Exploring the impact of ursodeoxycholic acid therapy on COVID‐19 in a real‐world setting
et al., Journal of Medical Virology, doi:10.1002/jmv.29418, Jan 2024
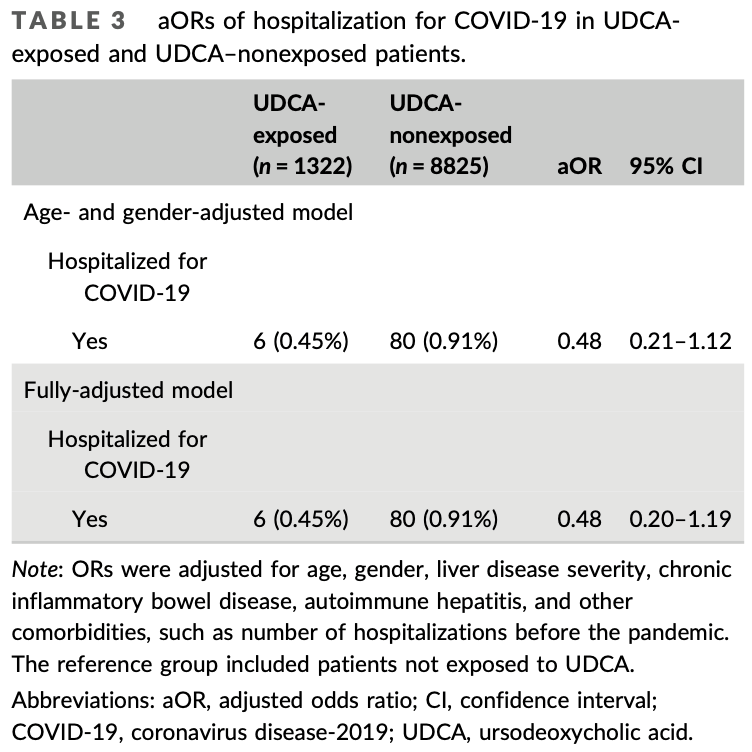
Retrospective cohort study of 10,147 chronic liver disease patients in France, with 1,322 exposed to ursodeoxycholic acid (UDCA), showing lower risk of hospitalization for COVID-19 with UDCA exposure, without statistical significance (adjusted OR 0.48, 95% CI 0.20-1.19). A case-control analysis of 88 hospitalized patients and 840 matched controls showed no significant difference, and there was no significant difference for ICU admission and mortality. The study is underpowered due to the low number of COVID-19 hospitalizations.
|
risk of death, 54.0% higher, RR 1.54, p = 0.45, treatment 3 of 1,322 (0.2%), control 13 of 8,825 (0.1%).
|
|
risk of ICU admission, 19.1% lower, RR 0.81, p = 1.00, treatment 4 of 1,322 (0.3%), control 33 of 8,825 (0.4%), NNT 1401.
|
|
risk of hospitalization, 40.2% lower, RR 0.60, p = 0.17, treatment 1,322, control 8,825, adjusted per study, combination of cohort and case control analyses.
|
|
risk of hospitalization, 51.8% lower, RR 0.48, p = 0.11, treatment 6 of 1,322 (0.5%), control 80 of 8,825 (0.9%), NNT 221, adjusted per study, odds ratio converted to relative risk, multivariable.
|
|
risk of hospitalization, 7.0% lower, OR 0.93, p = 0.92, treatment 7 of 88 (8.0%) cases,
58 of 840 (6.9%) controls, adjusted per study, case control OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Corpechot et al., 19 Jan 2024, retrospective, France, peer-reviewed, 5 authors, study period 1 March, 2020 - 31 December, 2020.
Contact: christophe.corpechot@aphp.fr.
Exploring the impact of ursodeoxycholic acid therapy on COVID‐19 in a real‐word setting
Journal of Medical Virology, doi:10.1002/jmv.29418
Recent data suggest that ursodeoxycholic acid (UDCA) therapy may reduce susceptibility to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and even improve clinical outcomes when coronavirus disease-2019 (COVID-19) was diagnosed. However, clinical evidence of UDCA's ability to prevent severe forms of COVID-19 remains limited and contradictory. We evaluated the association between UDCA exposure and the risk of hospitalization for COVID-19 in a large multicenter population of patients with chronic liver disease (CLD) followed during the pandemic period before vaccination. An exposed/unexposed cohort study and a nested case-control study were performed. The primary endpoint was severe COVID-19, defined as SARS-CoV2 infection requiring hospitalization. The secondary endpoint was COVID-19-associated intensive care unit (ICU) admission or death. Adjusted odds ratios (aOR) and their confidence intervals (CI) were determined after controlling for age, gender, comorbidities at risk for COVID-19, severity of CLD, and prior hospitalizations. A total of 10 147 patients, including 1322 exposed and 8825 not exposed to UDCA, totaling 21 867 person-years of follow-up, were included in the cohort analysis, while 88 patients hospitalized for COVID-19 and 840 matched controls were eligible for the nested case-control analysis. In both analyses, exposure to UDCA was not associated with a significant reduction in the risk of hospitalization for COVID-19, with aOR (95% confidence interval) values of 0.48 (0.20-1.19) and 0.93 (0.26-3.29), respectively. Furthermore, there was no significant reduction in the risk of ICU admission or death. In this large population of patients with CLD, UDCA exposure was not associated with a reduced risk of severe COVID-19.
AUTHOR CONTRIBUTIONS Christophe Corpechot, Jean-Charles Duclos-Vallée, and Lamiae Grimaldi conceived and designed the study. Data were extracted and analyzed by Marie Verdoux and Lamiae Grimaldi. The first draft of the study was written by Christophe Corpechot and Lamiae Grimaldi. All authors participated in the revision of the manuscript, approved the submitted versions, and confirmed the accuracy and completeness of the data and the fidelity of the analysis to the protocol.
CONFLICT OF INTEREST STATEMENT The authors declare no conflict of interest.
SUPPORTING INFORMATION Additional supporting information can be found online in the Supporting Information section at the end of this article.
References
Ampuero, Lucena, Hernández-Guerra, Primary biliary cholangitis and SARS-CoV-2 infection: incidence, susceptibility and outcomes, Gut, doi:10.1136/gutjnl-2021-325700
Brat, Weber, Gehlenborg, International electronic health record-derived COVID-19 clinical course profiles: the 4CE consortium, npj Digit Med
Brevini, Maes, Webb, FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2, Nature
Colapietro, Angelotti, Masetti, Ursodeoxycholic acid does not improve COVID-19 outcome in hospitalized patients, Viruses
Daniel, Serre, Orlova, Bréant, Paris et al., Initializing a hospital-wide data quality program. The AP-HP experience, Comput Methods Programs Biomed
Hu, Zhang, Huang, Effect of ursodeoxycholic acid on preventing SARS-CoV-2 infection in patients with liver transplantation: a multicenter retrospective cohort study, QJM, doi:10.1093/qjmed/hcad254
John, Bastaich, Webb, Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis, J Intern Med
Li, Zhu, Cui, Lin, Li, Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease, Front Cell Infect Microbiol
Liu, Wang, Ursodeoxycholic acid administration did not reduce susceptibility to SARS-CoV-2 infection in children, Liver Int
Marrone, Covino, Merra, Ursodeoxycholic acid does not affect the clinical outcome of SARS-CoV-2 infection: a retrospective study of propensity score-matched cohorts, Liver Int
Neuraz, Lerner, Digan, Natural language processing for rapid response to emergent diseases: case study of calcium channel blockers and hypertension in the COVID-19 pandemic, J Med Internet Res
Zecher, Buescher, Willemse, Prevalence of COVID-19 in patients with autoimmune liver disease in Europe: a patient-oriented online survey, United Eur Gastroenterol J
DOI record:
{
"DOI": "10.1002/jmv.29418",
"ISSN": [
"0146-6615",
"1096-9071"
],
"URL": "http://dx.doi.org/10.1002/jmv.29418",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Recent data suggest that ursodeoxycholic acid (UDCA) therapy may reduce susceptibility to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection and even improve clinical outcomes when coronavirus disease‐2019 (COVID‐19) was diagnosed. However, clinical evidence of UDCA's ability to prevent severe forms of COVID‐19 remains limited and contradictory. We evaluated the association between UDCA exposure and the risk of hospitalization for COVID‐19 in a large multicenter population of patients with chronic liver disease (CLD) followed during the pandemic period before vaccination. An exposed/unexposed cohort study and a nested case–control study were performed. The primary endpoint was severe COVID‐19, defined as SARS‐CoV2 infection requiring hospitalization. The secondary endpoint was COVID‐19‐associated intensive care unit (ICU) admission or death. Adjusted odds ratios (aOR) and their confidence intervals (CI) were determined after controlling for age, gender, comorbidities at risk for COVID‐19, severity of CLD, and prior hospitalizations. A total of 10 147 patients, including 1322 exposed and 8825 not exposed to UDCA, totaling 21 867 person‐years of follow‐up, were included in the cohort analysis, while 88 patients hospitalized for COVID‐19 and 840 matched controls were eligible for the nested case–control analysis. In both analyses, exposure to UDCA was not associated with a significant reduction in the risk of hospitalization for COVID‐19, with aOR (95% confidence interval) values of 0.48 (0.20–1.19) and 0.93 (0.26–3.29), respectively. Furthermore, there was no significant reduction in the risk of ICU admission or death. In this large population of patients with CLD, UDCA exposure was not associated with a reduced risk of severe COVID‐19.</jats:p>",
"alternative-id": [
"10.1002/jmv.29418"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-12-08"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2024-01-09"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2024-01-19"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7920-9784",
"affiliation": [
{
"name": "Reference Center for Inflammatory Biliary Diseases and Autoimmune Hepatitis, European Reference Network on Hepatological Diseases (ERN Rare‐Liver), Saint‐Antoine Hospital Assistance Publique‐Hôpitaux de Paris (AP‐HP) Paris France"
},
{
"name": "Inserm UMR_S938, Saint‐Antoine Research Center Sorbonne University Paris France"
}
],
"authenticated-orcid": false,
"family": "Corpechot",
"given": "Christophe",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Clinical Research Unit, Direction of Clinical Research, Bicêtre Hospital, AP‐HP Paris‐Saclay University Le Kremlin‐Bicêtre France"
}
],
"family": "Verdoux",
"given": "Marie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Informatics Department, Bicêtre Hospital, AP‐HP Paris‐Saclay University Le Kremlin‐Bicêtre France"
}
],
"family": "Frank‐Soltysiak",
"given": "Marie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fédération Hospitalo‐Universitaire Hépatinov, Inserm UMR_S 1193, Paul Brousse Hospital, AP‐HP Paris‐Saclay University Villejuif France"
}
],
"family": "Duclos‐Vallée",
"given": "Jean‐Charles",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Research Unit, Direction of Clinical Research, Bicêtre Hospital, AP‐HP Paris‐Saclay University Le Kremlin‐Bicêtre France"
},
{
"name": "Inserm UMR1018 Anti‐Infective Evasion and Pharmacoepidemiology, Simone Veil School of Medicine Paris‐Saclay University Montigny‐Le‐Bretonneux France"
}
],
"family": "Grimaldi",
"given": "Lamiae",
"sequence": "additional"
}
],
"container-title": "Journal of Medical Virology",
"container-title-short": "Journal of Medical Virology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2024,
1,
19
]
],
"date-time": "2024-01-19T12:18:39Z",
"timestamp": 1705666719000
},
"deposited": {
"date-parts": [
[
2024,
1,
19
]
],
"date-time": "2024-01-19T12:18:43Z",
"timestamp": 1705666723000
},
"indexed": {
"date-parts": [
[
2024,
1,
20
]
],
"date-time": "2024-01-20T00:26:19Z",
"timestamp": 1705710379938
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2024,
1
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 18,
"start": {
"date-parts": [
[
2024,
1,
19
]
],
"date-time": "2024-01-19T00:00:00Z",
"timestamp": 1705622400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/jmv.29418",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2024,
1
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
19
]
]
},
"published-print": {
"date-parts": [
[
2024,
1
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1038/s41586-022-05594-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.1111/joim.13630",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"DOI": "10.3389/fcimb.2023.1178590",
"article-title": "Protective effect of ursodeoxycholic acid on COVID‐19 in patients with chronic liver disease",
"author": "Li Y",
"doi-asserted-by": "crossref",
"journal-title": "Front Cell Infect Microbiol",
"key": "e_1_2_10_4_1",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1093/qjmed/hcad254",
"article-title": "Effect of ursodeoxycholic acid on preventing SARS‐CoV‐2 infection in patients with liver transplantation: a multicenter retrospective cohort study",
"author": "Hu L",
"doi-asserted-by": "crossref",
"journal-title": "QJM",
"key": "e_1_2_10_5_1",
"year": "2023"
},
{
"DOI": "10.3390/v15081738",
"article-title": "Ursodeoxycholic acid does not improve COVID‐19 outcome in hospitalized patients",
"author": "Colapietro F",
"doi-asserted-by": "crossref",
"first-page": "1738",
"issue": "8",
"journal-title": "Viruses",
"key": "e_1_2_10_6_1",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1111/liv.15736",
"article-title": "Ursodeoxycholic acid does not affect the clinical outcome of SARS‐CoV‐2 infection: a retrospective study of propensity score‐matched cohorts",
"author": "Marrone G",
"doi-asserted-by": "crossref",
"first-page": "83",
"journal-title": "Liver Int",
"key": "e_1_2_10_7_1",
"volume": "44",
"year": "2023"
},
{
"DOI": "10.1111/liv.15660",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1016/j.cmpb.2018.10.016",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1038/s41746-020-00308-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.2196/20773",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1136/gutjnl-2021-325700",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.1002/ueg2.12100",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
}
],
"reference-count": 12,
"references-count": 12,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/jmv.29418"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Exploring the impact of ursodeoxycholic acid therapy on COVID‐19 in a real‐word setting",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "96"
}