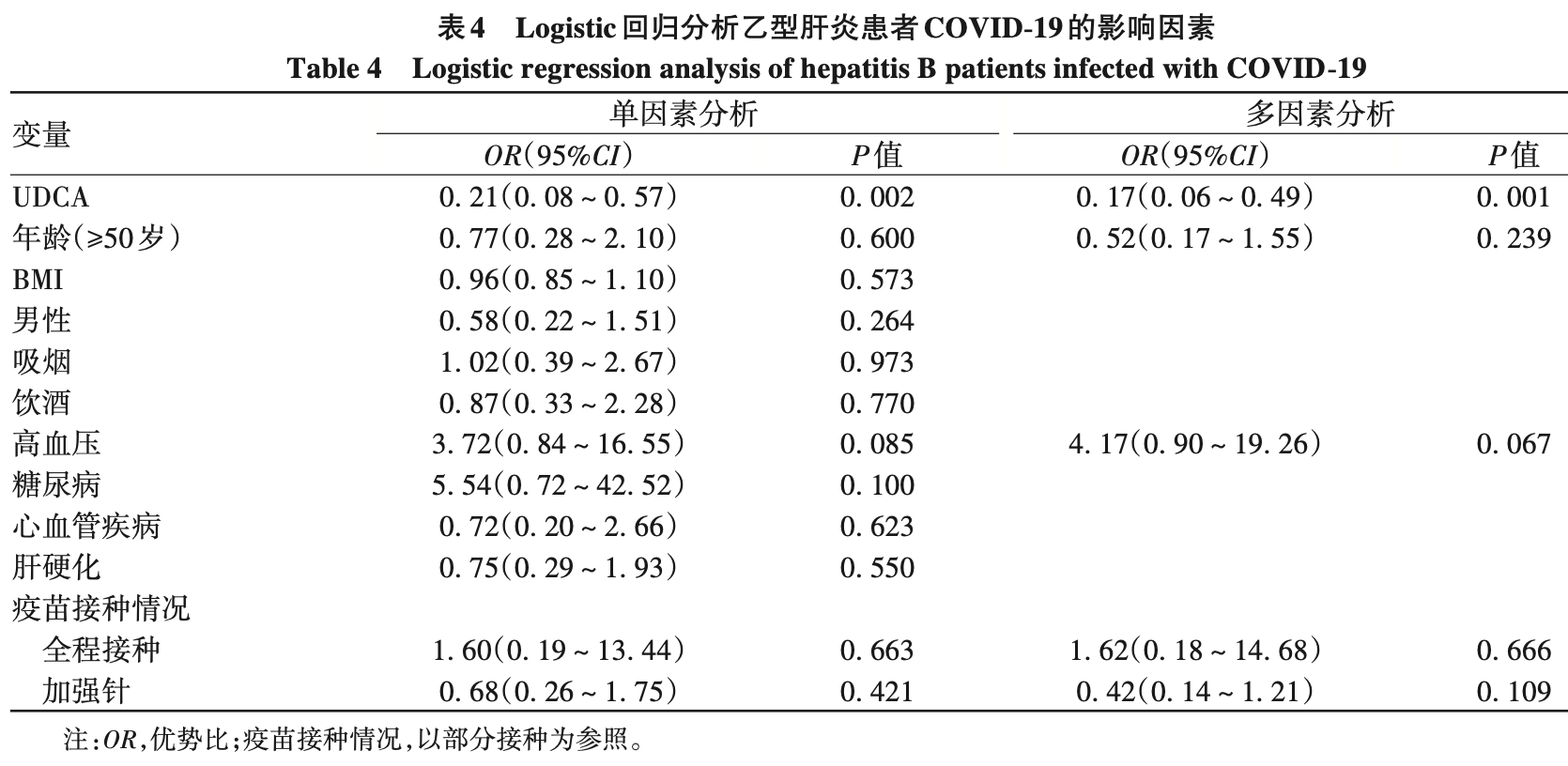
Efficacy of ursodeoxycholic acid in the prevention and treatment of COVID-19 in patients with chronic hepatitis B
et al., Journal of Clinical Hepatology, doi:10.12449/JCH240309, Mar 2024
Retrospective 215 patients with chronic hepatitis B in China, showing lower risk of COVID-19 infection, milder symptoms, and faster recovery with ursodeoxycholic acid (UDCA) treatment.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
recovery time >7 days, 47.8% lower, RR 0.52, p = 0.010, treatment 13 of 64 (20.3%), control 51 of 131 (38.9%), NNT 5.4, propensity score matching.
|
|
risk of case, 83.0% lower, OR 0.17, p = 0.001, treatment 78, control 137, adjusted per study, propensity score matching, multivariable, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Cui et al., 20 Mar 2024, retrospective, China, peer-reviewed, 5 authors.
, 缩短恢复时间, 在防治 COVID-19 方面具有重要价值。 关键词: 乙型肝炎, 慢性; 新型冠状病毒; 熊去氧胆酸; 倾向性评分 基金项目: 北京市自然科学基金(7212053) Efficacy of ursodeoxycholic acid in the prevention and treatment of COVID-19 in patients with chronic hepatitis B
doi:10.12449/JCH240309
摘要: 目的 探讨慢性乙型肝炎患者服用熊去氧胆酸后对新型冠状病毒感染
References
Bergwerk, Gonen, Lustig, Covid-19 breakthrough infections in vaccinated health care workers, J]. N Engl J Med, doi:10.1056/NEJMoa2109072
Beuers U, Trauner, Jansen, New paradigms in the treatment of hepatic cholestasis: From UDCA to FXR, PXR and be• yond[J], J Hepatol, doi:10.1016/j.jhep.2015.02.023
Brevini, Maes, Gj, FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2[J], Nature, doi:10.1038/s41586-022-05594-0
Chen Yc, Huang Wx, Expert consensus on the application of azvu• dine in the treatment of SARS-CoV-2 infection, J]. China Pharm, doi:10.3969/j.issn.1006-4931.2023.03.001
Davies, Morden, Rosseau, Outcomes of laboratoryconfirmed SARS-CoV-2 infection during resurgence driven by Omicron lineages BA.4 and BA.5 compared with previous waves in the Western Cape Province, South Africa[J], Int J Infect Dis, doi:10.1016/j.ijid.2022.11.024
Fuchs Cd, Trauner, Role of bile acids and their receptors in gas• trointestinal and hepatic pathophysiology[J], Nat Rev Gastroenterol Hepatol, doi:10.1038/s41575-021-00566-7
Garg, Hospitalization rates and charac• teristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 -COVID-NET, 14 States
Gaziano, Giambartolomei, Pereira Ac, Actionable druggable genome-wide Mendelian randomization identifies repur• posing opportunities for COVID-19, J]. Nat Med, doi:10.1038/s41591-021-01310-z
Levin Eg, Lustig, Cohen, Waning immune humoral re• sponse to BNT162b2 Covid-19 vaccine over 6 months, J]. N Engl J Med, doi:10.1056/NEJMoa2114583
Li Q, Guan, Wu, Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia[J], N Engl J Med, doi:10.1056/NEJMoa2001316
Li Yg, Zh, Efficacy of tenofovir combined with ursodeoxycholic acid in outpatient treatment of chronic hepatitis B[J], Health Med Res Prac, doi:10.11986/j.issn.1673-873X.2022.04.006
Li, Therapeutic options for the 2019 novel coronavi• rus (2019-nCoV) [J], Nat Rev Drug Discov, doi:10.1038/d41573-020-00016-0
Liu, Feng, Ren, The influencing factors of abnormal liver function in patients with COVID-19 and its dynamic changes in different drug treatments[J], J Capit Med Univ, doi:10.3969/j.issn.1006-7795.2022.03.017
Reiffel, Propensity score matching: The 'devil is in the details' where more may be hidden than you know[J], Am J Med, doi:10.1016/j.amjmed.2019.08.055
Sj, Treatment of chronic hepatitis B focuses on improving disease resistance[J], Guangming J Chin Med, doi:10.3969/j.issn.1003-8914.2012.08.003
Sl, Yt, Cai Xj, Research progress on anticoro• navirus drugs[J], Chin J Comp Med, doi:10.3969/j.issn.1671-7856.2022.06.016
Sun Xl, Hu, Yt, Clinical application of ursodeoxycholic acid [J], Chin J Pharmacov, doi:10.19803/j.1672-8629.20210604
Sy, Peng, Xh, Pathogenic mechanism of liver injury caused by coronavirus disease 2019 and protective strategies for pa• tients with viral hepatitis cirrhosis[J], J Clin Hepatol, doi:10.3969/j.issn.1001-5256.2020.07.037
Van Den Bossche L, Hindryckx, Devisscher, Urso• deoxycholic acid and its taurine-or glycine-conjugated species re• duce colitogenic dysbiosis and equally suppress experimental coli• tis in mice[J], Appl Environ Microbiol, doi:10.1128/AEM.02766-16
Verity R, Lc, Dorigatti, Estimates of the severity of coronavirus disease 2019: a model-based analysis, J]. Lancet Infect Dis, doi:10.1016/S1473-3099(20)30243-7
Wang Ch, Yao, Wang R, The latest research progress of novel coronavirus "Omicron sub-variant BA. 5, J Hanan Med Coll, doi:10.13210/j.cnki.jhmu.20220909.002
Wang Q, Guo, Iketani, Antibody evasion by SARS-CoV-2 Omicron subvariants BA, BA.4 and BA, doi:10.1038/s41586-022-05053-w
Who, Weekly epidemiological update on COVID-19 -22
Xy, Yy, Zhu, Efficacy of ursodeoxycholic acid in the prevention and treatment of COVID-19 in patients with chronic hepatitis B[J], J Clin Hepatol
Yang, Xu, Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a singlecentered, retrospective, observational study[J], Lancet Respir Med, doi:10.1016/S2213-2600(20)30079-5
Ye, Sw, Clinical evaluation of taurine ursodeoxycholic acid in the treatment of chronic hepatitis B with overlapping hepatitis E infec• tion[J], Chin Foreign Med Res, doi:10.14033/j.cnki.cfmr.2019.08.022
Zampino R, Mele, Ll, Liver injury in remdesivirtreated COVID-19 patients, J]. Hepatol Int, doi:10.1007/s12072-020-10077-3
Zhang, Xuqing: Can patients with liver disease be vacci• nated with COVID-19 vaccine?, J]. Liver Doctor
崔心宇, 朱娜, 效 果 分 []. 临 床 肝 胆 病 杂 志