UDCA treatment against COVID‐19: Do we have enough clinical evidence for drug repurposing?
et al., Journal of Internal Medicine, doi:10.1111/joim.13711, NCT05659654, Aug 2023
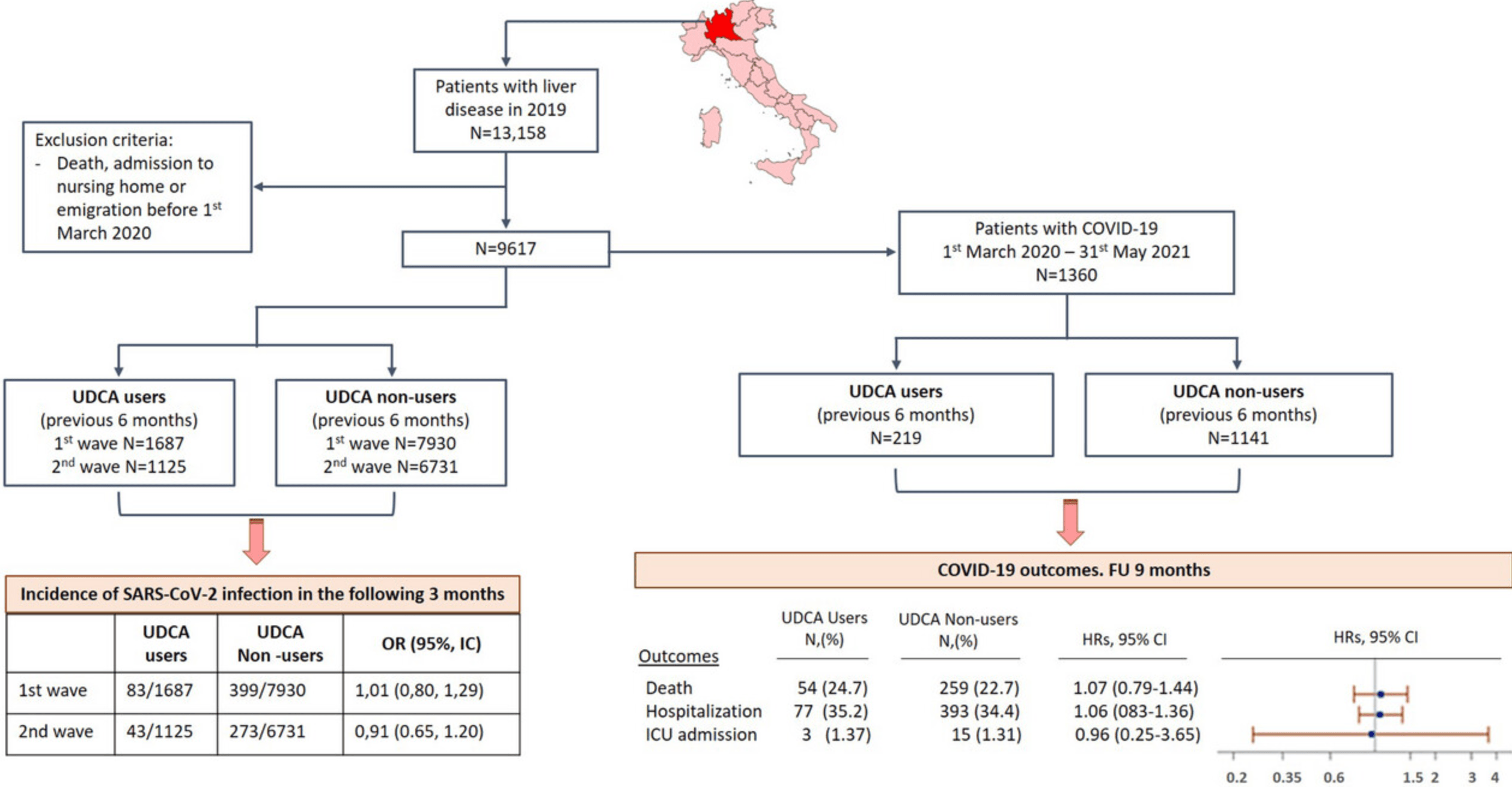
Retrospective cohort study of 9,617 patients with liver disease in Italy, divided into UDCA users and non-users. UDCA exposure was not associated with reduced SARS-CoV-2 infection or improved COVID-19 outcomes including death, hospitalization, and ICU admission in this unvaccinated cohort. The large sample size provides power, but administrative data limitations include lack of important confounders like BMI and hypertension.
|
risk of death, 7.0% higher, HR 1.07, p = 0.67, treatment 54 of 219 (24.7%), control 259 of 1,141 (22.7%), adjusted per study, multivariable, Cox proportional hazards.
|
|
risk of ICU admission, 4.0% lower, HR 0.96, p = 0.96, treatment 3 of 219 (1.4%), control 15 of 1,141 (1.3%), adjusted per study, multivariable, Cox proportional hazards.
|
|
risk of hospitalization, 6.0% higher, HR 1.06, p = 0.66, treatment 77 of 219 (35.2%), control 393 of 1,141 (34.4%), adjusted per study, multivariable, Cox proportional hazards.
|
|
risk of case, 2.8% lower, OR 0.97, p = 0.77, wave 1 and wave 2 combined, RR approximated with OR.
|
|
risk of case, 0.9% higher, RR 1.01, p = 0.94, treatment 83 of 1,687 (4.9%), control 399 of 7,930 (5.0%), NNT 896, odds ratio converted to relative risk, wave 1.
|
|
risk of case, 8.7% lower, RR 0.91, p = 0.56, treatment 43 of 1,125 (3.8%), control 273 of 6,731 (4.1%), NNT 428, odds ratio converted to relative risk, wave 2.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ojeda-Fernández et al., 22 Aug 2023, retrospective, Italy, peer-reviewed, 11 authors, study period 1 March, 2020 - 31 May, 2021, trial NCT05659654 (history).
Contact: luisa.ojeda@marionegri.it.
Abstract: Research Letter
doi: 10.1111/joim.13711
UDCA treatment against COVID-19: Do we have
enough clinical evidence for drug repurposing?
Dear Editor,
Ursodeoxycholic acid (UDCA), an off-patent drug
used to treat liver disease [1–3], is able to block
severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) entry into the cells downregulating
ACE2 expression [4], a promising strategy to protect against infection. In this light, John et al.
[5] have recently demonstrated that in patients
with cirrhosis, UDCA exposure was associated with
both a decrease in SARS-CoV-2 infection and a
reduction in coronavirus disease 2019 (COVID19) severity confirming previous data published by
Brevini et al. [4]. To investigate the impact of UDCA
treatment in SARS-CoV-2 infection and COVID-19
outcomes in an unselected population of COVID19 patients, we used the administrative databases
from Lombardy (Northern Italy), the first region of
the Western world to experience a rapid increase in
the number of COVID-19 cases and related deaths
and the most populated Italian region. The analysis included patients from the first and second
pandemic waves, where wild-type and alfa variants
were predominant.
To test the association of UDCA exposure in the
prevention of SARS-CoV-2 infection, we selected
9617 patients with a diagnosis of liver diseases
alive on 1 March 2020 (for more details on data
source, see the Supporting Information section).
The cohort was divided into two groups: UDCA
users and UDCA non-users whether they received
or not at least one prescription of UDCA before
entering the cohort (Fig. 1, left side). Demographic
data were recorded at the time of inclusion. History of comorbidities was collected in the four years
before inclusion using hospital records as primary
diagnosis and up to five co-existing conditions.
Exposures to medications of interest were traced
in the 12 months before entering the cohort (Supplementary Materials, ATC and ICD-9-CM codes).
We used logistic regression models to estimate the
incidence of SARS-CoV-2 infection in the following
3 months after UDCA exposure. Odds ratios (ORs)
with 95% confidence intervals (CIs) were adjusted
for baseline characteristics (age, sex, pre-existing
conditions and medications) and by inverse probability treatment weighting (IPTW). Baseline characteristics of the UDCA users and UDCA non-users
groups, with standardized mean differences (SMD)
were described before and after IPTW (Tables S2
and S3). Good balance was observed after IPTW
with SMD equally or less than 0.10 (Figs. S1 and
S2). Our model demonstrated good goodness of fit
(p-value for Hosmer–Lemeshow test were 0.61 and
0.08 for first and second pandemic wave, respectively) and led to a small variance of the effect
estimate as the average of the inverse predicted
probabilities was approximately equal to 1: IPTW
[mean, median (q1, q3)] were [0.99, 0.97 (0.93,
1.05)] and [1, 0.98 (0.94, 1.04)] for first and second
pandemic wave, respectively. No differences in risk
of SARS-CoV-2 infection were observed between
UDCA users and UDCA non-users according to the
pandemic waves: first wave (OR 1.01; 95% CI 0.80–
1.29) and second wave (OR 0.91; 95% CI 0.65–
1.27).
We also evaluate the impact of UDCA treatment on
COVID-19 outcomes in patients becoming positive
for SARS-CoV-2 between 1 March 2020 and 31
May 2021 (Fig. 1, right side) using a Cox proportional hazard model. Hazard ratios (HRs) and
95% CIs were adjusted for baseline characteristics
(sex, age,..
DOI record:
{
"DOI": "10.1111/joim.13711",
"ISSN": [
"0954-6820",
"1365-2796"
],
"URL": "http://dx.doi.org/10.1111/joim.13711",
"alternative-id": [
"10.1111/joim.13711"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-08-22"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-2920-1669",
"affiliation": [
{
"name": "Istituto di Ricerche Farmacologiche Mario Negri IRCCS Milan Italy"
}
],
"authenticated-orcid": false,
"family": "Ojeda‐Fernández",
"given": "Luisa",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Istituto di Ricerche Farmacologiche Mario Negri IRCCS Milan Italy"
}
],
"family": "Baviera",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Istituto di Ricerche Farmacologiche Mario Negri IRCCS Milan Italy"
}
],
"family": "Macaluso",
"given": "Giulia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Istituto di Ricerche Farmacologiche Mario Negri IRCCS Milan Italy"
}
],
"family": "Schena",
"given": "Simone",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Istituto di Ricerche Farmacologiche Mario Negri IRCCS Milan Italy"
}
],
"family": "Tettamanti",
"given": "Mauro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Istituto di Ricerche Farmacologiche Mario Negri IRCCS Milan Italy"
}
],
"family": "Cartabia",
"given": "Massimo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Istituto di Ricerche Farmacologiche Mario Negri IRCCS Milan Italy"
}
],
"family": "Foresta",
"given": "Andreana",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1915-3897",
"affiliation": [
{
"name": "Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico Angelo Bianchi Bonomi Hemophilia and Thrombosis Center and University of Milan Milan Italy"
}
],
"authenticated-orcid": false,
"family": "Manucci",
"given": "Pier Manuccio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7813-0572",
"affiliation": [
{
"name": "Istituto di Ricerche Farmacologiche Mario Negri IRCCS Milan Italy"
}
],
"authenticated-orcid": false,
"family": "Nobili",
"given": "Alessandro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Istituto di Ricerche Farmacologiche Mario Negri IRCCS Milan Italy"
}
],
"family": "Remuzzi",
"given": "Giuseppe",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Istituto di Ricerche Farmacologiche Mario Negri IRCCS Milan Italy"
}
],
"family": "Roncaglioni",
"given": "Carla",
"sequence": "additional"
}
],
"container-title": "Journal of Internal Medicine",
"container-title-short": "J Intern Med",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
8,
18
]
],
"date-time": "2023-08-18T08:29:08Z",
"timestamp": 1692347348000
},
"deposited": {
"date-parts": [
[
2023,
8,
23
]
],
"date-time": "2023-08-23T06:17:55Z",
"timestamp": 1692771475000
},
"indexed": {
"date-parts": [
[
2023,
8,
24
]
],
"date-time": "2023-08-24T20:11:20Z",
"timestamp": 1692907880013
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
8,
22
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
22
]
],
"date-time": "2023-08-22T00:00:00Z",
"timestamp": 1692662400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/joim.13711",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1111",
"published": {
"date-parts": [
[
2023,
8,
22
]
]
},
"published-online": {
"date-parts": [
[
2023,
8,
22
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/j.jhep.2009.04.009",
"article-title": "EASL Clinical Practice Guidelines: management of cholestatic liver diseases",
"author": "European Association for the Study of the Liver",
"doi-asserted-by": "crossref",
"first-page": "237",
"journal-title": "J Hepatol.",
"key": "e_1_2_5_2_1",
"volume": "51",
"year": "2009"
},
{
"DOI": "10.1002/hep.22906",
"article-title": "American Association for Study of Liver Diseases: primary biliary cirrhosis",
"author": "Lindor KD",
"doi-asserted-by": "crossref",
"first-page": "291",
"journal-title": "Hepatology.",
"key": "e_1_2_5_3_1",
"volume": "50",
"year": "2009"
},
{
"DOI": "10.1016/j.jhep.2017.03.022",
"article-title": "EASL Clinical Practice Guidelines: the diagnosis and management of patients with primary biliary cholangitis",
"author": "European Association for the Study of the Liver",
"doi-asserted-by": "crossref",
"first-page": "145",
"journal-title": "J Hepatol.",
"key": "e_1_2_5_4_1",
"volume": "67",
"year": "2017"
},
{
"DOI": "10.1038/s41586-022-05594-0",
"article-title": "FXR inhibition may protect from SARS‐CoV‐2 infection by reducing ACE2",
"author": "Brevini T",
"doi-asserted-by": "crossref",
"first-page": "134",
"journal-title": "Nature.",
"key": "e_1_2_5_5_1",
"volume": "615",
"year": "2023"
},
{
"DOI": "10.1111/joim.13630",
"article-title": "Ursodeoxycholic acid is associated with a reduction in SARS‐CoV‐2 infection and reduced severity of COVID‐19 in patients with cirrhosis",
"author": "John BV",
"doi-asserted-by": "crossref",
"first-page": "636",
"journal-title": "J Intern Med.",
"key": "e_1_2_5_6_1",
"volume": "293",
"year": "2023"
},
{
"DOI": "10.3390/vaccines8020320",
"article-title": "Merit of an ursodeoxycholic acid clinical trial in COVID‐19 patients",
"author": "Subramanian S",
"doi-asserted-by": "crossref",
"first-page": "320",
"journal-title": "Vaccines (Basel).",
"key": "e_1_2_5_7_1",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/j.mehy.2020.109897",
"article-title": "Ursodeoxycholic acid as a candidate therapeutic to alleviate and/or prevent COVID‐19‐associated cytokine storm",
"author": "Abdulrab S",
"doi-asserted-by": "crossref",
"journal-title": "Med Hypotheses.",
"key": "e_1_2_5_8_1",
"volume": "143",
"year": "2020"
},
{
"key": "e_1_2_5_9_1",
"unstructured": "FobarR.China promotes bear bile as coronavirus treatment alarming wildlife advocates.National Geographic. 2020 March 25.https://www.nationalgeographic.com/animals/article/chinese-government-promotes-bear-bile-as-coronavirus-covid19-treatment"
},
{
"DOI": "10.1111/j.1524-4733.2009.00671.x",
"article-title": "Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals",
"author": "Xu S",
"doi-asserted-by": "crossref",
"first-page": "273",
"journal-title": "Value Health.",
"key": "e_1_2_5_10_1",
"volume": "13",
"year": "2010"
}
],
"reference-count": 9,
"references-count": 9,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/joim.13711"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Internal Medicine"
],
"subtitle": [],
"title": "UDCA treatment against COVID‐19: Do we have enough clinical evidence for drug repurposing?",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy"
}