FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2
et al., Nature, doi:10.1038/s41586-022-05594-0, Dec 2022
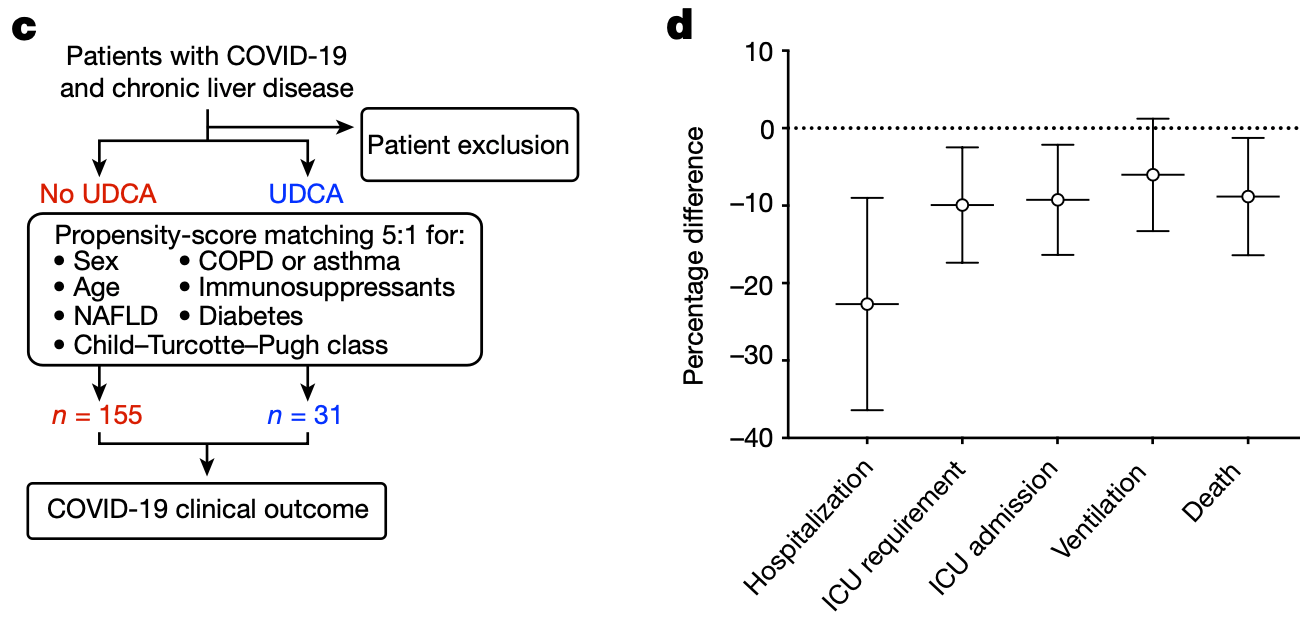
Retrospective study from two registries of 1,096 COVID-19 patients with chronic liver disease, including 31 treated with ursodeoxycholic acid (UDCA). Propensity score matching was used to compare outcomes between UDCA-treated and untreated patients. The analysis found that UDCA treatment was associated with reduced hospitalization, ICU admission, ventilation, and death from COVID-19. The authors suggest that UDCA may decrease susceptibility to SARS-CoV-2 infection by downregulating the host receptor ACE2 through inhibition of the farnesoid X receptor.
Authors also show that UDCA-mediated downregulation of ACE2 reduces susceptibility to SARS-CoV-2 infection in vitro, in vivo and in human lungs and livers perfused ex situ; and that UDCA reduces the expression of ACE2 in the nasal epithelium in humans.
4 preclinical studies support the efficacy of ursodeoxycholic acid for COVID-19:
Ursodeoxycholic acid reduced ACE2 expression and blocked pseudovirus
infection in Calu-3 cells2, protected against Omicron infection in hamsters by
downregulating ACE2 expression via FXR inhibition, leading to reduced
viral load in the upper respiratory tract and prevention of weight
loss4, may reduce SARS-CoV-2 infection by interfering with
spike protein binding to ACE2 receptors on B cells1, and inhibited SARS-CoV-2 infection by downregulating
ACE2 expression via FXR inhibition in multiple tissues (respiratory,
biliary, and intestinal), reduced viral transmission in a hamster model,
and decreased viral replication in human organ perfusion models3.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments5.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 94.4% lower, RR 0.06, p = 0.13, treatment 0 of 31 (0.0%), control 14 of 155 (9.0%), NNT 11, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), propensity score matching.
|
|
risk of mechanical ventilation, 66.7% lower, RR 0.33, p = 0.48, treatment 1 of 31 (3.2%), control 15 of 155 (9.7%), NNT 16, propensity score matching.
|
|
risk of ICU admission, 75.0% lower, RR 0.25, p = 0.21, treatment 1 of 31 (3.2%), control 20 of 155 (12.9%), NNT 10, propensity score matching.
|
|
risk of hospitalization, 39.6% lower, RR 0.60, p = 0.03, treatment 11 of 31 (35.5%), control 91 of 155 (58.7%), NNT 4.3, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Park et al., Ursodeoxycholic Acid Attenuates B Cell Susceptibility to SARS-CoV-2 Spike Protein by Interfering Its Binding to ACE2, Biomolecules & Therapeutics, doi:10.4062/biomolther.2025.149.
2.
Tong et al., Ursodeoxycholic acid reduces ACE-2 activity in COVID-19 patients and Calu- 3 cells, Research Square, doi:10.21203/rs.3.rs-5317838/v1.
3.
Brevini et al., FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2, Nature, doi:10.1038/s41586-022-05594-0.
Brevini et al., 5 Dec 2022, retrospective, United Kingdom, peer-reviewed, 80 authors.
FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2
Nature, doi:10.1038/s41586-022-05594-0
prophylaxis against COVID-19 2 . Therefore, there is a pressing need for novel prophylactic agents that reduce the risk of severe disease 3 , are less susceptible to viral resistance and are compatible with healthcare systems in low-and middle-income countries. Viral host receptors represent logical therapeutic targets, because they are essential for SARS-CoV-2 cellular entry and infection 1 . Among these, ACE2 is particularly appealing 1 . ACE2 is a transmembrane carboxypeptidase with a broad substrate specificity, including angiotensin II, that acts as the main receptor for SARS-CoV-2. It directly binds to the spike proteins of different coronaviruses, with a high affinity for SARS-CoV-2, rendering it indispensable for viral entry 11 . Accordingly, COVID-19 predominantly affects tissues that express ACE2, such as the lungs, the cardiovascular system, the digestive tract and the biliary tree 12, 13 . Modifying the expression of ACE2 could impede viral entry and protect against infection with SARS-CoV-2 and potentially other coronaviruses that use the same receptor. Furthermore, because ACE2 is a host-cell protein, its expression is not likely to be affected by mutations in the virus. Therefore, therapies that modulate ACE2 expression may be effective against multiple SARS-CoV-2 variants with a higher genetic barrier to resistance. However, the mechanisms that control ACE2 expression remain unclear. Here we use human cholangiocyte organoids as a proof-of-principle system to demonstrate that the bile acid receptor FXR controls the expression of ACE2. We show that this mechanism applies in several SARS-CoV-2-affected tissues, including gastrointestinal and respiratory epithelia. Subsequently, we demonstrate that suppressing FXR signalling, by using the approved drug UDCA or the over-the-counter phytosteroid z-guggulsterone (ZGG), reduces ACE2 expression and SARS-CoV-2 infection in vitro and in an airborne transmission model in golden Syrian hamsters. We repeat our experiments in human lungs and livers perfused ex situ and show that administering UDCA at physiologically relevant concentrations reduces ACE2 and viral infection in both organs ex vivo. We then demonstrate a reduction in the levels of ACE2 in the nasal epithelium of volunteers receiving clinically approved doses of UDCA. Finally, we interrogate an international registry cohort of patients with COVID-19 and chronic liver disease, identify a correlation between UDCA therapy and better clinical outcomes from COVID-19 and reproduce these results in a second independent cohort of liver-transplant recipients.
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reporting summary Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. Cambridge BRC Cell Phenotyping Hub for their help with flow cytometry and processing of samples; the building staff of the Jeffrey Cheah Biomedical Centre for maintaining the institute open and safe during the period of lockdown; K. Füssel for coordinating the volunteer study and sample collection at the University Medical Centre Hamburg-Eppendorf; J. Hails
References
Andreasson, Dark, Fisher, Ex vivo lung perfusion in clinical lung transplantation-state of the art, Eur. J. Cardiothorac. Surg
Balasubramaniyan, Luo, Sun, Suchy, SUMOylation of the farnesoid X receptor (FXR) regulates the expression of FXR target genes, J. Biol. Chem
Barron-Millar, The serum proteome and ursodeoxycholic acid response in primary biliary cholangitis, Hepatology
Bartoszko, Prophylaxis against covid-19: living systematic review and network meta-analysis, Br. Med. J
Beigel, Remdesivir for the treatment of Covid-19-final report, N. Engl. J. Med
Bjerknes, Cheng, Methods for the isolation of intact epithelium from the mouse intestine, Anat. Rec
Callaway, The unequal scramble for coronavirus vaccines-by the numbers, Nature
Cantuti-Castelvetri, Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity, Science
Cao, Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature
Caron, Cariou, Staels, FXR: more than a bile acid receptor?, Endocrinology
Chen, Bile acids induce activation of alveolar epithelial cells and lung fibroblasts through farnesoid X receptor-dependent and independent pathways, Respirology
Collier, Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies, Nature
Comeglio, Anti-fibrotic effects of chronic treatment with the selective FXR agonist obeticholic acid in the bleomycin-induced rat model of pulmonary fibrosis, J. Steroid Biochem. Mol. Biol
Dyer, Covid-19: countries are learning what others paid for vaccines, Br. Med. J
Easl Clinical, Guidelines, The diagnosis and management of patients with primary biliary cholangitis, J. Hepatol
Evangelou, Pulmonary infection by SARS-CoV-2 induces senescence accompanied by an inflammatory phenotype in severe COVID-19: possible implications for viral mutagenesis, Eur. Respir. J
Fickert, Effects of ursodeoxycholic and cholic acid feeding on hepatocellular transporter expression in mouse liver, Gastroenterology
Fickert, Wagner, Biliary bile acids in hepatobiliary injury-what is the link?, J. Hepatol
Fiorucci, Biagioli, Zampella, Distrutti, Bile acids activated receptors regulate innate immunity, Front. Immunol
Fuchs, Trauner, Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology, Nat. Rev. Gastroenterol. Hepatol
Gadaleta, Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease, Gut
Gaziano, Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19, Nat. Med
Gerber, A protease-activatable luminescent biosensor and reporter cell line for authentic SARS-CoV-2 infection, PLoS Pathog
Guido, Furin cleavage of SARS-CoV-2 Spike promotes but is not essential for infection and cell-cell fusion, PLoS Pathog
Gupta, Extrapulmonary manifestations of COVID-19, Nat. Med
Jiang, Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease, J. Clin. Invest
John, Effectiveness of COVID-19 viral vector Ad.26.COV2.S vaccine and comparison with mRNA vaccines in cirrhosis, Clin. Gastroenterol. Hepatol
John, Ursodeoxycholic acid use and outcomes of Coronavirus 2019 in patients with liver disease, Hepatology
Kemp, SARS-CoV-2 evolution during treatment of chronic infection, Nature
Lamers, SARS-CoV-2 productively infects human gut enterocytes, Science
Lee, Pharmacokinetics of ursodeoxycholic acid in elderly volunteers compared with younger adults in a Korean population, J. Clin. Pharmacol
Levin, Intramuscular AZD7442 (tixagevimab-cilgavimab) for prevention of Covid-19, N. Engl. J. Med
Marjot, Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study, J. Hepatol
Marjot, SARS-CoV-2 infection in patients with autoimmune hepatitis, J. Hepatol
Mohty, Prophylactic, preemptive, and curative treatment for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a position statement from an international expert group, Bone Marrow Transplant
Morrison, Use of phosphodiesterase inhibition during ex-vivo lung perfusion of donor lungs unsuitable for transplantation, J. Heart Lung Transplant
Nasralla, A randomized trial of normothermic preservation in liver transplantation, Nature
Patel, Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial, Hepatology
Patterson, Methods of inactivation of SARS-CoV-2 for downstream biological assays, J. Infect. Dis
Qin, Perioperative presentation of COVID-19 disease in a liver transplant recipient, Hepatology
Sachs, Long-term expanding human airway organoids for disease modeling, EMBO J
Sampaziotis, Cholangiocyte organoids can repair bile ducts after transplantation in the human liver, Science
Sampaziotis, Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids, Nat. Med
Sharma, Ursodeoxycholic acid amides as novel glucocorticoid receptor modulators, J. Med. Chem
Sridhar, A blueprint for the implementation of a validated approach for the detection of SARS-Cov2 in clinical samples in academic facilities, Wellcome Open Res
Sun, Cai, Gonzalez, The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer, Nat. Rev. Gastroenterol. Hepatol
Sun, Gut microbiota and intestinal FXR mediate the clinical benefits of metformin, Nat. Med
Sungnak, SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes, Nat. Med
Tysoe, Isolation and propagation of primary human cholangiocyte organoids for the generation of bioengineered biliary tissue, Nat. Protoc
Tysoe, Isolation and propagation of primary human cholangiocyte organoids for the generation of bioengineered biliary tissue, Nat. Protoc
Urizar, A natural product that lowers cholesterol as an antagonist ligand for FXR, Science
Vabret, Immunology of COVID-19: current state of the science, Immunity
Watson, Observations on the ex situ perfusion of livers for transplantation, Am. J. Transplant
Webb, Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study, Lancet Gastroenterol. Hepatol
Weinreich, REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19, N. Engl. J. Med
Youk, Three-dimensional human alveolar stem cell culture models reveal infection response to SARS-CoV-2, Cell Stem Cell
Zhao, Activation of FXR protects against renal fibrosis via suppressing Smad3 expression, Sci. Rep
Zhou, A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature
DOI record:
{
"DOI": "10.1038/s41586-022-05594-0",
"ISSN": [
"0028-0836",
"1476-4687"
],
"URL": "http://dx.doi.org/10.1038/s41586-022-05594-0",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Preventing SARS-CoV-2 infection by modulating viral host receptors, such as angiotensin-converting enzyme 2 (ACE2)<jats:sup>1</jats:sup>, could represent a new chemoprophylactic approach for COVID-19 that complements vaccination<jats:sup>2,3</jats:sup>. However, the mechanisms that control the expression of ACE2 remain unclear. Here we show that the farnesoid X receptor (FXR) is a direct regulator of <jats:italic>ACE2</jats:italic> transcription in several tissues affected by COVID-19, including the gastrointestinal and respiratory systems. We then use the over-the-counter compound z-guggulsterone and the off-patent drug ursodeoxycholic acid (UDCA) to reduce FXR signalling and downregulate ACE2 in human lung, cholangiocyte and intestinal organoids and in the corresponding tissues in mice and hamsters. We show that the UDCA-mediated downregulation of ACE2 reduces susceptibility to SARS-CoV-2 infection in vitro, in vivo and in human lungs and livers perfused ex situ. Furthermore, we reveal that UDCA reduces the expression of ACE2 in the nasal epithelium in humans. Finally, we identify a correlation between UDCA treatment and positive clinical outcomes after SARS-CoV-2 infection using retrospective registry data, and confirm these findings in an independent validation cohort of recipients of liver transplants. In conclusion, we show that FXR has a role in controlling ACE2 expression and provide evidence that modulation of this pathway could be beneficial for reducing SARS-CoV-2 infection, paving the way for future clinical trials.</jats:p>",
"alternative-id": [
"5594"
],
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "3 May 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "23 November 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "5 December 2022"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "F.S., L.V. and K.S.-P. are founders and shareholders of Bilitech. L.V. is a founder and shareholder of DEFINIGEN. The remaining authors declare no competing interests."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-3581-5379",
"affiliation": [],
"authenticated-orcid": false,
"family": "Brevini",
"given": "Teresa",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-0266-6557",
"affiliation": [],
"authenticated-orcid": false,
"family": "Maes",
"given": "Mailis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Webb",
"given": "Gwilym J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "John",
"given": "Binu V.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fuchs",
"given": "Claudia D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buescher",
"given": "Gustav",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4418-8602",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wang",
"given": "Lu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Griffiths",
"given": "Chelsea",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6463-0288",
"affiliation": [],
"authenticated-orcid": false,
"family": "Brown",
"given": "Marnie L.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1515-0514",
"affiliation": [],
"authenticated-orcid": false,
"family": "Scott",
"given": "William E.",
"sequence": "additional",
"suffix": "III"
},
{
"affiliation": [],
"family": "Pereyra-Gerber",
"given": "Pehuén",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gelson",
"given": "William T. H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown",
"given": "Stephanie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dillon",
"given": "Scott",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Muraro",
"given": "Daniele",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8482-5736",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sharp",
"given": "Jo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4960-2139",
"affiliation": [],
"authenticated-orcid": false,
"family": "Neary",
"given": "Megan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Box",
"given": "Helen",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9448-8876",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tatham",
"given": "Lee",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8928-2037",
"affiliation": [],
"authenticated-orcid": false,
"family": "Stewart",
"given": "James",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Curley",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pertinez",
"given": "Henry",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Forrest",
"given": "Sally",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mlcochova",
"given": "Petra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Varankar",
"given": "Sagar S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0226-2621",
"affiliation": [],
"authenticated-orcid": false,
"family": "Darvish-Damavandi",
"given": "Mahnaz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mulcahy",
"given": "Victoria L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kuc",
"given": "Rhoda E.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1051-0595",
"affiliation": [],
"authenticated-orcid": false,
"family": "Williams",
"given": "Thomas L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Heslop",
"given": "James A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rossetti",
"given": "Davide",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tysoe",
"given": "Olivia C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galanakis",
"given": "Vasileios",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vila-Gonzalez",
"given": "Marta",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0951-4588",
"affiliation": [],
"authenticated-orcid": false,
"family": "Crozier",
"given": "Thomas W. M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9304-3573",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bargehr",
"given": "Johannes",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5900-1209",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sinha",
"given": "Sanjay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Upponi",
"given": "Sara S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fear",
"given": "Corrina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Swift",
"given": "Lisa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0633-3696",
"affiliation": [],
"authenticated-orcid": false,
"family": "Saeb-Parsy",
"given": "Kourosh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Davies",
"given": "Susan E.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3634-6591",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wester",
"given": "Axel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hagström",
"given": "Hannes",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6903-6878",
"affiliation": [],
"authenticated-orcid": false,
"family": "Melum",
"given": "Espen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Clements",
"given": "Darran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Humphreys",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Herriott",
"given": "Jo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kijak",
"given": "Edyta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cox",
"given": "Helen",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8274-457X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Bramwell",
"given": "Chloe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valentijn",
"given": "Anthony",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Illingworth",
"given": "Christopher J. R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dahman",
"given": "Bassam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bastaich",
"given": "Dustin R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferreira",
"given": "Raphaella D.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marjot",
"given": "Thomas",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0860-0831",
"affiliation": [],
"authenticated-orcid": false,
"family": "Barnes",
"given": "Eleanor",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7163-2062",
"affiliation": [],
"authenticated-orcid": false,
"family": "Moon",
"given": "Andrew M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barritt",
"given": "Alfred S.",
"sequence": "additional",
"suffix": "IV"
},
{
"ORCID": "http://orcid.org/0000-0001-9751-1808",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gupta",
"given": "Ravindra K.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-1308-5755",
"affiliation": [],
"authenticated-orcid": false,
"family": "Baker",
"given": "Stephen",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2096-3117",
"affiliation": [],
"authenticated-orcid": false,
"family": "Davenport",
"given": "Anthony P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Corbett",
"given": "Gareth",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9001-4112",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gorgoulis",
"given": "Vassilis G.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2975-416X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Buczacki",
"given": "Simon J. A.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7364-6422",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lee",
"given": "Joo-Hyeon",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3318-1851",
"affiliation": [],
"authenticated-orcid": false,
"family": "Matheson",
"given": "Nicholas J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1275-6425",
"affiliation": [],
"authenticated-orcid": false,
"family": "Trauner",
"given": "Michael",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4822-7223",
"affiliation": [],
"authenticated-orcid": false,
"family": "Fisher",
"given": "Andrew J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gibbs",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Butler",
"given": "Andrew J.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0590-4901",
"affiliation": [],
"authenticated-orcid": false,
"family": "Watson",
"given": "Christopher J. E.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mells",
"given": "George F.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dougan",
"given": "Gordon",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9819-7651",
"affiliation": [],
"authenticated-orcid": false,
"family": "Owen",
"given": "Andrew",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lohse",
"given": "Ansgar W.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3848-2602",
"affiliation": [],
"authenticated-orcid": false,
"family": "Vallier",
"given": "Ludovic",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0812-7586",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sampaziotis",
"given": "Fotios",
"sequence": "additional"
},
{
"affiliation": [],
"name": "UK-PBC Consortium",
"sequence": "additional"
}
],
"container-title": "Nature",
"container-title-short": "Nature",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
12,
5
]
],
"date-time": "2022-12-05T17:03:08Z",
"timestamp": 1670259788000
},
"deposited": {
"date-parts": [
[
2023,
2,
8
]
],
"date-time": "2023-02-08T05:06:20Z",
"timestamp": 1675832780000
},
"indexed": {
"date-parts": [
[
2023,
2,
9
]
],
"date-time": "2023-02-09T09:13:59Z",
"timestamp": 1675934039577
},
"is-referenced-by-count": 11,
"issued": {
"date-parts": [
[
2022,
12,
5
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
5
]
],
"date-time": "2022-12-05T00:00:00Z",
"timestamp": 1670198400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
5
]
],
"date-time": "2022-12-05T00:00:00Z",
"timestamp": 1670198400000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41586-022-05594-0.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41586-022-05594-0",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41586-022-05594-0.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2022,
12,
5
]
]
},
"published-online": {
"date-parts": [
[
2022,
12,
5
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1038/s41591-021-01310-z",
"author": "L Gaziano",
"doi-asserted-by": "publisher",
"first-page": "668",
"journal-title": "Nat. Med.",
"key": "5594_CR1",
"unstructured": "Gaziano, L. et al. Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19. Nat. Med. 27, 668–676 (2021).",
"volume": "27",
"year": "2021"
},
{
"key": "5594_CR2",
"unstructured": "World Health Organization. WHO Guidelines: Drugs to Prevent COVID-19 https://www.who.int/publications/i/item/WHO-2019-nCoV-prophylaxes-2021-1 (WHO, 2021)."
},
{
"DOI": "10.1136/bmj.n949",
"author": "JJ Bartoszko",
"doi-asserted-by": "publisher",
"first-page": "n949",
"journal-title": "Br. Med. J.",
"key": "5594_CR3",
"unstructured": "Bartoszko, J. J. et al. Prophylaxis against covid-19: living systematic review and network meta-analysis. Br. Med. J. 373, n949 (2021).",
"volume": "373",
"year": "2021"
},
{
"key": "5594_CR4",
"unstructured": "World Health Organization. Therapeutics and COVID-19: living guideline (version 9.3, 3 March 2022) (WHO, 2022)."
},
{
"DOI": "10.1056/NEJMoa2021436",
"author": "The RECOVERY Collaborative Group.",
"doi-asserted-by": "publisher",
"first-page": "693",
"journal-title": "N. Engl. J. Med.",
"key": "5594_CR5",
"unstructured": "The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 384, 693–704 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"author": "JH Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"journal-title": "N. Engl. J. Med.",
"key": "5594_CR6",
"unstructured": "Beigel, J. H. et al. Remdesivir for the treatment of Covid-19—final report. N. Engl. J. Med. 383, 1813–1826 (2020).",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-04385-3",
"author": "Y Cao",
"doi-asserted-by": "publisher",
"first-page": "657",
"journal-title": "Nature",
"key": "5594_CR7",
"unstructured": "Cao, Y. et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 602, 657–663 (2022).",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-03412-7",
"author": "DA Collier",
"doi-asserted-by": "publisher",
"first-page": "136",
"journal-title": "Nature",
"key": "5594_CR8",
"unstructured": "Collier, D. A. et al. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature 593, 136–141 (2021).",
"volume": "593",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n281",
"author": "O Dyer",
"doi-asserted-by": "publisher",
"first-page": "n281",
"journal-title": "Br. Med. J.",
"key": "5594_CR9",
"unstructured": "Dyer, O. Covid-19: countries are learning what others paid for vaccines. Br. Med. J. 372, n281 (2021).",
"volume": "372",
"year": "2021"
},
{
"DOI": "10.1038/d41586-020-02450-x",
"author": "E Callaway",
"doi-asserted-by": "publisher",
"first-page": "506",
"journal-title": "Nature",
"key": "5594_CR10",
"unstructured": "Callaway, E. The unequal scramble for coronavirus vaccines—by the numbers. Nature 584, 506–507 (2020).",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"author": "P Zhou",
"doi-asserted-by": "publisher",
"first-page": "270",
"journal-title": "Nature",
"key": "5594_CR11",
"unstructured": "Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020).",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0868-6",
"author": "W Sungnak",
"doi-asserted-by": "publisher",
"first-page": "681",
"journal-title": "Nat. Med.",
"key": "5594_CR12",
"unstructured": "Sungnak, W. et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 26, 681–687 (2020).",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1183/13993003.02951-2021",
"author": "K Evangelou",
"doi-asserted-by": "publisher",
"first-page": "2102951",
"journal-title": "Eur. Respir. J.",
"key": "5594_CR13",
"unstructured": "Evangelou, K. et al. Pulmonary infection by SARS-CoV-2 induces senescence accompanied by an inflammatory phenotype in severe COVID-19: possible implications for viral mutagenesis. Eur. Respir. J. 60, 2102951 (2022).",
"volume": "60",
"year": "2022"
},
{
"DOI": "10.1126/science.aaz6964",
"author": "F Sampaziotis",
"doi-asserted-by": "publisher",
"first-page": "839",
"journal-title": "Science",
"key": "5594_CR14",
"unstructured": "Sampaziotis, F. et al. Cholangiocyte organoids can repair bile ducts after transplantation in the human liver. Science 371, 839–846 (2021).",
"volume": "371",
"year": "2021"
},
{
"DOI": "10.1038/s41596-019-0168-0",
"author": "OC Tysoe",
"doi-asserted-by": "publisher",
"first-page": "1884",
"journal-title": "Nat. Protoc.",
"key": "5594_CR15",
"unstructured": "Tysoe, O. C. et al. Isolation and propagation of primary human cholangiocyte organoids for the generation of bioengineered biliary tissue. Nat. Protoc. 14, 1884–1925 (2019).",
"volume": "14",
"year": "2019"
},
{
"DOI": "10.1038/nm.4360",
"author": "F Sampaziotis",
"doi-asserted-by": "publisher",
"first-page": "954",
"journal-title": "Nat. Med.",
"key": "5594_CR16",
"unstructured": "Sampaziotis, F. et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat. Med. 23, 954–963 (2017).",
"volume": "23",
"year": "2017"
},
{
"DOI": "10.1038/s41575-020-00404-2",
"author": "L Sun",
"doi-asserted-by": "publisher",
"first-page": "335",
"journal-title": "Nat. Rev. Gastroenterol. Hepatol.",
"key": "5594_CR17",
"unstructured": "Sun, L., Cai, J. & Gonzalez, F. J. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 18, 335–347 (2021).",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1126/science.1072891",
"author": "NL Urizar",
"doi-asserted-by": "publisher",
"first-page": "1703",
"journal-title": "Science",
"key": "5594_CR18",
"unstructured": "Urizar, N. L. et al. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science 296, 1703–1706 (2002).",
"volume": "296",
"year": "2002"
},
{
"DOI": "10.1038/s41591-018-0222-4",
"author": "L Sun",
"doi-asserted-by": "publisher",
"first-page": "1919",
"journal-title": "Nat. Med.",
"key": "5594_CR19",
"unstructured": "Sun, L. et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 24, 1919–1929 (2018).",
"volume": "24",
"year": "2018"
},
{
"DOI": "10.1111/resp.12815",
"author": "B Chen",
"doi-asserted-by": "publisher",
"first-page": "1075",
"journal-title": "Respirology",
"key": "5594_CR20",
"unstructured": "Chen, B. et al. Bile acids induce activation of alveolar epithelial cells and lung fibroblasts through farnesoid X receptor-dependent and independent pathways. Respirology 21, 1075–1080 (2016).",
"volume": "21",
"year": "2016"
},
{
"DOI": "10.1016/j.jsbmb.2017.01.010",
"author": "P Comeglio",
"doi-asserted-by": "publisher",
"first-page": "26",
"journal-title": "J. Steroid Biochem. Mol. Biol.",
"key": "5594_CR21",
"unstructured": "Comeglio, P. et al. Anti-fibrotic effects of chronic treatment with the selective FXR agonist obeticholic acid in the bleomycin-induced rat model of pulmonary fibrosis. J. Steroid Biochem. Mol. Biol. 168, 26–37 (2017).",
"volume": "168",
"year": "2017"
},
{
"DOI": "10.1016/j.jhep.2017.04.026",
"author": "P Fickert",
"doi-asserted-by": "publisher",
"first-page": "619",
"journal-title": "J. Hepatol.",
"key": "5594_CR22",
"unstructured": "Fickert, P. & Wagner, M. Biliary bile acids in hepatobiliary injury—what is the link? J. Hepatol. 67, 619–631 (2017).",
"volume": "67",
"year": "2017"
},
{
"DOI": "10.1038/s41591-020-0968-3",
"author": "A Gupta",
"doi-asserted-by": "publisher",
"first-page": "1017",
"journal-title": "Nat. Med.",
"key": "5594_CR23",
"unstructured": "Gupta, A. et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 26, 1017–1032 (2020).",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1016/j.stem.2020.10.004",
"author": "J Youk",
"doi-asserted-by": "publisher",
"first-page": "905",
"journal-title": "Cell Stem Cell",
"key": "5594_CR24",
"unstructured": "Youk, J. et al. Three-dimensional human alveolar stem cell culture models reveal infection response to SARS-CoV-2. Cell Stem Cell 27, 905–919 (2020).",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1126/science.abc1669",
"author": "MM Lamers",
"doi-asserted-by": "publisher",
"first-page": "50",
"journal-title": "Science",
"key": "5594_CR25",
"unstructured": "Lamers, M. M. et al. SARS-CoV-2 productively infects human gut enterocytes. Science 3, 50–54 (2020).",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1016/j.jhep.2017.03.022",
"author": "EASL Clinical Practice Guidelines.",
"doi-asserted-by": "publisher",
"first-page": "145",
"journal-title": "J. Hepatol.",
"key": "5594_CR26",
"unstructured": "EASL Clinical Practice Guidelines.The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 67, 145–172 (2017).",
"volume": "67",
"year": "2017"
},
{
"DOI": "10.1021/jm100860s",
"author": "R Sharma",
"doi-asserted-by": "publisher",
"first-page": "122",
"journal-title": "J. Med. Chem.",
"key": "5594_CR27",
"unstructured": "Sharma, R. et al. Ursodeoxycholic acid amides as novel glucocorticoid receptor modulators. J. Med. Chem. 54, 122–130 (2011).",
"volume": "54",
"year": "2011"
},
{
"DOI": "10.1371/journal.ppat.1010265",
"author": "PP Gerber",
"doi-asserted-by": "publisher",
"first-page": "e1010265",
"journal-title": "PLoS Pathog.",
"key": "5594_CR28",
"unstructured": "Gerber, P. P. et al. A protease-activatable luminescent biosensor and reporter cell line for authentic SARS-CoV-2 infection. PLoS Pathog. 18, e1010265 (2022).",
"volume": "18",
"year": "2022"
},
{
"DOI": "10.1002/jcph.1409",
"author": "S Lee",
"doi-asserted-by": "publisher",
"first-page": "1085",
"journal-title": "J. Clin. Pharmacol.",
"key": "5594_CR29",
"unstructured": "Lee, S. et al. Pharmacokinetics of ursodeoxycholic acid in elderly volunteers compared with younger adults in a Korean population. J. Clin. Pharmacol. 59, 1085–1092 (2019).",
"volume": "59",
"year": "2019"
},
{
"DOI": "10.1038/s41586-018-0047-9",
"author": "D Nasralla",
"doi-asserted-by": "publisher",
"first-page": "50",
"journal-title": "Nature",
"key": "5594_CR30",
"unstructured": "Nasralla, D. et al. A randomized trial of normothermic preservation in liver transplantation. Nature 557, 50–56 (2018).",
"volume": "557",
"year": "2018"
},
{
"DOI": "10.1093/ejcts/ezu228",
"author": "ASI Andreasson",
"doi-asserted-by": "publisher",
"first-page": "779",
"journal-title": "Eur. J. Cardiothorac. Surg.",
"key": "5594_CR31",
"unstructured": "Andreasson, A. S. I., Dark, J. H. & Fisher, A. J. Ex vivo lung perfusion in clinical lung transplantation—state of the art. Eur. J. Cardiothorac. Surg. 46, 779–788 (2014).",
"volume": "46",
"year": "2014"
},
{
"DOI": "10.1111/ajt.14687",
"author": "CJE Watson",
"doi-asserted-by": "publisher",
"first-page": "2005",
"journal-title": "Am. J. Transplant.",
"key": "5594_CR32",
"unstructured": "Watson, C. J. E. et al. Observations on the ex situ perfusion of livers for transplantation. Am. J. Transplant. 18, 2005–2020 (2018).",
"volume": "18",
"year": "2018"
},
{
"DOI": "10.1002/hep.32011",
"author": "B Barron‐Millar",
"doi-asserted-by": "publisher",
"first-page": "3269",
"journal-title": "Hepatology",
"key": "5594_CR33",
"unstructured": "Barron‐Millar, B. et al. The serum proteome and ursodeoxycholic acid response in primary biliary cholangitis. Hepatology 74, 3269–3283 (2021).",
"volume": "74",
"year": "2021"
},
{
"DOI": "10.1016/S2468-1253(20)30271-5",
"author": "GJ Webb",
"doi-asserted-by": "publisher",
"first-page": "1008",
"journal-title": "Lancet Gastroenterol. Hepatol.",
"key": "5594_CR34",
"unstructured": "Webb, G. J. et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol. Hepatol. 5, 1008–1016 (2020).",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.jhep.2020.09.024",
"author": "T Marjot",
"doi-asserted-by": "publisher",
"first-page": "567",
"journal-title": "J. Hepatol.",
"key": "5594_CR35",
"unstructured": "Marjot, T. et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J. Hepatol. 74, 567–577 (2021).",
"volume": "74",
"year": "2021"
},
{
"DOI": "10.1016/j.cgh.2022.05.038",
"author": "BV John",
"doi-asserted-by": "publisher",
"first-page": "2405",
"journal-title": "Clin. Gastroenterol. Hepatol.",
"key": "5594_CR36",
"unstructured": "John, B. V. et al. Effectiveness of COVID-19 viral vector Ad.26.COV2.S vaccine and comparison with mRNA vaccines in cirrhosis. Clin. Gastroenterol. Hepatol. 20, 2405–2408 (2022).",
"volume": "20",
"year": "2022"
},
{
"key": "5594_CR37",
"unstructured": "John, B. V. et al. Ursodeoxycholic acid use and outcomes of Coronavirus 2019 in patients with liver disease. Hepatology 76, S557–S558 (2022)."
},
{
"DOI": "10.1002/hep.31205",
"author": "K Patel",
"doi-asserted-by": "publisher",
"first-page": "58",
"journal-title": "Hepatology",
"key": "5594_CR38",
"unstructured": "Patel, K. et al. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology 72, 58–71 (2020).",
"volume": "72",
"year": "2020"
},
{
"DOI": "10.1172/JCI76738",
"author": "C Jiang",
"doi-asserted-by": "publisher",
"first-page": "386",
"journal-title": "J. Clin. Invest.",
"key": "5594_CR39",
"unstructured": "Jiang, C. et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest. 125, 386–402 (2015).",
"volume": "125",
"year": "2015"
},
{
"DOI": "10.1038/srep37234",
"author": "K Zhao",
"doi-asserted-by": "publisher",
"first-page": "37234",
"journal-title": "Sci. Rep.",
"key": "5594_CR40",
"unstructured": "Zhao, K. et al. Activation of FXR protects against renal fibrosis via suppressing Smad3 expression. Sci. Rep. 6, 37234 (2016).",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.1136/gut.2010.212159",
"author": "RM Gadaleta",
"doi-asserted-by": "publisher",
"first-page": "463",
"journal-title": "Gut",
"key": "5594_CR41",
"unstructured": "Gadaleta, R. M. et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60, 463–472 (2011).",
"volume": "60",
"year": "2011"
},
{
"DOI": "10.1210/en.2006-0701",
"author": "S Caron",
"doi-asserted-by": "publisher",
"first-page": "4022",
"journal-title": "Endocrinology",
"key": "5594_CR42",
"unstructured": "Caron, S., Cariou, B. & Staels, B. FXR: more than a bile acid receptor? Endocrinology 147, 4022–4024 (2006).",
"volume": "147",
"year": "2006"
},
{
"DOI": "10.1056/NEJMoa2035002",
"author": "DM Weinreich",
"doi-asserted-by": "publisher",
"first-page": "238",
"journal-title": "N. Engl. J. Med.",
"key": "5594_CR43",
"unstructured": "Weinreich, D. M. et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19. N. Engl. J. Med. 384, 238–251 (2021).",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2116620",
"author": "MJ Levin",
"doi-asserted-by": "publisher",
"first-page": "2188",
"journal-title": "N. Engl. J. Med.",
"key": "5594_CR44",
"unstructured": "Levin, M. J. et al. Intramuscular AZD7442 (tixagevimab–cilgavimab) for prevention of Covid-19. N. Engl. J. Med. 386, 2188–2200 (2022).",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-03291-y",
"author": "SA Kemp",
"doi-asserted-by": "publisher",
"first-page": "277",
"journal-title": "Nature",
"key": "5594_CR45",
"unstructured": "Kemp, S. A. et al. SARS-CoV-2 evolution during treatment of chronic infection. Nature 592, 277–282 (2021).",
"volume": "592",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2018.01853",
"author": "S Fiorucci",
"doi-asserted-by": "publisher",
"first-page": "1853",
"journal-title": "Front. Immunol.",
"key": "5594_CR46",
"unstructured": "Fiorucci, S., Biagioli, M., Zampella, A. & Distrutti, E. Bile acids activated receptors regulate innate immunity. Front. Immunol. 13, 1853 (2018).",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1038/s41575-021-00566-7",
"author": "CD Fuchs",
"doi-asserted-by": "publisher",
"first-page": "432",
"journal-title": "Nat. Rev. Gastroenterol. Hepatol.",
"key": "5594_CR47",
"unstructured": "Fuchs, C. D. & Trauner, M. Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat. Rev. Gastroenterol. Hepatol. 19, 432–450 (2022).",
"volume": "19",
"year": "2022"
},
{
"DOI": "10.1016/j.immuni.2020.05.002",
"author": "N Vabret",
"doi-asserted-by": "publisher",
"first-page": "910",
"journal-title": "Immunity",
"key": "5594_CR48",
"unstructured": "Vabret, N. et al. Immunology of COVID-19: current state of the science. Immunity 52, 910–941 (2020).",
"volume": "52",
"year": "2020"
},
{
"DOI": "10.1038/s41409-019-0705-z",
"author": "M Mohty",
"doi-asserted-by": "publisher",
"first-page": "485",
"journal-title": "Bone Marrow Transplant.",
"key": "5594_CR49",
"unstructured": "Mohty, M. et al. Prophylactic, preemptive, and curative treatment for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a position statement from an international expert group. Bone Marrow Transplant. 55, 485–495 (2020).",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1002/hep.31257",
"author": "J Qin",
"doi-asserted-by": "publisher",
"first-page": "1491",
"journal-title": "Hepatology",
"key": "5594_CR50",
"unstructured": "Qin, J. et al. Perioperative presentation of COVID-19 disease in a liver transplant recipient. Hepatology 72, 1491–1493 (2020).",
"volume": "72",
"year": "2020"
},
{
"DOI": "10.1038/s41596-019-0168-0",
"author": "OC Tysoe",
"doi-asserted-by": "publisher",
"first-page": "1884",
"journal-title": "Nat. Protoc.",
"key": "5594_CR51",
"unstructured": "Tysoe, O. C. et al. Isolation and propagation of primary human cholangiocyte organoids for the generation of bioengineered biliary tissue. Nat. Protoc. 14, 1884–1925 (2019).",
"volume": "14",
"year": "2019"
},
{
"DOI": "10.1002/ar.1091990412",
"author": "M Bjerknes",
"doi-asserted-by": "publisher",
"first-page": "565",
"journal-title": "Anat. Rec.",
"key": "5594_CR52",
"unstructured": "Bjerknes, M. & Cheng, H. Methods for the isolation of intact epithelium from the mouse intestine. Anat. Rec. 199, 565–574 (1981).",
"volume": "199",
"year": "1981"
},
{
"DOI": "10.1371/journal.ppat.1009246",
"author": "P Guido",
"doi-asserted-by": "publisher",
"first-page": "e1009246",
"journal-title": "PLoS Pathog.",
"key": "5594_CR53",
"unstructured": "Guido, P. et al. Furin cleavage of SARS-CoV-2 Spike promotes but is not essential for infection and cell–cell fusion. PLoS Pathog. 17, e1009246 (2021).",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1074/jbc.M112.443937",
"author": "N Balasubramaniyan",
"doi-asserted-by": "publisher",
"first-page": "13850",
"journal-title": "J. Biol. Chem.",
"key": "5594_CR54",
"unstructured": "Balasubramaniyan, N., Luo, Y., Sun, A. Q. & Suchy, F. J. SUMOylation of the farnesoid X receptor (FXR) regulates the expression of FXR target genes. J. Biol. Chem. 288, 13850–13862 (2013).",
"volume": "288",
"year": "2013"
},
{
"DOI": "10.1093/infdis/jiaa507",
"author": "EI Patterson",
"doi-asserted-by": "publisher",
"first-page": "1462",
"journal-title": "J. Infect. Dis.",
"key": "5594_CR55",
"unstructured": "Patterson, E. I. et al. Methods of inactivation of SARS-CoV-2 for downstream biological assays. J. Infect. Dis. 222, 1462–1467 (2020).",
"volume": "222",
"year": "2020"
},
{
"DOI": "10.1126/science.abd2985",
"author": "L Cantuti-Castelvetri",
"doi-asserted-by": "publisher",
"first-page": "856",
"journal-title": "Science",
"key": "5594_CR56",
"unstructured": "Cantuti-Castelvetri, L. et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370, 856–860 (2020).",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.12688/wellcomeopenres.15937.2",
"author": "S Sridhar",
"doi-asserted-by": "publisher",
"first-page": "110",
"journal-title": "Wellcome Open Res.",
"key": "5594_CR57",
"unstructured": "Sridhar, S. et al. A blueprint for the implementation of a validated approach for the detection of SARS-Cov2 in clinical samples in academic facilities. Wellcome Open Res. 5, 110 (2020).",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1053/gast.2001.25542",
"author": "P Fickert",
"doi-asserted-by": "publisher",
"first-page": "170",
"journal-title": "Gastroenterology",
"key": "5594_CR58",
"unstructured": "Fickert, P. et al. Effects of ursodeoxycholic and cholic acid feeding on hepatocellular transporter expression in mouse liver. Gastroenterology 121, 170–183 (2001).",
"volume": "121",
"year": "2001"
},
{
"DOI": "10.1016/j.healun.2019.01.1302",
"author": "MI Morrison",
"doi-asserted-by": "publisher",
"first-page": "S321",
"journal-title": "J. Heart Lung Transplant.",
"key": "5594_CR59",
"unstructured": "Morrison, M. I. et al. Use of phosphodiesterase inhibition during ex-vivo lung perfusion of donor lungs unsuitable for transplantation. J. Heart Lung Transplant. 38, S321 (2019).",
"volume": "38",
"year": "2019"
},
{
"DOI": "10.15252/embj.2018100300",
"author": "N Sachs",
"doi-asserted-by": "publisher",
"first-page": "e100300",
"journal-title": "EMBO J.",
"key": "5594_CR60",
"unstructured": "Sachs, N. et al. Long‐term expanding human airway organoids for disease modeling. EMBO J. 38, e100300 (2019).",
"volume": "38",
"year": "2019"
},
{
"DOI": "10.1016/j.jhep.2021.01.021",
"author": "T Marjot",
"doi-asserted-by": "publisher",
"first-page": "1335",
"journal-title": "J. Hepatol.",
"key": "5594_CR61",
"unstructured": "Marjot, T. et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J. Hepatol. 74, 1335–1343 (2021).",
"volume": "74",
"year": "2021"
}
],
"reference-count": 61,
"references-count": 61,
"relation": {
"has-review": [
{
"asserted-by": "object",
"id": "10.3410/f.742438702.793597353",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41586-022-05594-0"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy"
}