Ursodeoxycholic acid administration did not reduce susceptibility to SARS‐CoV‐2 infection in children
et al., Liver International, doi:10.1111/liv.15660, Jun 2023
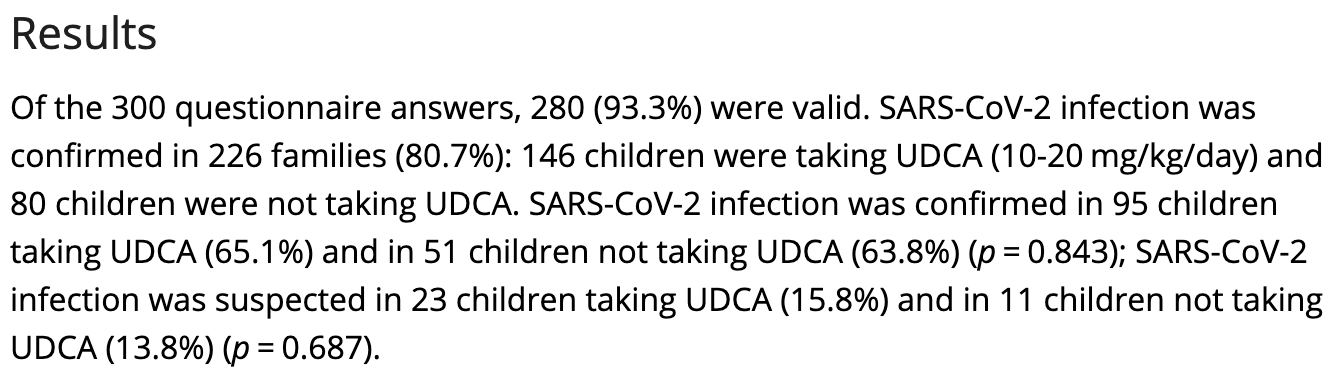
Retrospective 280 Chinese families with children previously seen in a liver clinic assessing whether ursodeoxycholic acid (UDCA) reduced SARS-CoV-2 infection risk. Among infected families, the study found no significant difference in confirmed or suspected SARS-CoV-2 infections between children taking UDCA (80.9%) and those not taking it (77.6%) (p=0.843).
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
risk of case, 2.1% higher, RR 1.02, p = 0.88, treatment 95 of 146 (65.1%), control 51 of 80 (63.7%), confirmed.
|
|
risk of case, 4.3% higher, RR 1.04, p = 0.61, treatment 118 of 146 (80.8%), control 62 of 80 (77.5%), confirmed + suspected.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Liu et al., 29 Jun 2023, retrospective, China, peer-reviewed, survey, 2 authors.
DOI record:
{
"DOI": "10.1111/liv.15660",
"ISSN": [
"1478-3223",
"1478-3231"
],
"URL": "http://dx.doi.org/10.1111/liv.15660",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background and Aims</jats:title><jats:p>A recent study suggested that administration of ursodeoxycholic acid (UDCA) at dosages usually employed clinically may reduce rates of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. A recent surge of SARS‐CoV‐2 omicron infection in China allowed study of whether UDCA administration reduced susceptibility to SARS‐CoV‐2 infection in children with liver disease.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Through WeChat groups, a questionnaire was distributed to families (<jats:italic>n</jats:italic> = 300) in which a child had been admitted to our liver service in the past 5 years. Among the families/households in which someone was infected with SARS‐CoV‐2, the proportion in which a child taking UDCA was infected was compared with the proportion in which a child not taking UDCA was infected.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Of the 300 questionnaire answers, 280 (93.3%) were valid. SARS‐CoV‐2 infection was confirmed in 226 families (80.7%): 146 children were taking UDCA (10‐20 mg/kg/day) and 80 children were not taking UDCA. SARS‐CoV‐2 infection was confirmed in 95 children taking UDCA (65.1%) and in 51 children not taking UDCA (63.8%) (<jats:italic>p</jats:italic> = 0.843); SARS‐CoV‐2 infection was suspected in 23 children taking UDCA (15.8%) and in 11 children not taking UDCA (13.8%) (<jats:italic>p</jats:italic> = 0.687).</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>These results indicate that UDCA administration does not reduce susceptibility to SARS‐CoV‐2 infection in children with liver disease.</jats:p></jats:sec>",
"alternative-id": [
"10.1111/liv.15660"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-01-12"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-06-15"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-06-29"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0858-2151",
"affiliation": [
{
"name": "The Center for Pediatric Liver Diseases Children's Hospital of Fudan University Shanghai China"
},
{
"name": "Department of Pediatrics Shanghai Medical College, Fudan University Shanghai China"
}
],
"authenticated-orcid": false,
"family": "Liu",
"given": "Teng",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-0823-586X",
"affiliation": [
{
"name": "The Center for Pediatric Liver Diseases Children's Hospital of Fudan University Shanghai China"
},
{
"name": "Department of Pediatrics Shanghai Medical College, Fudan University Shanghai China"
}
],
"authenticated-orcid": false,
"family": "Wang",
"given": "Jian‐She",
"sequence": "additional"
}
],
"container-title": "Liver International",
"container-title-short": "Liver International",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
6,
29
]
],
"date-time": "2023-06-29T07:35:17Z",
"timestamp": 1688024117000
},
"deposited": {
"date-parts": [
[
2023,
8,
12
]
],
"date-time": "2023-08-12T06:45:21Z",
"timestamp": 1691822721000
},
"funder": [
{
"DOI": "10.13039/501100001809",
"award": [
"82201898"
],
"doi-asserted-by": "publisher",
"name": "National Natural Science Foundation of China"
}
],
"indexed": {
"date-parts": [
[
2023,
8,
15
]
],
"date-time": "2023-08-15T16:30:14Z",
"timestamp": 1692117014062
},
"is-referenced-by-count": 1,
"issue": "9",
"issued": {
"date-parts": [
[
2023,
6,
29
]
]
},
"journal-issue": {
"issue": "9",
"published-print": {
"date-parts": [
[
2023,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
6,
29
]
],
"date-time": "2023-06-29T00:00:00Z",
"timestamp": 1687996800000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/liv.15660",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "1950-1954",
"prefix": "10.1111",
"published": {
"date-parts": [
[
2023,
6,
29
]
]
},
"published-online": {
"date-parts": [
[
2023,
6,
29
]
]
},
"published-print": {
"date-parts": [
[
2023,
9
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1038/s41586-021-04385-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_2_1"
},
{
"DOI": "10.1038/s41591-021-01310-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_3_1"
},
{
"DOI": "10.3762/bjoc.14.33",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_4_1"
},
{
"DOI": "10.1038/s41586-022-05594-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_5_1"
},
{
"key": "e_1_2_14_6_1",
"unstructured": "YanL.https://www.ecns.cn/news/society/2022‐12‐28/detail‐ihciertf7993989.shtml. Accessed December 28 2022"
},
{
"DOI": "10.1002/hep.1840190629",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_7_1"
},
{
"DOI": "10.1056/NEJMc2005073",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_8_1"
},
{
"DOI": "10.1038/s41579-020-00459-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_9_1"
},
{
"DOI": "10.1016/j.it.2020.10.004",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_10_1"
},
{
"DOI": "10.1016/j.biopha.2022.113021",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_11_1"
},
{
"DOI": "10.1097/01.mpg.0000226386.79483.7b",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_12_1"
},
{
"DOI": "10.1016/j.puhe.2021.12.003",
"doi-asserted-by": "publisher",
"key": "e_1_2_14_13_1"
}
],
"reference-count": 12,
"references-count": 12,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/liv.15660"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Hepatology"
],
"subtitle": [],
"title": "Ursodeoxycholic acid administration did not reduce susceptibility to <scp>SARS‐CoV</scp>‐2 infection in children",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "43"
}