserted-by": "crossref",
"key": "ref_12",
"unstructured": "Tolouian, R., Mulla, Z.D., Jamaati, H., Babamahmoodi, A., Marjani, M., Eskandari, R., and Dastan, F. (2021). Effect of bromhexine in hospitalized patients with COVID-19. J. Investig. Med., 1–6."
},
{
"DOI": "10.34172/bi.2020.27",
"article-title": "Effect of bromhexine on clinical outcomes and mortality in COVID-19 patients: A randomized clinical trial",
"author": "Ansarin",
"doi-asserted-by": "crossref",
"first-page": "209",
"journal-title": "Bioimpacts",
"key": "ref_13",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1155/2022/4693121",
"article-title": "Bromhexine hydrochloride prophylaxis of COVID-19 for medical personnel: A randomized open-label study",
"author": "Mikhaylov",
"doi-asserted-by": "crossref",
"first-page": "4693121",
"journal-title": "Interdiscip. Perspect. Infect. Dis.",
"key": "ref_14",
"volume": "2022",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(20)30567-5",
"article-title": "How will country-based mitigation measures influence the course of the COVID-19 epidemic?",
"author": "Anderson",
"doi-asserted-by": "crossref",
"first-page": "931",
"journal-title": "Lancet",
"key": "ref_15",
"volume": "395",
"year": "2020"
},
{
"key": "ref_16",
"unstructured": "Sociedad Española de Médicos de Atención Primaria (SEMERGEN) (2020). Protocolo de Actuación en Pacientes con COVID-19 Asistidos en Atención Primaria, Sociedad Española de Médicos de Atención Primaria (SEMERGEN). Available online: https://www.semergen.es/files/docs/COVID-19/Documentos/ferrer-covid-19.pdf."
},
{
"key": "ref_17",
"unstructured": "World Health Organization (2022, November 22). COVID-19 Clinical Management. This Document Is the Update of an Interim Guidance Originally Published under the Title “Clinical Management of COVID-19: Interim Guidance, 27 May 2020”. World Health Organization 2021. WHO Reference Number: WHO/2019-nCoV/clinical/2021.1. Available online: https://www.semergen.es/files/docs/COVID-19/Documentos/who-guide-covig192021.1-eng.pdf."
},
{
"DOI": "10.1007/s00228-020-02971-4",
"article-title": "Re-recognizing bromhexine hydrochloride: Pharmaceutical properties and its possible role in treating pediatric COVID-19",
"author": "Fu",
"doi-asserted-by": "crossref",
"first-page": "261",
"journal-title": "Eur. J. Clin. Pharmacol.",
"key": "ref_18",
"volume": "77",
"year": "2021"
},
{
"DOI": "10.1016/j.phrs.2020.104837",
"article-title": "Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection",
"author": "Maggio",
"doi-asserted-by": "crossref",
"first-page": "104837",
"journal-title": "Pharmacol. Res.",
"key": "ref_19",
"volume": "157",
"year": "2020"
},
{
"DOI": "10.1016/j.phrs.2020.104853",
"article-title": "Possible use of the mucolytic drug, bromhexine hydrochloride, as a prophylactic agent against SARS-CoV-2 infection based on its action on the Transmembrane Serine Protease 2",
"author": "Habtemariam",
"doi-asserted-by": "crossref",
"first-page": "104853",
"journal-title": "Pharmacol. Res.",
"key": "ref_20",
"volume": "157",
"year": "2020"
},
{
"DOI": "10.36233/0507-4088-106",
"article-title": "Bromhexine is a potential drug for COVID-19; From hypothesis to clinical trials",
"author": "Bahadoram",
"doi-asserted-by": "crossref",
"first-page": "126",
"journal-title": "Probl. Virol.",
"key": "ref_21",
"volume": "67",
"year": "2022"
},
{
"key": "ref_22",
"unstructured": "Ministerio de Sanidad (2022, November 22). Enfermedad por Coronavirus, COVID-19. Actualización 12 de Noviembre de 2020. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/ITCoronavirus/home.htm."
},
{
"DOI": "10.1097/MD.0000000000022114",
"article-title": "Evaluating the efficacy and safety of bromhexine hydrochloride tablets in treating pediatric COVID-19: A protocol for meta-analysis and systematic review",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "e22114",
"journal-title": "Medicine",
"key": "ref_23",
"volume": "99",
"year": "2020"
},
{
"key": "ref_24",
"unstructured": "(2022, November 22). Actualización de la Situación Epidemiológica de las Variantes SARS-CoV-2 en España. 26 de Septiembre de 2022. Ministerio de Sanidad. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/COVID19_Actualizacion_variantes_20220926.pdf."
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2077-0383/12/1/142"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Efficacy of Bromhexine versus Standard of Care in Reducing Viral Load in Patients with Mild-to-Moderate COVID-19 Disease Attended in Primary Care: A Randomized Open-Label Trial",
"type": "journal-article",
"volume": "12"
}
