Ursodeoxycholic acid and severe COVID-19 outcomes in a cohort study using the OpenSAFELY platform
et al., Communications Medicine, doi:10.1038/s43856-024-00664-y, Dec 2023 (preprint)
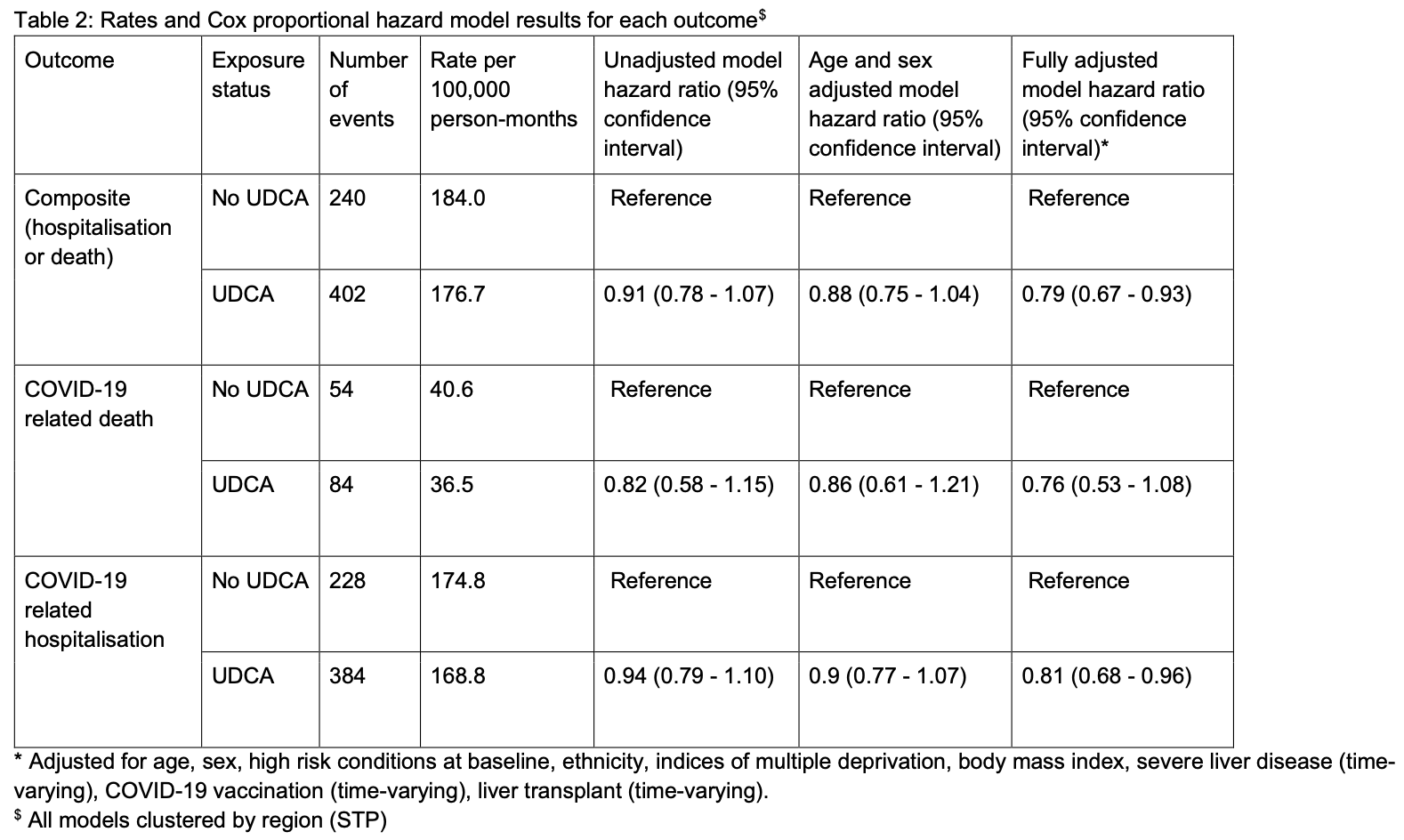
OpenSAFELY retrospective 11,305 primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC) patients showing lower risk of COVID-19 hospitalization or death with ursodeoxycholic acid (UDCA) treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments1.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 24.0% lower, HR 0.76, p = 0.13, treatment 7,225, control 4,080.
|
|
risk of hospitalization, 19.0% lower, HR 0.81, p = 0.02, treatment 7,225, control 4,080.
|
|
risk of death/hospitalization, 21.0% lower, HR 0.79, p = 0.005, treatment 7,225, control 4,080.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Costello et al., 13 Dec 2023, retrospective, United Kingdom, peer-reviewed, 19 authors, study period 1 March, 2020 - 31 December, 2022.
Ursodeoxycholic acid and severe COVID-19 outcomes in people with liver disease: a cohort study using the OpenSAFELY platform
doi:10.1101/2023.12.11.23299191
Biological evidence suggests ursodeoxycholic acid (UDCA) -a common treatment of cholestatic liver disease -may prevent severe COVID-19 outcomes. With the approval of NHS England, we conducted a population-based cohort study using primary care records, linked to death registration data and hospital records through the OpenSAFELY-TPP platform. We estimated the hazard of COVID-19 hospitalisation or death between 1 March 2020 and 31 December 2022, comparing UDCA treatment to no UDCA treatment in a population with indication. Of 11,320 eligible individuals, 642 were hospitalised or died with COVID-19 during follow-up, 402 (63%) events among UDCA users. After confounder adjustment, UDCA was associated with a 21% (95% CI 7%-33%) relative reduction in the hazard of COVID-19 hospitalisation or death, consistent with an absolute risk reduction of 1.3% (95% CI 1.0%-1.6%). Our findings support calls for clinical trials investigating UDCA as a preventative measure for severe COVID-19 outcomes. .
Conflicts of interest BG has received research funding from the Bennett Foundation, the Laura and John Arnold Foundation, the NHS National Institute for Health Research (NIHR), the NIHR School of Primary Care Research, NHS England, the NIHR Oxford Biomedical Research Centre, the Mohn-Westlake Foundation, NIHR Applied Research Collaboration Oxford and Thames Valley, the Wellcome Trust, the Good Thinking Foundation, Health Data Research UK, the Health Foundation, the World Health Organisation, UKRI MRC, Asthma UK, the British Lung Foundation, and the Longitudinal Health and Wellbeing strand of the National Core Studies programme; he is a Non-Executive Director at NHS Digital; he also receives personal income from speaking and writing for lay audiences on the misuse of science. BMK is also employed by NHS England working on medicines policy and clinical lead for primary care medicines data. AM is a member of RCGP health informatics group and the NHS Digital GP data Professional Advisory Group, and received consulting fee from Induction Healthcare. LAT has received research funding from MRC, Wellcome, NIHR and GSK, consulted for Bayer in relation to an observational study of chronic kidney disease (unpaid), and is a member of 4 non-industry funded (NIHR/MRC) trial advisory committees (unpaid) and MHRA Expert advisory group (Women's Health). REC has shares in AstraZeneca. VM received a grant from NIHR. AS is employed by LSHTM on a fellowship sponsored by GSK. JT received an..
References
Andrews, Schultze, Curtis, Hulme, Tazare et al., OpenSAFELY: Representativeness of electronic health record platform OpenSAFELY-TPP data compared to the population of England, Wellcome Open Res, doi:10.12688/wellcomeopenres.18010.1
Brevini, Maes, Webb, John, Fuchs et al., FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2, Nature, doi:10.1038/s41586-022-05594-0
Canales, Mayoral, Hernández-Huerta, Navarro, Cervantes et al., Interaction of Spike protein and lipid membrane of SARS-CoV-2 with Ursodeoxycholic acid, an in-silico analysis, Sci Rep, doi:10.1038/s41598-021-01705-5
Carey, Ali, Lindor, Primary biliary cirrhosis, The Lancet, doi:10.1016/S0140-6736(15)00154-3
Colapietro, Angelotti, Masetti, Shiffer, Pugliese et al., Ursodeoxycholic Acid Does Not Improve COVID-19 Outcome in Hospitalized Patients, Viruses, doi:10.3390/v15081738
De Vries, Groenwold, Bias of time-varying exposure effects due to time-varying covariate measurement strategies, Pharmacoepidemiol Drug Saf, doi:10.1002/pds.5328
Dyson, Beuers, Jones, Lohse, Hudson, Primary sclerosing cholangitis, The Lancet, doi:10.1016/S0140-6736(18)30300-3
Hu, Zhang, Huang, Shen, Feng et al., Effect of Ursodeoxycholic Acid on Preventing SARS-CoV-2 Infection in Patients With Liver Transplantation: a multicenter retrospective cohort study, QJM Mon J Assoc Physicians, doi:10.1093/qjmed/hcad254
John, Bastaich, Webb, Brevini, Moon et al., Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis, J Intern Med, doi:10.1111/joim.13630
Karlsen, Folseraas, Thorburn, Vesterhus, Primary sclerosing cholangitisa comprehensive review, J Hepatol, doi:10.1016/j.jhep.2017.07.022
Lamontagne, Agarwal, Rochwerg, Siemieniuk, Agoritsas et al., A living WHO guideline on drugs for covid-19, BMJ, doi:10.1136/bmj.m3379
Lee, Wong, Chai, Lee, Lee et al., Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis, BMJ, doi:10.1136/bmj-2021-068632
Li, Zhu, Cui, Lin, Li, Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease, Front Cell Infect Microbiol
Marrone, Covino, Merra, Piccioni, Amodeo et al., Ursodeoxycholic acid does not affect the clinical outcome of SARS-CoV-2 infection: A retrospective study of propensity score-matched cohorts, Liver Int n.d, doi:10.1111/liv.15736
Mclennan, Noble, Noble, Plunkett, Wright et al., English Indices of Deprivation
Ojeda-Fernández, Baviera, Macaluso, Schena, Tettamanti et al., UDCA treatment against COVID-19: Do we have enough clinical evidence for drug repurposing, J Intern Med n.d, doi:10.1111/joim.13711
Poupon, Lindor, Parés, Chazouillères, Poupon et al., Combined analysis of the effect of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis, J Hepatol, doi:10.1016/s0168-8278(03)00192-2
Rowan, Bates, Hulme, Evans, Davy et al., A comprehensive high cost drugs dataset from the NHS in England -An OpenSAFELY-TPP Short Data Report, Wellcome Open Res, doi:10.12688/wellcomeopenres.17360.1
Sivakumar, Gandhi, Shakweh, Li, Safinia et al., Widespread gaps in the quality of care for primary biliary cholangitis in UK, Frontline Gastroenterol, doi:10.1136/flgastro-2020-101713
Subramanian, Iles, Ikramuddin, Steer, Merit of an Ursodeoxycholic Acid Clinical Trial in COVID-19 Patients, Vaccines, doi:10.3390/vaccines8020320
Thuy, Bao, Moon, Ursodeoxycholic acid ameliorates cell migration retarded by the SARS-CoV-2 spike protein in BEAS-2B human bronchial epithelial cells, Biomed Pharmacother, doi:10.1016/j.biopha.2022.113021
Williamson, Walker, Bhaskaran, Bacon, Bates et al., Factors associated with COVID-19-related death using OpenSAFELY, Nature, doi:10.1038/s41586-020-2521-4
You, Ma, Efe, Wang, Jeong et al., APASL clinical practice guidance: the diagnosis and management of patients with primary biliary cholangitis, Hepatol Int, doi:10.1007/s12072-021-10276-6
DOI record:
{
"DOI": "10.1038/s43856-024-00664-y",
"ISSN": [
"2730-664X"
],
"URL": "http://dx.doi.org/10.1038/s43856-024-00664-y",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Biological evidence suggests ursodeoxycholic acid (UDCA)—a common treatment of cholestatic liver disease—may prevent severe COVID-19 outcomes. We aimed to compare the hazard of COVID-19 hospitalisation or death between UDCA users versus non-users in a population with primary biliary cholangitis (PBC) or primary sclerosing cholangitis (PSC).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>With the approval of NHS England, we conducted a population-based cohort study using primary care records between 1 March 2020 and 31 December 2022, linked to death registration data and hospital records through the OpenSAFELY-TPP platform. Cox proportional hazards regression was used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the association between time-varying UDCA exposure and COVID-19 related hospitalisation or death, stratified by geographical region and considering models unadjusted and fully adjusted for pre-specified confounders.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>We identify 11,305 eligible individuals, 640 were hospitalised or died with COVID-19 during follow-up, 400 (63%) events among UDCA users. After confounder adjustment, UDCA is associated with a 21% relative reduction in the hazard of COVID-19 hospitalisation or death (HR 0.79, 95% CI 0.67–0.93), consistent with an absolute risk reduction of 1.35% (95% CI 1.07%–1.69%).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>We found evidence that UDCA is associated with a lower hazard of COVID-19 related hospitalisation and death, support calls for clinical trials investigating UDCA as a preventative measure for severe COVID-19 outcomes.</jats:p>\n </jats:sec>",
"alternative-id": [
"664"
],
"article-number": "238",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "15 January 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "5 November 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "19 November 2024"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "B.G. has received research funding from the Bennett Foundation, the Laura and John Arnold Foundation, the NHS National Institute for Health Research (NIHR), the NIHR School of Primary Care Research, NHS England, the NIHR Oxford Biomedical Research Centre, the Mohn–Westlake Foundation, NIHR Applied Research Collaboration Oxford and Thames Valley, the Wellcome Trust, the Good Thinking Foundation, Health Data Research UK, the Health Foundation, the World Health Organisation, UKRI MRC, Asthma UK, the British Lung Foundation, and the Longitudinal Health and Wellbeing strand of the National Core Studies programme; he is a Non-Executive Director at NHS Digital; he also receives personal income from speaking and writing for lay audiences on the misuse of science. BMK is also employed by NHS England working on medicines policy and clinical lead for primary care medicines data. A.M. is a member of RCGP health informatics group and the NHS Digital GP data Professional Advisory Group, and received consulting fee from Induction Healthcare. L.A.T. has received research funding from MRC, Wellcome, NIHR and GSK, consulted for Bayer in relation to an observational study of chronic kidney disease (unpaid), and is a member of 4 non-industry funded (NIHR/MRC) trial advisory committees (unpaid) and MHRA Expert advisory group (Women’s Health). R.E.C. has shares in AstraZeneca. V.M. received a grant from NIHR. A.S. is employed by LSHTM on a fellowship sponsored by GSK. J.T. received an AstraZeneca grant for unrelated COVID-19 research. G.F.M. and R.S. have received research funding from Intercept Pharmaceuticals and Advanz Pharma. All other authors declare no conflicts of interest."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-2709-6666",
"affiliation": [],
"authenticated-orcid": false,
"family": "Costello",
"given": "Ruth E.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Waller",
"given": "Karen M. J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Rachel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mells",
"given": "George F.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8618-7333",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wong",
"given": "Angel Y. S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schultze",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahalingasivam",
"given": "Viyaasan",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9425-644X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Herrett",
"given": "Emily",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zheng",
"given": "Bang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Liang-Yu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "MacKenna",
"given": "Brian",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2098-1278",
"affiliation": [],
"authenticated-orcid": false,
"family": "Mehrkar",
"given": "Amir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bacon",
"given": "Sebastian C. J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goldacre",
"given": "Ben",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8848-9493",
"affiliation": [],
"authenticated-orcid": false,
"family": "Tomlinson",
"given": "Laurie A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tazare",
"given": "John",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1408-7907",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rentsch",
"given": "Christopher T.",
"sequence": "additional"
},
{
"affiliation": [],
"name": "the OpenSAFELY collaborative",
"sequence": "additional"
},
{
"affiliation": [],
"name": "the LH&W NCS (or CONVALESCENCE) Collaborative",
"sequence": "additional"
}
],
"container-title": "Communications Medicine",
"container-title-short": "Commun Med",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
11,
19
]
],
"date-time": "2024-11-19T11:41:38Z",
"timestamp": 1732016498000
},
"deposited": {
"date-parts": [
[
2024,
11,
20
]
],
"date-time": "2024-11-20T00:08:20Z",
"timestamp": 1732061300000
},
"funder": [
{
"DOI": "10.13039/501100000272",
"award": [
"COV-LT-0009",
"NIHR135559"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000272",
"id-type": "DOI"
}
],
"name": "DH | National Institute for Health Research"
},
{
"name": "National core studies programme"
}
],
"indexed": {
"date-parts": [
[
2024,
11,
20
]
],
"date-time": "2024-11-20T05:41:42Z",
"timestamp": 1732081302948,
"version": "3.28.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
11,
19
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
11,
19
]
],
"date-time": "2024-11-19T00:00:00Z",
"timestamp": 1731974400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
11,
19
]
],
"date-time": "2024-11-19T00:00:00Z",
"timestamp": 1731974400000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s43856-024-00664-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s43856-024-00664-y",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s43856-024-00664-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2024,
11,
19
]
]
},
"published-online": {
"date-parts": [
[
2024,
11,
19
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1007/s12072-021-10276-6",
"author": "H You",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Hepatol. Int.",
"key": "664_CR1",
"unstructured": "You, H. et al. APASL clinical practice guidance: the diagnosis and management of patients with primary biliary cholangitis. Hepatol. Int. 16, 1–23 (2022).",
"volume": "16",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(15)00154-3",
"author": "EJ Carey",
"doi-asserted-by": "publisher",
"first-page": "1565",
"journal-title": "Lancet",
"key": "664_CR2",
"unstructured": "Carey, E. J., Ali, A. H. & Lindor, K. D. Primary biliary cirrhosis. Lancet 386, 1565–1575 (2015).",
"volume": "386",
"year": "2015"
},
{
"DOI": "10.1016/j.jhep.2017.07.022",
"author": "TH Karlsen",
"doi-asserted-by": "publisher",
"first-page": "1298",
"journal-title": "J. Hepatol.",
"key": "664_CR3",
"unstructured": "Karlsen, T. H., Folseraas, T., Thorburn, D. & Vesterhus, M. Primary sclerosing cholangitis – a comprehensive review. J. Hepatol. 67, 1298–1323 (2017).",
"volume": "67",
"year": "2017"
},
{
"DOI": "10.1016/S0140-6736(18)30300-3",
"author": "JK Dyson",
"doi-asserted-by": "publisher",
"first-page": "2547",
"journal-title": "Lancet",
"key": "664_CR4",
"unstructured": "Dyson, J. K., Beuers, U., Jones, D. E. J., Lohse, A. W. & Hudson, M. Primary sclerosing cholangitis. Lancet 391, 2547–2559 (2018).",
"volume": "391",
"year": "2018"
},
{
"DOI": "10.1016/S0168-8278(03)00192-2",
"author": "RE Poupon",
"doi-asserted-by": "publisher",
"first-page": "12",
"journal-title": "J. Hepatol.",
"key": "664_CR5",
"unstructured": "Poupon, R. E. et al. Combined analysis of the effect of treatment with ursodeoxycholic acid on histologic progression in primary biliary cirrhosis. J. Hepatol. 39, 12–16 (2003).",
"volume": "39",
"year": "2003"
},
{
"DOI": "10.1136/flgastro-2020-101713",
"author": "M Sivakumar",
"doi-asserted-by": "publisher",
"first-page": "32",
"journal-title": "Frontline Gastroenterol.",
"key": "664_CR6",
"unstructured": "Sivakumar, M. et al. Widespread gaps in the quality of care for primary biliary cholangitis in UK. Frontline Gastroenterol. 13, 32–38 (2022).",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41586-022-05594-0",
"doi-asserted-by": "publisher",
"key": "664_CR7",
"unstructured": "Brevini T. et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature,1–9 https://doi.org/10.1038/s41586-022-05594-0 (2022)."
},
{
"DOI": "10.1038/s41598-021-01705-5",
"author": "FJ Rodal Canales",
"doi-asserted-by": "publisher",
"journal-title": "Sci. Rep.",
"key": "664_CR8",
"unstructured": "Rodal Canales, F. J. et al. Interaction of Spike protein and lipid membrane of SARS-CoV-2 with Ursodeoxycholic acid, an in-silico analysis. Sci. Rep. 11, 22288 (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.biopha.2022.113021",
"author": "PX Thuy",
"doi-asserted-by": "publisher",
"first-page": "113021",
"journal-title": "Biomed. Pharmacother.",
"key": "664_CR9",
"unstructured": "Thuy, P. X., Bao, T. D. D. & Moon, E.-Y. Ursodeoxycholic acid ameliorates cell migration retarded by the SARS-CoV-2 spike protein in BEAS-2B human bronchial epithelial cells. Biomed. Pharmacother. 150, 113021 (2022).",
"volume": "150",
"year": "2022"
},
{
"DOI": "10.1111/joim.13630",
"author": "BV John",
"doi-asserted-by": "publisher",
"first-page": "636",
"journal-title": "J. Intern. Med.",
"key": "664_CR10",
"unstructured": "John, B. V. et al. Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis. J. Intern. Med. 293, 636–647 (2023).",
"volume": "293",
"year": "2023"
},
{
"DOI": "10.3389/fcimb.2023.1178590",
"author": "Y Li",
"doi-asserted-by": "publisher",
"first-page": "1178590",
"journal-title": "Front. Cell. Infect. Microbiol.",
"key": "664_CR11",
"unstructured": "Li, Y., Zhu, N., Cui, X., Lin, Y. & Li, X. Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease. Front. Cell. Infect. Microbiol. 13, 1178590 (2023).",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1111/joim.13711",
"doi-asserted-by": "publisher",
"key": "664_CR12",
"unstructured": "Ojeda-Fernández L. et al. UDCA treatment against COVID-19: do we have enough clinical evidence for drug repurposing? J. Intern. Med.https://doi.org/10.1111/joim.13711."
},
{
"DOI": "10.1111/liv.15736",
"doi-asserted-by": "publisher",
"key": "664_CR13",
"unstructured": "Marrone G. et al. Ursodeoxycholic acid does not affect the clinical outcome of SARS-CoV-2 infection: a retrospective study of propensity score-matched cohorts. Liver Int.https://doi.org/10.1111/liv.15736."
},
{
"DOI": "10.3390/v15081738",
"author": "F Colapietro",
"doi-asserted-by": "publisher",
"first-page": "1738",
"journal-title": "Viruses",
"key": "664_CR14",
"unstructured": "Colapietro, F. et al. Ursodeoxycholic acid does not improve COVID-19 outcome in hospitalized patients. Viruses 15, 1738 (2023).",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1093/qjmed/hcad254",
"doi-asserted-by": "crossref",
"key": "664_CR15",
"unstructured": "Hu L. et al. Effect of ursodeoxycholic acid on preventing SARS-CoV-2 infection in patients with liver transplantation: a multicenter retrospective cohort study. QJM, 117, 339–347 (2024)."
},
{
"DOI": "10.12688/wellcomeopenres.18010.1",
"author": "C Andrews",
"doi-asserted-by": "publisher",
"journal-title": "Wellcome Open Res.",
"key": "664_CR16",
"unstructured": "Andrews, C. et al. OpenSAFELY: representativeness of electronic health record platform OpenSAFELY-TPP data compared to the population of England. Wellcome Open Res.https://doi.org/10.12688/wellcomeopenres.18010.1 (2022).",
"year": "2022"
},
{
"key": "664_CR17",
"unstructured": "McLennan D. et al. English Indices of Deprivation 2019: technical report. https://www.gov.uk/government/publications/english-indices-of-deprivation-2019-technical-report."
},
{
"DOI": "10.12688/wellcomeopenres.17360.1",
"author": "A Rowan",
"doi-asserted-by": "publisher",
"first-page": "360",
"journal-title": "Wellcome Open Res.",
"key": "664_CR18",
"unstructured": "Rowan, A. et al. A comprehensive high cost drugs dataset from the NHS in England - an OpenSAFELY-TPP short data report. Wellcome Open Res. 6, 360 (2021).",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1002/pds.5328",
"author": "BBL Penning de Vries",
"doi-asserted-by": "publisher",
"first-page": "22",
"journal-title": "Pharmacoepidemiol. Drug Saf.",
"key": "664_CR19",
"unstructured": "Penning de Vries, B. B. L. & Groenwold, R. H. H. Bias of time-varying exposure effects due to time-varying covariate measurement strategies. Pharmacoepidemiol. Drug Saf. 31, 22–27 (2022).",
"volume": "31",
"year": "2022"
},
{
"DOI": "10.1038/s41586-020-2521-4",
"author": "EJ Williamson",
"doi-asserted-by": "publisher",
"first-page": "430",
"journal-title": "Nature",
"key": "664_CR20",
"unstructured": "Williamson, E. J. et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 584, 430–436 (2020).",
"volume": "584",
"year": "2020"
},
{
"key": "664_CR21",
"unstructured": "COVID-19 vaccine surveillance report - week 39."
},
{
"key": "664_CR22",
"unstructured": "The NHS England OpenSAFELY COVID-19 service - privacy notice. NHS Digit (accessed 24 August 2023); https://digital.nhs.uk/coronavirus/coronavirus-covid-19-response-information-governance-hub/the-nhs-england-opensafely-covid-19-service-privacy-notice."
},
{
"key": "664_CR23",
"unstructured": "Data Security and Protection Toolkit. NHS Digit (accessed 16 August 2023); https://digital.nhs.uk/data-and-information/looking-after-information/data-security-and-information-governance/data-security-and-protection-toolkit."
},
{
"DOI": "10.1136/bmj-2021-068632",
"author": "ARYB Lee",
"doi-asserted-by": "publisher",
"first-page": "e068632",
"journal-title": "BMJ",
"key": "664_CR24",
"unstructured": "Lee, A. R. Y. B., Wong, S. Y., Chai, L. Y. A., Lee, S. C., Lee, M. X. & Muthiah, M. D. et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ 376, e068632 (2022).",
"volume": "376",
"year": "2022"
},
{
"author": "F Lamontagne",
"first-page": "m3379",
"journal-title": "BMJ",
"key": "664_CR25",
"unstructured": "Lamontagne, F. et al. A living WHO guideline on drugs for covid-19. BMJ 370, m3379 (2020).",
"volume": "370",
"year": "2020"
},
{
"key": "664_CR26",
"unstructured": "Ursodeoxycholic acid | Interactions | BNF content published by NICE (accessed 24 April 2024); https://bnf.nice.org.uk/interactions/ursodeoxycholic-acid/."
},
{
"DOI": "10.1001/jamanetworkopen.2022.7970",
"author": "V Conti",
"doi-asserted-by": "publisher",
"first-page": "e227970",
"journal-title": "JAMA Netw. Open",
"key": "664_CR27",
"unstructured": "Conti, V. et al. Identification of drug interaction adverse events in patients with COVID-19: a systematic review. JAMA Netw. Open 5, e227970 (2022).",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.3390/vaccines8020320",
"author": "S Subramanian",
"doi-asserted-by": "publisher",
"first-page": "320",
"journal-title": "Vaccines",
"key": "664_CR28",
"unstructured": "Subramanian, S., Iles, T., Ikramuddin, S. & Steer, C. J. Merit of an ursodeoxycholic acid clinical trial in COVID-19 patients. Vaccines 8, 320 (2020).",
"volume": "8",
"year": "2020"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s43856-024-00664-y"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Ursodeoxycholic acid and severe COVID-19 outcomes in a cohort study using the OpenSAFELY platform",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "4"
}