Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease
et al., Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2023.1178590, May 2023
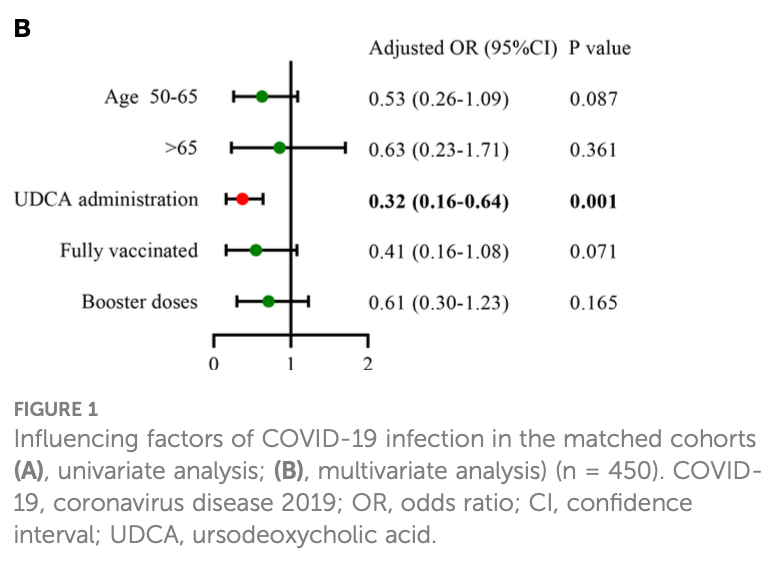
Retrospective propensity score matched cohort study of 225 chronic liver disease patients on UDCA therapy matched to 225 controls without UDCA in China. UDCA use was associated with lower COVID-19 infection rate (85% vs 94%), lower maximum temperature, less severe symptoms, shorter recovery time (5 vs 7 days median), and lower risk of infection on regression (OR 0.32). The results rely on patient self-report rather than lab confirmed COVID-19 diagnosis.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
risk of hospitalization, 40.0% lower, RR 0.60, p = 0.72, treatment 3 of 225 (1.3%), control 5 of 225 (2.2%), NNT 112.
|
|
hospitalization time, 28.6% lower, relative time 0.71, p < 0.001, treatment median 5.0 IQR 3.0 n=225, control median 7.0 IQR 3.0 n=225.
|
|
risk of severe case, 80.0% lower, RR 0.20, p = 0.22, treatment 1 of 225 (0.4%), control 5 of 225 (2.2%), NNT 56.
|
|
risk of moderate/severe case, 80.0% lower, RR 0.20, p < 0.001, treatment 10 of 225 (4.4%), control 50 of 225 (22.2%), NNT 5.6.
|
|
risk of case, 10.9% lower, RR 0.89, p = 0.05, treatment 192 of 225 (85.3%), control 212 of 225 (94.2%), NNT 11, odds ratio converted to relative risk, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Li et al., 3 May 2023, retrospective, China, peer-reviewed, mean age 53.0, 5 authors, study period January 2022 - December 2022.
Contact: leaxin@ccmu.edu.cn.
Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease
Frontiers in Cellular and Infection Microbiology, doi:10.3389/fcimb.2023.1178590
Objective: Ursodeoxycholic acid (UDCA) may reduce susceptibility to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection by downregulating angiotensin-converting enzyme 2 (ACE2), based on recent experimental investigation. This study aimed to determine the potential protective effect of UDCA against SARS-CoV-2 infection in patients with chronic liver disease. Methods: Patients with chronic liver disease receiving UDCA (taking UDCA ≥1 month) at Beijing Ditan Hospital between January 2022 and December 2022 were consecutively enrolled. These patients were matched in a 1:1 ratio to those with liver disease not receiving UDCA during the same period by using a propensity score matching analysis with nearest neighbor matching algorithm. We conducted a phone survey of coronavirus disease 2019 (COVID-19) infection during the early phase of the pandemic liberation (from 15 December 2022 to 15 January 2023). The risk of COVID-19 was compared in two matched cohorts of 225 UDCA users and 225 non-UDCA users based on patient self-report. Results: In the adjusted analysis, the control group was superior to the UDCA group in COVID-19 vaccination rates and liver function indicators, including gglutamyl transpeptidase and alkaline phosphatase (p < 0.05). UDCA was associated with a lower incidence of SARS-CoV-2 infection (UDCA 85.3% vs. control 94.2%, p = 0.002), more mild cases (80.0% vs. 72.0%, p = 0.047), and shorter median time from infection to recovery (5 vs. 7 days, p < 0.001). Logistic regression analysis showed that UDCA was a significant protective factor against COVID-19 infection (OR: 0.32, 95%CI: 0.16-0.64, p = 0.001). Furthermore, diabetes mellitus (OR: 2.48, 95%CI: 1.11-5.54, p = 0.027) and moderate/severe infection (OR: 8.94, 95%CI: 1.07-74.61, p = 0.043) were more likely to prolong the time from infection to recovery.
Ethics statement The studies involving human participants were reviewed and approved by The Ethical Review Committee of the Beijing Ditan Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions YLi was the first author of this study. Study design: YLi. Data collection: NZ. Analysis of data: YLin and XC. Drafting of the manuscript: YLi. Critical reversion of the manuscript: XL. Material and technical support, and study supervision: XL. All authors have read and approved the manuscript.
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ fcimb.2023.1178590/full#supplementary-material SUPPLEMENTARY FIGURE 1 Influence..
References
Al-Kuraishy, Al-Gareeb, Alblihed, Guerreiro, Cruz-Martins et al., COVID-19 in relation to hyperglycemia and diabetes mellitus, Front. Cardiovasc. Med, doi:10.3389/fcvm.2021.644095
Ampuero, Lucena, Hernańdez-Guerra, Moreno-Moraleda, Arenas et al., Primary biliary cholangitis and SARS-CoV-2 infection: incidence, susceptibility and outcomes, Gut, doi:10.1136/gutjnl-2021-325700
Bartoszko, Siemieniuk, Kum, Qasim, Zeraatkar et al., Prophylaxis against covid-19: living systematic review and network metaanalysis, BMJ, doi:10.1136/bmj.n949
Brevini, Maes, Webb, John, Fuchs et al., FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2, Nature, doi:10.1038/s41586-022-05594-0
Cao, Wang, Jian, Xiao, Song et al., Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature, doi:10.1038/s41586-021-04385-3
Chen, Zhou, Dong, Qu, Gong et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in wuhan, China: a descriptive study, Lancet, doi:10.1016/S0140-6736(20)30211-7
Chenchula, Karunakaran, Sharma, Chavan, Current evidence on efficacy of COVID-19 booster dose vaccination against the omicron variant: a systematic review, J. Med. Virol, doi:10.1002/jmv.27697
Dufour, Marjot, Becchetti, Tilg, COVID-19 and liver disease, Gut, doi:10.1136/gutjnl-2021-326792
Efe, Dhanasekaran, Lammert, Ebik, Higuera-De, Association for the Study of the Liver2017 EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis, doi:10.1016/j.jhep.2017.03.022
Hu, Ni, Fan, Men, Yang, Past hepatitis b virus infection was not associated with poorer response or the UK-PBC risk score in ursodeoxycholic acid-treated patients with primary biliary cirrhosis, Eur. J. Gastroenterol. Hepatol, doi:10.1097/MEG.0000000000001320
Hu, Zhang, Zhang, Zhao, Lian et al., COVID-19 is more severe in patients with hypertension; ACEI/ARB treatment does not influence clinical severity and outcome, J. Infect, doi:10.1016/j.jinf.2020.05.056
Ji, Zhang, Yang, Mu, Zhao et al., Effect of COVID-19 on patients with compensated chronic liver diseases, Hepatol. Int, doi:10.1007/s12072-020-10058-6
Kim, Lee, Choi, Um, Lee et al., Clinical characteristics of 40 patients infected with the SARS-CoV-2 omicron variant in Korea, J. Kor. Med. Sci, doi:10.3346/jkms.2022.37.e31
Lai, Wang, Chen, Chen, Wang, The clinical efficacy and safety of anti-viral agents for non-hospitalized patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials, Viruses, doi:10.3390/v14081706
Li, Peng, Duan, Wang, Yu et al., Older age and depressive state are risk factors for re-positivity with SARS-CoV-2 omicron variant, Front. Public Health, doi:10.3389/fpubh.2022.1014470
Li, Wang, Ndiwane, Orner, Palacios et al., The association of COVID-19 occurrence and severity with the use of angiotensin converting enzyme inhibitors or angiotensin-II receptor blockers in patients with hypertension, PloS One, doi:10.1371/journal.pone.0248652
Lindor, Bowlus, Boyer, Levy, Mayo, Primary biliary cholangitis: 2018 practice guidance from the American association for the study of liver diseases, Hepatology, doi:10.1002/hep.30145
Marjot, Moon, Cook, Abd-Elsalam, Aloma et al., Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study, J. Hepatol, doi:10.1016/j.jhep.2020.09.024
Moon, Webb, Aloman, Armstrong, Cargill et al., High mortality rates for SARS-CoV-2 infection in patients with preexisting chronic liver disease and cirrhosis: preliminary results from an international registry, J. Hepatol, doi:10.1016/j.jhep.2020.05.013
Onder, Rezza, Brusaferro, Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy, JAMA, doi:10.1001/jama.2020.4683
Raman, Patel, Ranjanm, COVID-19: unmasking emerging SARS-CoV-2 variants, vaccines and therapeutic strategies, Biomolecules, doi:10.3390/biom11070993
Shrestha, Foster, Rawlinson, Tedla, Bull, Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: implications for immune escape and transmission, Rev. Med. Virol, doi:10.1002/rmv.2381
Suzuki, Yamasoba, Kimura, Wang, Kishimoto et al., Attenuated fusogenicity and pathogenicity of SARS-CoV-2 omicron variant, Nature, doi:10.1038/s41586-022-04462-1
Wang, Xu, Liu, Liu, Zhang et al., Multiomics: unraveling the panoramic landscapes of SARS-CoV-2 infection, Cell Mol. Immunol, doi:10.1038/s41423-021-00754-0
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among communitydwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet, doi:10.1016/S0140-6736(22)01586-0
Yang, Wang, Du, Liu, Liu et al., Patients with COVID-19 and HBV coinfection are at risk of poor prognosis, Infect. Dis. Ther, doi:10.1007/s40121-022-00638-4
Ye, Zhang, Chen, Wu, Chen et al., Ursodeoxycholic acid alleviates experimental liver fibrosis involving inhibition of autophagy, Life Sci, doi:10.1016/j.lfs.2019.117175
Zou, Fang, Li, Wu, Gao et al., Characteristics of liver function in patients with SARS-CoV-2 and chronic HBV coinfection, Clin. Gastroenterol. Hepatol, doi:10.1016/j.cgh.2020.06.017
DOI record:
{
"DOI": "10.3389/fcimb.2023.1178590",
"ISSN": [
"2235-2988"
],
"URL": "http://dx.doi.org/10.3389/fcimb.2023.1178590",
"abstract": "<jats:sec><jats:title>Objective</jats:title><jats:p>Ursodeoxycholic acid (UDCA) may reduce susceptibility to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection by downregulating angiotensin-converting enzyme 2 (ACE2), based on recent experimental investigation. This study aimed to determine the potential protective effect of UDCA against SARS-CoV-2 infection in patients with chronic liver disease.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Patients with chronic liver disease receiving UDCA (taking UDCA ≥1 month) at Beijing Ditan Hospital between January 2022 and December 2022 were consecutively enrolled. These patients were matched in a 1:1 ratio to those with liver disease not receiving UDCA during the same period by using a propensity score matching analysis with nearest neighbor matching algorithm. We conducted a phone survey of coronavirus disease 2019 (COVID-19) infection during the early phase of the pandemic liberation (from 15 December 2022 to 15 January 2023). The risk of COVID-19 was compared in two matched cohorts of 225 UDCA users and 225 non-UDCA users based on patient self-report.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>In the adjusted analysis, the control group was superior to the UDCA group in COVID-19 vaccination rates and liver function indicators, including γ-glutamyl transpeptidase and alkaline phosphatase (p &lt; 0.05). UDCA was associated with a lower incidence of SARS-CoV-2 infection (UDCA 85.3% <jats:italic>vs.</jats:italic> control 94.2%, p = 0.002), more mild cases (80.0% <jats:italic>vs.</jats:italic> 72.0%, p = 0.047), and shorter median time from infection to recovery (5 <jats:italic>vs.</jats:italic> 7 days, p &lt; 0.001). Logistic regression analysis showed that UDCA was a significant protective factor against COVID-19 infection (OR: 0.32, 95%CI: 0.16–0.64, p = 0.001). Furthermore, diabetes mellitus (OR: 2.48, 95%CI: 1.11–5.54, p = 0.027) and moderate/severe infection (OR: 8.94, 95%CI: 1.07–74.61, p = 0.043) were more likely to prolong the time from infection to recovery.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>UDCA therapy may be beneficial in reducing COVID-19 infection risk, alleviating symptoms, and shortening the recovery time in patients with chronic liver disease. However, it should be emphasized that the conclusions were based on patient self-report rather than classical COVID-19 detection by experimental investigations. Further large clinical and experimental studies are needed to validate these findings.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fcimb.2023.1178590"
],
"author": [
{
"affiliation": [],
"family": "Li",
"given": "Yanyan",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Na",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cui",
"given": "Xinyu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Yingying",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Xin",
"sequence": "additional"
}
],
"container-title": "Frontiers in Cellular and Infection Microbiology",
"container-title-short": "Front. Cell. Infect. Microbiol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
5,
3
]
],
"date-time": "2023-05-03T05:24:06Z",
"timestamp": 1683091446000
},
"deposited": {
"date-parts": [
[
2023,
5,
3
]
],
"date-time": "2023-05-03T05:24:11Z",
"timestamp": 1683091451000
},
"indexed": {
"date-parts": [
[
2023,
5,
4
]
],
"date-time": "2023-05-04T04:39:36Z",
"timestamp": 1683175176179
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
5,
3
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
5,
3
]
],
"date-time": "2023-05-03T00:00:00Z",
"timestamp": 1683072000000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fcimb.2023.1178590/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
5,
3
]
]
},
"published-online": {
"date-parts": [
[
2023,
5,
3
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.3389/fcvm.2021.644095",
"article-title": "COVID-19 in relation to hyperglycemia and diabetes mellitus",
"author": "Al-Kuraishy",
"doi-asserted-by": "publisher",
"journal-title": "Front. Cardiovasc. Med.",
"key": "B1",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1136/gutjnl-2021-325700",
"article-title": "Primary biliary cholangitis and SARS-CoV-2 infection: incidence, susceptibility and outcomes",
"author": "Ampuero",
"doi-asserted-by": "publisher",
"first-page": "gutjnl-2021-3257002021",
"journal-title": "Gut",
"key": "B2",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n949",
"article-title": "Prophylaxis against covid-19: living systematic review and network meta-analysis",
"author": "Bartoszko",
"doi-asserted-by": "publisher",
"journal-title": "BMJ",
"key": "B3",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.1038/s41586-022-05594-0",
"article-title": "FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2",
"author": "Brevini",
"doi-asserted-by": "publisher",
"first-page": "134",
"journal-title": "Nature",
"key": "B4",
"volume": "615",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-04385-3",
"article-title": "Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies",
"author": "Cao",
"doi-asserted-by": "publisher",
"first-page": "657",
"journal-title": "Nature",
"key": "B5",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"article-title": "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in wuhan, China: a descriptive study",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "507",
"journal-title": "Lancet",
"key": "B6",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1002/jmv.27697",
"article-title": "Current evidence on efficacy of COVID-19 booster dose vaccination against the omicron variant: a systematic review",
"author": "Chenchula",
"doi-asserted-by": "publisher",
"first-page": "2969",
"journal-title": "J. Med. Virol.",
"key": "B7",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1136/gutjnl-2021-326792",
"article-title": "COVID-19 and liver disease",
"author": "Dufour",
"doi-asserted-by": "publisher",
"first-page": "2350",
"journal-title": "Gut",
"key": "B8",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1002/hep.31797",
"article-title": "Outcome of COVID-19 in patients with autoimmune hepatitis: an international multicenter study",
"author": "Efe",
"doi-asserted-by": "publisher",
"first-page": "2099",
"journal-title": "Hepatology",
"key": "B9",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1016/j.jhep.2017.03.022",
"article-title": "EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis",
"doi-asserted-by": "publisher",
"first-page": "145",
"journal-title": "J. Hepatol.",
"key": "B10",
"volume": "67",
"year": "2017"
},
{
"key": "B11",
"unstructured": "Diagnosis and treatment plan for SARS-CoV-2 infection (Tenth version on trial)2023"
},
{
"DOI": "10.1097/MEG.0000000000001320",
"article-title": "Past hepatitis b virus infection was not associated with poorer response or the UK-PBC risk score in ursodeoxycholic acid-treated patients with primary biliary cirrhosis",
"author": "Hu",
"doi-asserted-by": "publisher",
"first-page": "277",
"journal-title": "Eur. J. Gastroenterol. Hepatol.",
"key": "B12",
"volume": "31",
"year": "2019"
},
{
"DOI": "10.1016/j.jinf.2020.05.056",
"article-title": "COVID-19 is more severe in patients with hypertension; ACEI/ARB treatment does not influence clinical severity and outcome",
"author": "Hu",
"doi-asserted-by": "publisher",
"first-page": "979",
"journal-title": "J. Infect.",
"key": "B13",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1007/s12072-020-10058-6",
"article-title": "Effect of COVID-19 on patients with compensated chronic liver diseases",
"author": "Ji",
"doi-asserted-by": "publisher",
"first-page": "701",
"journal-title": "Hepatol. Int.",
"key": "B14",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.3346/jkms.2022.37.e31",
"article-title": "Clinical characteristics of 40 patients infected with the SARS-CoV-2 omicron variant in Korea",
"author": "Kim",
"doi-asserted-by": "publisher",
"journal-title": "J. Kor. Med. Sci.",
"key": "B15",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.3390/v14081706",
"article-title": "The clinical efficacy and safety of anti-viral agents for non-hospitalized patients with COVID-19: a systematic review and network meta-analysis of randomized controlled trials",
"author": "Lai",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "B16",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3389/fpubh.2022.1014470",
"article-title": "Older age and depressive state are risk factors for re-positivity with SARS-CoV-2 omicron variant",
"author": "Li",
"doi-asserted-by": "publisher",
"journal-title": "Front. Public Health",
"key": "B17",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0248652",
"article-title": "The association of COVID-19 occurrence and severity with the use of angiotensin converting enzyme inhibitors or angiotensin-II receptor blockers in patients with hypertension",
"author": "Li",
"doi-asserted-by": "publisher",
"journal-title": "PloS One",
"key": "B18",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1002/hep.30145",
"article-title": "Primary biliary cholangitis: 2018 practice guidance from the American association for the study of liver diseases",
"author": "Lindor",
"doi-asserted-by": "publisher",
"first-page": "394",
"journal-title": "Hepatology",
"key": "B19",
"volume": "69",
"year": "2019"
},
{
"DOI": "10.1016/j.jhep.2020.09.024",
"article-title": "Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study",
"author": "Marjot",
"doi-asserted-by": "publisher",
"first-page": "567",
"journal-title": "J. Hepatol.",
"key": "B20",
"volume": "74",
"year": "2022"
},
{
"DOI": "10.1016/j.jhep.2020.05.013",
"article-title": "High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: preliminary results from an international registry",
"author": "Moon",
"doi-asserted-by": "publisher",
"first-page": "705",
"journal-title": "J. Hepatol.",
"key": "B21",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.4683",
"article-title": "Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy",
"author": "Onder",
"doi-asserted-by": "publisher",
"first-page": "1775",
"journal-title": "JAMA",
"key": "B22",
"volume": "323",
"year": "2020"
},
{
"key": "B23",
"unstructured": "A report on SARS-CoV-2 infections based on big data model estimates2023"
},
{
"DOI": "10.3390/biom11070993",
"article-title": "COVID-19: unmasking emerging SARS-CoV-2 variants, vaccines and therapeutic strategies",
"author": "Raman",
"doi-asserted-by": "publisher",
"journal-title": "Biomolecules",
"key": "B24",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1002/rmv.2381",
"article-title": "Evolution of the SARS-CoV-2 omicron variants BA.1 to BA.5: implications for immune escape and transmission",
"author": "Shrestha",
"doi-asserted-by": "publisher",
"first-page": "e2381",
"journal-title": "Rev. Med. Virol.",
"key": "B25",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-04462-1",
"article-title": "Attenuated fusogenicity and pathogenicity of SARS-CoV-2 omicron variant",
"author": "Suzuki",
"doi-asserted-by": "publisher",
"first-page": "700",
"journal-title": "Nature",
"key": "B26",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1038/s41423-021-00754-0",
"article-title": "Multiomics: unraveling the panoramic landscapes of SARS-CoV-2 infection",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "2313",
"journal-title": "Cell Mol. Immunol.",
"key": "B27",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"article-title": "Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study",
"author": "Wong",
"doi-asserted-by": "publisher",
"first-page": "1213",
"journal-title": "Lancet",
"key": "B28",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1007/s40121-022-00638-4",
"article-title": "Patients with COVID-19 and HBV coinfection are at risk of poor prognosis",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "1229",
"journal-title": "Infect. Dis. Ther.",
"key": "B29",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1016/j.lfs.2019.117175",
"article-title": "Ursodeoxycholic acid alleviates experimental liver fibrosis involving inhibition of autophagy",
"author": "Ye",
"doi-asserted-by": "publisher",
"journal-title": "Life Sci.",
"key": "B30",
"volume": "242",
"year": "2020"
},
{
"DOI": "10.1016/j.cgh.2020.06.017",
"article-title": "Characteristics of liver function in patients with SARS-CoV-2 and chronic HBV coinfection",
"author": "Zou",
"doi-asserted-by": "publisher",
"first-page": "597",
"journal-title": "Clin. Gastroenterol. Hepatol.",
"key": "B31",
"volume": "19",
"year": "2021"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fcimb.2023.1178590/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"Immunology",
"Microbiology"
],
"subtitle": [],
"title": "Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "13"
}