Nasal spray of an IgM‐like ACE2 fusion protein HH‐120 prevents SARS‐CoV‐2 infection: Two investigator‐initiated postexposure prophylaxis trials
et al., Journal of Medical Virology, doi:10.1002/jmv.29275, NCT05747677, Dec 2023
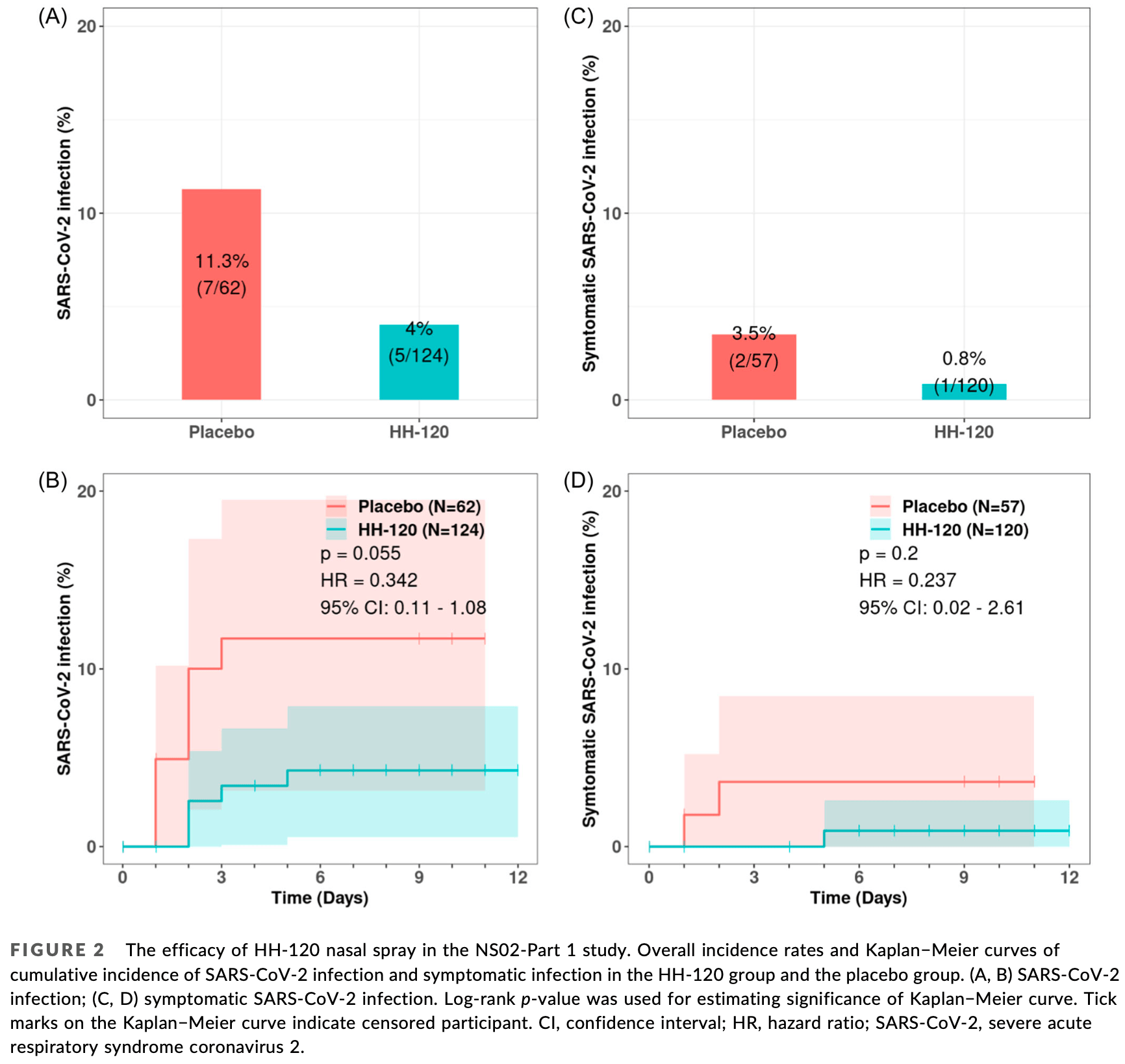
RCT 269 participants showing significantly reduced risk of infection and symptomatic infection with IgM-like ACE2 fusion protein HH-120 nasal spray used as post-exposure prophylaxis. Participants self-administered HH-120 or placebo 5-10 times daily for up to 10 days. HH-120 reduced risk of infection by 64.6% in general contacts and 43.8% in close contacts, and reduced risk of symptomatic infection by 77.1% and 72.5%, respectively.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
risk of symptomatic case, 75.9% lower, HR 0.24, p = 0.02, treatment 120, control 57, both parts combined.
|
|
risk of symptomatic case, 76.3% lower, HR 0.24, p = 0.20, treatment 1 of 120 (0.8%), control 2 of 57 (3.5%), NNT 37, Cox proportional hazards, Part 1.
|
|
risk of symptomatic case, 75.8% lower, HR 0.24, p = 0.03, treatment 3 of 40 (7.5%), control 6 of 22 (27.3%), NNT 5.1, Cox proportional hazards, Part 2.
|
|
risk of case, 58.2% lower, HR 0.42, p = 0.03, treatment 124, control 62, both parts combined.
|
|
risk of case, 65.8% lower, HR 0.34, p = 0.06, treatment 5 of 124 (4.0%), control 7 of 62 (11.3%), NNT 14, Cox proportional hazards, Part 1.
|
|
risk of case, 50.4% lower, HR 0.50, p = 0.18, treatment 7 of 41 (17.1%), control 7 of 23 (30.4%), NNT 7.5, Cox proportional hazards, Part 2.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Song et al., 6 Dec 2023, retrospective, placebo-controlled, China, peer-reviewed, mean age 36.0, 18 authors, study period June 2022 - December 2022, trial NCT05747677 (history).
Contact: liwenhui@nibs.ac.cn, ronghuajin@ccmu.edu.cn.
Nasal spray of an IgM‐like ACE2 fusion protein HH‐120 prevents SARS‐CoV‐2 infection: Two investigator‐initiated postexposure prophylaxis trials
Journal of Medical Virology, doi:10.1002/jmv.29275
HH-120, an IgM-like angiotensin converting enzyme 2 (ACE2) fusion protein, has been developed as a nasal spray against Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is currently undergoing human trials. HH-120 nasal spray was assessed for postexposure prophylaxis (PEP) in two investigatorinitiated (NS01 and NS02) trials with different risk levels of SARS-CoV-2 exposure. NS01 enrolled family caregiver participants who had continuous contacts with laboratory-confirmed index cases; NS02 enrolled participants who had general contacts (Part 1) or close contacts (Part 2) with index cases. The primary endpoints were safety and laboratory-confirmed and/or symptomatic SARS-CoV-2 infection. In NS01 trial (14 participants), the SARS-CoV-2 infection rates were 25% in the HH-120 group and 83.3% in the external control group (relative risk reduction [RRR]: 70.0%). In NS02-Part 1 (193 participants), the infection rates were 4% (HH-120) versus 11.3% (placebo), symptomatic infection rates were 0.8% versus 3.5%, hence with a RRR of 64.6% and 77.1%, respectively. In Part 2 (76 participants), the infection rates were 17.1% (HH-120) versus 30.4% (placebo), symptomatic infection rates were 7.5% versus 27.3%, with a RRR of 43.8% and 72.5%, respectively. No HH-120-related serious adverse effects were observed. The HH-120 nasal spray used as PEP was safe and effective in preventing laboratory-confirmed and symptomatic SARS-CoV-2 infection.
While this study provides valuable insights into the potential benefits of the HH-120 nasal spray in preventing SARS-CoV-2 infection, there are several limitations that should be taken into consideration. First, the sample sizes were relatively small, and the number of events were relatively low, despite all participants received consecutive NAAT for SARS-CoV-2 to capture every event after enrollment. Second, these trials were conducted during a period when there were strict policies of intensive contact tracing and quarantine, and in closed settings in China. Therefore, the realworld efficacy of the HH-120 nasal spray in post-pandemic with no strict surveillance remains to be assessed. Third, in the NS02 trial, the exact durations of participants' exposure to the index cases before enrollment could not be accurately measured, which could potentially introduce bias into the study. Variations in participants' exposure durations arise from the nature of this type of study, which involves retrospective data collection or relies on participants' self-reporting. Finally, all the participants were in a quarantine status, which prevented us from assessing the ability of the HH-120 nasal spray to produce a beneficial effect in reducing the transmission of SARS-CoV-2. In summary, our study provides compelling preliminary evidence that the HH-120 nasal spray is highly effective in protecting individuals who had been exposed to SARS-CoV-2 from the laboratory-confirmed and symptomatic..
References
Ahn, Kim, Hong, Nasal ciliated cells are primary targets for SARS-CoV-2 replication in the early stage of COVID-19, J Clin Invest
Andrews, Stowe, Kirsebom, Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant, N Engl J Med
Baker, Nakayama, Hegarty, SARS-CoV-2 B.1.1.529 (Omicron) variant transmission within households-four US Jurisdictions, November 2021 to February 2022, MMWR Morb Mortal Wkly Rep
Cao, Jian, Wang, Imprinted SARS-CoV-2 humoral immunity induces convergent Omicron RBD evolution, Nature
Carabelli, Peacock, Thorne, SARS-CoV-2 variant biology: immune escape, transmission and fitness, Nat Rev Microbiol
Center For, Control, Guidelines for investigation and management of close contacts of COVID-19 cases, China CDC Weekly
Del Águila-Mejía, Wallmann, Calvo-Montes, Rodríguez-Lozano, Valle-Madrazo et al., Secondary attack rate, transmission and incubation periods, and serial interval of SARS-CoV-2 omicron variant, Spain, Emerging Infect Dis
Fiege, Thiede, Nanda, Single cell resolution of SARS-CoV-2 tropism, antiviral responses, and susceptibility to therapies in primary human airway epithelium, PLoS Pathog
Glasgow, Glasgow, Limonta, Engineered ACE2 receptor traps potently neutralize SARS-CoV-2, Proc Natl Acad Sci
Harvey, Carabelli, Jackson, SARS-CoV-2 variants, spike mutations and immune escape, Nat Rev Microbiol
Health, Services, Common terminology criteria for adverse events
Hou, Okuda, Edwards, SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract, Cell
Jørgensen, Nygård, Kacelnik, Telle, Secondary attack rates for Omicron and Delta variants of SARS-CoV-2 in Norwegian households, JAMA
Keam, Tixagevimab + cilgavimab: first approval, Drugs
Lau, Cheng, Leung, Real-world COVID-19 vaccine effectiveness against the Omicron BA.2 variant in a SARS-CoV-2 infection-naive population, Nature Med
Liu, Mao, Chen, An IgM-like inhalable ACE2 fusion protein broadly neutralizes SARS-CoV-2 variants, Nat Commun
Lu, Ling, Jiang, Primary assessment of the diversity of Omicron sublineages and the epidemiologic features of autumn/ winter 2022 COVID-19 wave in Chinese mainland, Front Med
Manica, Bellis, Guzzetta, Intrinsic generation time of the SARS-CoV-2 Omicron variant: an observational study of household transmission, Lancet Reg Health-Europe
Ogata, Tanaka, Tanaka, Increased secondary attack rates among the household contacts of patients with the omicron variant of the coronavirus disease 2019 in Japan, Int J Environ Res Public Health
Ravindra, Alfajaro, Gasque, Single-cell longitudinal analysis of SARS-CoV-2 infection in human airway epithelium identifies target cells, alterations in gene expression, and cell state changes, PLoS Biol
Slifka, Amanna, Passive immunization
Song, Chen, Li, Nasal spray of an IgM-like ACE2 fusion protein HH-120 accelerates SARS-CoV-2 clearance: a single-center propensity score-matched cohort study, J Med Virol
Takashita, Kinoshita, Yamayoshi, Efficacy of antibodies and antiviral drugs against COVID-19 Omicron Variant, N Engl J Med
Tanaka, Nelson, Olson, An ACE2 triple decoy that neutralizes SARS-CoV-2 shows enhanced affinity for virus variants, Sci Rep
Tao, Tzou, Nouhin, The biological and clinical significance of emerging SARS-CoV-2 variants, Nat Rev Genet
Wang, Jiang, Jiang, Neutralization of SARS-CoV-2 BQ.1.1, CH.1.1, and XBB.1.5 by breakthrough infection sera from previous and recent waves in China, Cell Discov
Wei, Ma, Wang, Household transmission of SARS-CoV-2 during the Omicron wave in Shanghai, China: a case-ascertained study, Influenza Other Respir Viruses
Wu, Lidsky, Xiao, SARS-CoV-2 replication in airway epithelia requires motile cilia and microvillar reprogramming, Cell
Yang, Peng, Lin, Human ACE2-functionalized gold "Virus-Trap" nanostructures for accurate capture of SARS-CoV-2 and single-virus SERS detection, Nano Micro Lett
Zhang, Dou, Zheng, Epidemiological characteristics of close contacts of COVID-19 cases and infection-related risk factors in Beijing, Zhonghua liu xing bing xue za zhi=Zhonghua liuxingbingxue zazhi
Zou, Ruan, Huang, SARS-CoV-2 viral load in upper respiratory specimens of infected patients, N Engl J Med
DOI record:
{
"DOI": "10.1002/jmv.29275",
"ISSN": [
"0146-6615",
"1096-9071"
],
"URL": "http://dx.doi.org/10.1002/jmv.29275",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:label /><jats:p>HH‐120, an IgM‐like angiotensin converting enzyme 2 (ACE2) fusion protein, has been developed as a nasal spray against Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and is currently undergoing human trials. HH‐120 nasal spray was assessed for postexposure prophylaxis (PEP) in two investigator‐initiated (NS01 and NS02) trials with different risk levels of SARS‐CoV‐2 exposure. NS01 enrolled family caregiver participants who had continuous contacts with laboratory‐confirmed index cases; NS02 enrolled participants who had general contacts (Part 1) or close contacts (Part 2) with index cases. The primary endpoints were safety and laboratory‐confirmed and/or symptomatic SARS‐CoV‐2 infection. In NS01 trial (14 participants), the SARS‐CoV‐2 infection rates were 25% in the HH‐120 group and 83.3% in the external control group (relative risk reduction [RRR]: 70.0%). In NS02‐Part 1 (193 participants), the infection rates were 4% (HH‐120) versus 11.3% (placebo), symptomatic infection rates were 0.8% versus 3.5%, hence with a RRR of 64.6% and 77.1%, respectively. In Part 2 (76 participants), the infection rates were 17.1% (HH‐120) versus 30.4% (placebo), symptomatic infection rates were 7.5% versus 27.3%, with a RRR of 43.8% and 72.5%, respectively. No HH‐120‐related serious adverse effects were observed. The HH‐120 nasal spray used as PEP was safe and effective in preventing laboratory‐confirmed and symptomatic SARS‐CoV‐2 infection.</jats:p></jats:sec>",
"alternative-id": [
"10.1002/jmv.29275"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-10-25"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-11-16"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-12-06"
}
],
"author": [
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University Beijing China"
}
],
"family": "Song",
"given": "Rui",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University Beijing China"
}
],
"family": "Chen",
"given": "Xiaoyou",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University Beijing China"
}
],
"family": "Li",
"given": "Baoliang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University Beijing China"
}
],
"family": "Ni",
"given": "Jun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University Beijing China"
}
],
"family": "Zhou",
"given": "Yunao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Huahui Health Ltd. Beijing China"
}
],
"family": "Zhang",
"given": "Hongbin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Huahui Health Ltd. Beijing China"
}
],
"family": "Liang",
"given": "Xiao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Huahui Health Ltd. Beijing China"
}
],
"family": "Zou",
"given": "Liangfeng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Huahui Health Ltd. Beijing China"
}
],
"family": "Liu",
"given": "Juan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Huahui Health Ltd. Beijing China"
}
],
"family": "Yang",
"given": "Fang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Huahui Health Ltd. Beijing China"
}
],
"family": "Li",
"given": "Guangyu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University Beijing China"
}
],
"family": "Guo",
"given": "Xiaodi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University Beijing China"
}
],
"family": "Liu",
"given": "Zhe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Huahui Health Ltd. Beijing China"
}
],
"family": "Mao",
"given": "Fengfeng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Huahui Health Ltd. Beijing China"
}
],
"family": "Lei",
"given": "Cong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute of Biological Sciences Beijing China"
},
{
"name": "Tsinghua Institute of Multidisciplinary Biomedical Research Tsinghua University Beijing China"
}
],
"family": "Sui",
"given": "Jianhua",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Institute of Biological Sciences Beijing China"
},
{
"name": "Tsinghua Institute of Multidisciplinary Biomedical Research Tsinghua University Beijing China"
}
],
"family": "Li",
"given": "Wenhui",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Beijing Ditan Hospital Capital Medical University Beijing China"
},
{
"name": "National Center for Infectious Diseases, Beijing Ditan Hospital Capital Medical University Beijing China"
},
{
"name": "Beijing Key Laboratory of Emerging Infectious Diseases, Institute of Infectious Diseases, Beijing Ditan Hospital Capital Medical University Beijing China"
}
],
"family": "Jin",
"given": "Ronghua",
"sequence": "additional"
}
],
"container-title": "Journal of Medical Virology",
"container-title-short": "Journal of Medical Virology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
12,
6
]
],
"date-time": "2023-12-06T17:12:38Z",
"timestamp": 1701882758000
},
"deposited": {
"date-parts": [
[
2023,
12,
6
]
],
"date-time": "2023-12-06T17:12:46Z",
"timestamp": 1701882766000
},
"funder": [
{
"DOI": "10.13039/501100009592",
"award": [
"Z221100007922014",
"Z221100007922020"
],
"doi-asserted-by": "publisher",
"name": "Beijing Municipal Science and Technology Commission"
}
],
"indexed": {
"date-parts": [
[
2023,
12,
7
]
],
"date-time": "2023-12-07T00:57:42Z",
"timestamp": 1701910662609
},
"is-referenced-by-count": 0,
"issue": "12",
"issued": {
"date-parts": [
[
2023,
12
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 5,
"start": {
"date-parts": [
[
2023,
12,
6
]
],
"date-time": "2023-12-06T00:00:00Z",
"timestamp": 1701820800000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.29275",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2023,
12
]
]
},
"published-online": {
"date-parts": [
[
2023,
12,
6
]
]
},
"published-print": {
"date-parts": [
[
2023,
12
]
]
},
"publisher": "Wiley",
"reference": [
{
"key": "e_1_2_9_2_1",
"unstructured": "Our World in Data. Coronavirus (COVID‐19) vaccinations.2023. Accessed July 8 2023.https://ourworldindata.org/covid-vaccinations"
},
{
"DOI": "10.1111/irv.13097",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_3_1"
},
{
"DOI": "10.1056/NEJMoa2119451",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_4_1"
},
{
"DOI": "10.1007/s40265-022-01731-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_5_1"
},
{
"article-title": "Imprinted SARS‐CoV‐2 humoral immunity induces convergent Omicron RBD evolution",
"author": "Cao Y",
"first-page": "521",
"issue": "7948",
"journal-title": "Nature",
"key": "e_1_2_9_6_1",
"volume": "614",
"year": "2023"
},
{
"DOI": "10.1038/s41421-023-00569-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_7_1"
},
{
"DOI": "10.1016/B978-0-323-35761-6.00008-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_8_1"
},
{
"DOI": "10.1038/s41576-021-00408-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_9_1"
},
{
"DOI": "10.1038/s41579-021-00573-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_10_1"
},
{
"article-title": "SARS‐CoV‐2 variant biology: immune escape, transmission and fitness",
"author": "Carabelli AM",
"first-page": "162",
"issue": "3",
"journal-title": "Nat Rev Microbiol",
"key": "e_1_2_9_11_1",
"volume": "21",
"year": "2023"
},
{
"DOI": "10.1073/pnas.2016093117",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_12_1"
},
{
"DOI": "10.1007/s40820-021-00620-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_13_1"
},
{
"DOI": "10.1038/s41598-021-91809-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_14_1"
},
{
"DOI": "10.1038/s41467-023-40933-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_15_1"
},
{
"DOI": "10.1016/j.cell.2020.05.042",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_16_1"
},
{
"DOI": "10.1056/NEJMc2001737",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_17_1"
},
{
"DOI": "10.1172/JCI148517",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_18_1"
},
{
"DOI": "10.1371/journal.pbio.3001143",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_19_1"
},
{
"DOI": "10.1371/journal.ppat.1009292",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_20_1"
},
{
"DOI": "10.1016/j.cell.2022.11.030",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_21_1"
},
{
"DOI": "10.1002/jmv.28805",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_22_1"
},
{
"DOI": "10.1007/s11684-022-0981-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_23_1"
},
{
"DOI": "10.3201/eid2806.220158",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_24_1"
},
{
"article-title": "[Epidemiological characteristics of close contacts of COVID‐19 cases and infection‐related risk factors in Beijing]",
"author": "Zhang YQ",
"first-page": "1757",
"issue": "10",
"journal-title": "Zhonghua liu xing bing xue za zhi=Zhonghua liuxingbingxue zazhi",
"key": "e_1_2_9_25_1",
"volume": "42",
"year": "2021"
},
{
"key": "e_1_2_9_26_1",
"unstructured": "National Health Commission of The People's Republic of China. Diagnosis and treatment of 2019 novel coronavirus‐associated pneumonia (Edition 9). 2022. Accessed July 8 2023.https://www.gov.cn/zhengce/zhengceku/2022-03/15/content_5679257.htm"
},
{
"DOI": "10.46234/ccdcw2020.084",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_27_1"
},
{
"key": "e_1_2_9_28_1",
"unstructured": "FDA. Assessing COVID‐19‐related symptoms in outpatient adult and adolescent subjects in clinical trials of drugs and biological products for COVID‐19 prevention or treatment.2020.https://www.fda.gov/regulatory-information/search-fda-guidance-documents/assessing-covid-19-related-symptoms-outpatient-adult-and-adolescent-subjects-clinical-trials-drugs"
},
{
"key": "e_1_2_9_29_1",
"unstructured": "HealthUDO ServicesH.Common terminology criteria for adverse events.2017."
},
{
"DOI": "10.1038/s41591-023-02219-5",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_30_1"
},
{
"DOI": "10.1056/NEJMc2119407",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_31_1"
},
{
"DOI": "10.15585/mmwr.mm7109e1",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_32_1"
},
{
"DOI": "10.1001/jama.2022.3780",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_33_1"
},
{
"DOI": "10.3390/ijerph19138068",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_34_1"
},
{
"DOI": "10.1016/j.lanepe.2022.100446",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_35_1"
}
],
"reference-count": 34,
"references-count": 34,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/jmv.29275"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Nasal spray of an IgM‐like ACE2 fusion protein HH‐120 prevents SARS‐CoV‐2 infection: Two investigator‐initiated postexposure prophylaxis trials",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "95"
}