Ursodeoxycholic Acid Does Not Improve COVID-19 Outcome in Hospitalized Patients
et al., Viruses, doi:10.3390/v15081738, Aug 2023
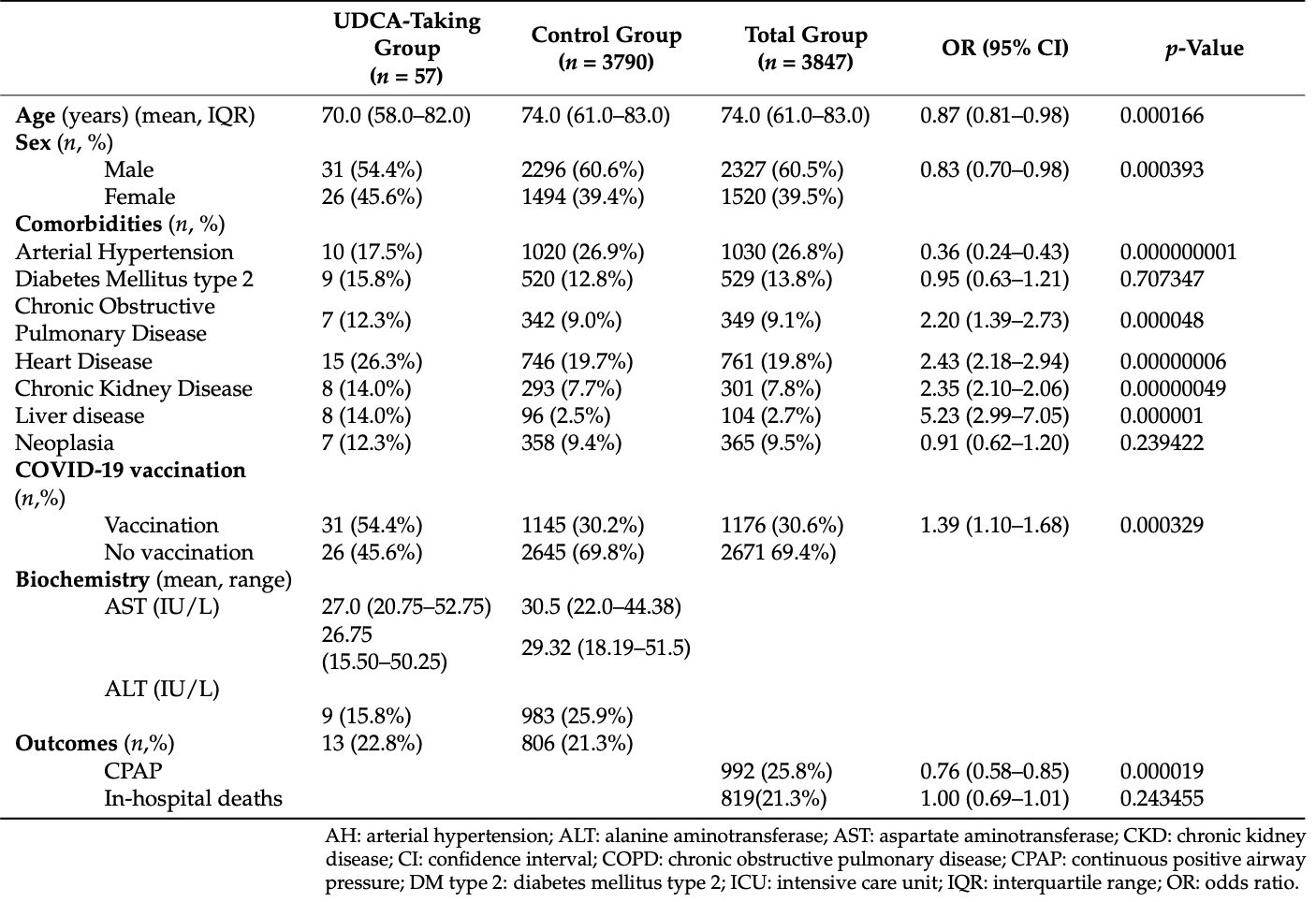
Retrospective 3,847 COVID-19 patients hospitalized in Italy, including 57 treated with UDCA. UDCA treatment was not associated with reduced mortality, however treatment was associated with a lower rate of CPAP use. It's not clear how the upper confidence interval for mortality can be so much closer to the point estimate (0.01 vs. 0.31). Conflicting values are given for the CPAP OR 0.88 and 0.76.
|
risk of death, no change, OR 1.00, p = 0.24, treatment 57, control 3,790, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Colapietro et al., 14 Aug 2023, retrospective, Italy, peer-reviewed, 19 authors, study period January 2020 - January 2023.
Contact: francesca.colapietro@humanitas.it (corresponding author), antonio.desai@humanitas.it, michele.bartoletti@humanitas.it, alessio.aghemo@hunimed.eu, monica.ormas@humanitas.it, paolo.omodei@humanitas.it.
Ursodeoxycholic Acid Does Not Improve COVID-19 Outcome in Hospitalized Patients
Viruses, doi:10.3390/v15081738
Ursodeoxycholic acid (UDCA) was demonstrated to reduce susceptibility to SARS-CoV-2 infection in vitro and improve infection course in chronic liver diseases. However, real-life evidence is lacking. We analyzed the impact of UDCA on COVID-19 outcomes in patients hospitalized in a tertiary center. Between January 2020 and January 2023, among 3847 patients consecutively hospitalized for COVID19, 57 (=UDCA group) were taking UDCA. The UDCA and the control groups (n = 3790) did not differ concerning comorbidities including diabetes mellitus type 2 (15.8% vs. 12.8%) and neoplasia (12.3% vs. 9.4%). Liver diseases and vaccination rate were more common in the UDCA group (14.0% vs. 2.5% and 54.4% vs. 30.2%, respectively). Overall mortality and CPAP treatment were 22.8 % and 15.7% in the UDCA, and 21.3% and 25.9% in the control group. Mortality was similar (p = 0.243), whereas UDCA was associated with a lower rate of CPAP treatment (OR = 0.76, p < 0.05). Treatment with UDCA was not an independent predictor of survival in patients hospitalized for COVID-19.
Funding: The authors did not receive any financial support in order to complete the study or write the manuscript.
Informed Consent Statement: Not applicable.
Conflicts of Interest: All the authors have given substantial contribution to the completion of this work and have seen and approved the text in the current version. None reported a conflict of interest with respect to this manuscript.
Abbreviations UDCA: ursodeoxycholic acid; PBC: primary biliary cholangitis; CPAP: continuous positive airway pressure; HER: electronic health record; SQL: Structured Query Language; AH: arterial hypertension; DM: diabetes mellitus; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; LFT: liver function test; PLT: platelets; OR: odds ratio; CI: confidence interval; ACE2: angiotensin-converting enzyme 2; FXR: farnesoid X receptor; ICU: intensive care unit; IQR: interquartile range.
References
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of COVID-19-Final Report, N. Engl. J. Med, doi:10.1056/NEJMoa2007764
Brevini, Maes, Webb, John, Fuchs et al., FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2, Nature, doi:10.1038/s41586-022-05594-0
Burki, The future of Paxlovid for COVID-19, Lancet Respir. Med, doi:10.1016/S2213-2600(22)00192-8
Cao, Wang, Jian, Xiao, Song et al., Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies, Nature, doi:10.1038/s41586-021-04385-3
Foisy, Yakiwchuk, Hughes, Induction effects of ritonavir: Implications for drug interactions, Ann. Pharmacother, doi:10.1345/aph.1K615
Goldman, Lye, Hui, Marks, Bruno et al., Remdesivir for 5 or 10 Days in Patients with Severe COVID-19, N. Engl. J. Med, doi:10.1056/NEJMoa2015301
Hofmann, Pharmacology of ursodeoxycholic acid, an enterohepatic drug, Scand. J. Gastroenterol, doi:10.3109/00365529409103618
John, Bastaich, Webb, Brevini, Moon et al., Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis, J. Intern. Med, doi:10.1111/joim.13630
Jones, Lee, Beschorner, Vogel, Grochow et al., Venoocclusive disease of the liver following bone marrow transplantation, Transplantation, doi:10.1097/00007890-198712000-00011
Li, Zhu, Cui, Lin, Li, Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease, Front. Cell. Infect. Microbiol, doi:10.3389/fcimb.2023.1178590
Liu, Wang, Ursodeoxycholic acid administration did not reduce susceptibility to SARS-CoV-2 infection in children, Liver Int, doi:10.1111/liv.15660
Lleo, Wang, Gershwin, Hirschfield, Primary biliary cholangitis, Lancet, doi:10.1016/S0140-6736(20)31607-X
Menéndez-Arias, Decoding molnupiravir-induced mutagenesis in SARS-CoV-2, J. Biol. Chem, doi:10.1016/j.jbc.2021.100867
Mohty, Malard, Abecasis, Aerts, Alaskar et al., Prophylactic, preemptive, and curative treatment for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: A position statement from an international expert group, Bone Marrow Transplant, doi:10.1038/s41409-019-0705-z
Mohty, Malard, Abecassis, Aerts, Alaskar et al., Sinusoidal obstruction syndrome/veno-occlusive disease: Current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT), Bone Marrow Transplant, doi:10.1038/bmt.2015.52
Pan, Peto, Henao-Restrepo, Preziosi, Sathiyamoorthy et al., Repurposed Antiviral Drugs for COVID-19-Interim WHO Solidarity Trial Results, N. Engl. J. Med, doi:10.1056/NEJMoa2023184
Rosas, Diaz, Gottlieb, Lobo, Robinson et al., Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: A randomized clinical trial, Intensive Care Med, doi:10.1007/s00134-021-06507-x
Sun, Lin, Wang, Gao, Ye, Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection, Lancet Infect. Dis, doi:10.1016/S1473-3099(22)00430-3
Tomalka, Suthar, Deeks, Sekaly, Fighting the SARS-CoV-2 pandemic requires a global approach to understanding the heterogeneity of vaccine responses, Nat. Immunol, doi:10.1038/s41590-022-01130-4
Wang, Yang, Song, Oral GS-441524 derivatives: Next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase, Front. Immunol, doi:10.3389/fimmu.2022.1015355
Wang, Zhang, Du, Du, Zhao et al., Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial, Lancet, doi:10.1016/S0140-6736(20)31022-9
Ward, Brogden, Heel, Speight, Avery, Ursodeoxycholic acid: A review of its pharmacological properties and therapeutic efficacy, Drugs, doi:10.2165/00003495-198427020-00001
Yang, Wang, Bench-to-bedside: Innovation of small molecule anti-SARS-CoV-2 drugs in China, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2023.115503
DOI record:
{
"DOI": "10.3390/v15081738",
"ISSN": [
"1999-4915"
],
"URL": "http://dx.doi.org/10.3390/v15081738",
"abstract": "<jats:p>Ursodeoxycholic acid (UDCA) was demonstrated to reduce susceptibility to SARS-CoV-2 infection in vitro and improve infection course in chronic liver diseases. However, real-life evidence is lacking. We analyzed the impact of UDCA on COVID-19 outcomes in patients hospitalized in a tertiary center. Between January 2020 and January 2023, among 3847 patients consecutively hospitalized for COVID19, 57 (=UDCA group) were taking UDCA. The UDCA and the control groups (n = 3790) did not differ concerning comorbidities including diabetes mellitus type 2 (15.8% vs. 12.8%) and neoplasia (12.3% vs. 9.4%). Liver diseases and vaccination rate were more common in the UDCA group (14.0% vs. 2.5% and 54.4% vs. 30.2%, respectively). Overall mortality and CPAP treatment were 22.8 % and 15.7% in the UDCA, and 21.3% and 25.9% in the control group. Mortality was similar (p = 0.243), whereas UDCA was associated with a lower rate of CPAP treatment (OR = 0.76, p < 0.05). Treatment with UDCA was not an independent predictor of survival in patients hospitalized for COVID-19.</jats:p>",
"alternative-id": [
"v15081738"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7520-744X",
"affiliation": [
{
"name": "Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, 20072 Milan, Italy"
},
{
"name": "Division of Internal Medicine and Hepatology, Department of Gastroenterology, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"authenticated-orcid": false,
"family": "Colapietro",
"given": "Francesca",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, 20072 Milan, Italy"
},
{
"name": "Humanitas Artificial Intelligence Center, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"family": "Angelotti",
"given": "Giovanni",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Internal Medicine and Hepatology, Department of Gastroenterology, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"family": "Masetti",
"given": "Chiara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, 20072 Milan, Italy"
},
{
"name": "Emergency Department, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"family": "Shiffer",
"given": "Dana",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-6466-1412",
"affiliation": [
{
"name": "Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, 20072 Milan, Italy"
},
{
"name": "Division of Internal Medicine and Hepatology, Department of Gastroenterology, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"authenticated-orcid": false,
"family": "Pugliese",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Internal Medicine and Hepatology, Department of Gastroenterology, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"family": "De Nicola",
"given": "Stella",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, 20072 Milan, Italy"
}
],
"family": "Carella",
"given": "Francesco",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4674-4905",
"affiliation": [
{
"name": "Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, 20072 Milan, Italy"
},
{
"name": "Emergency Department, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"authenticated-orcid": false,
"family": "Desai",
"given": "Antonio",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Internal Medicine and Hepatology, Department of Gastroenterology, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"family": "Ormas",
"given": "Monica",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4204-758X",
"affiliation": [
{
"name": "Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, 20072 Milan, Italy"
},
{
"name": "Nephrology and Dialysis Unit, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"authenticated-orcid": false,
"family": "Calatroni",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology, Division of Gastroenterology and Digestive Endoscopy, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"family": "Omodei",
"given": "Paolo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Respiratory Medicine, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"family": "Ciccarelli",
"given": "Michele",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, 20072 Milan, Italy"
},
{
"name": "Division of Respiratory Medicine, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"family": "Aliberti",
"given": "Stefano",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, 20072 Milan, Italy"
},
{
"name": "Nephrology and Dialysis Unit, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"family": "Reggiani",
"given": "Francesco",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, 20072 Milan, Italy"
},
{
"name": "Infectious Disease Unit, Humanitas Research Hospital IRCSS, 20089 Milan, Italy"
}
],
"family": "Bartoletti",
"given": "Michele",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, 20072 Milan, Italy"
},
{
"name": "Department of Anaesthesia and Intensive Care, Humanitas University IRCCS, 20090 Milan, Italy"
}
],
"family": "Cecconi",
"given": "Maurizio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0561-7902",
"affiliation": [
{
"name": "Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, 20072 Milan, Italy"
},
{
"name": "Division of Internal Medicine and Hepatology, Department of Gastroenterology, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"authenticated-orcid": false,
"family": "Lleo",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, 20072 Milan, Italy"
},
{
"name": "Division of Internal Medicine and Hepatology, Department of Gastroenterology, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"family": "Aghemo",
"given": "Alessio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7309-6929",
"affiliation": [
{
"name": "Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, 20072 Milan, Italy"
},
{
"name": "Emergency Department, Humanitas Research Hospital IRCCS, 20089 Milan, Italy"
}
],
"authenticated-orcid": false,
"family": "Voza",
"given": "Antonio",
"sequence": "additional"
}
],
"container-title": "Viruses",
"container-title-short": "Viruses",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
8,
14
]
],
"date-time": "2023-08-14T14:20:14Z",
"timestamp": 1692022814000
},
"deposited": {
"date-parts": [
[
2023,
8,
15
]
],
"date-time": "2023-08-15T15:40:14Z",
"timestamp": 1692114014000
},
"indexed": {
"date-parts": [
[
2023,
9,
22
]
],
"date-time": "2023-09-22T07:49:53Z",
"timestamp": 1695368993154
},
"is-referenced-by-count": 1,
"issue": "8",
"issued": {
"date-parts": [
[
2023,
8,
14
]
]
},
"journal-issue": {
"issue": "8",
"published-online": {
"date-parts": [
[
2023,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
14
]
],
"date-time": "2023-08-14T00:00:00Z",
"timestamp": 1691971200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4915/15/8/1738/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "1738",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
8,
14
]
]
},
"published-online": {
"date-parts": [
[
2023,
8,
14
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref_1",
"unstructured": "(2023, February 16). Therapeutics and COVID-19. Available online: https://www.who.int/teams/health-care-readiness/COVID-19/therapeutics."
},
{
"DOI": "10.1016/j.ejmech.2023.115503",
"article-title": "Bench-to-bedside: Innovation of small molecule anti-SARS-CoV-2 drugs in China",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "115503",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_2",
"volume": "257",
"year": "2023"
},
{
"DOI": "10.1038/s41590-022-01130-4",
"article-title": "Fighting the SARS-CoV-2 pandemic requires a global approach to understanding the heterogeneity of vaccine responses",
"author": "Tomalka",
"doi-asserted-by": "crossref",
"first-page": "360",
"journal-title": "Nat. Immunol.",
"key": "ref_3",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the Treatment of COVID-19—Final Report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N. Engl. J. Med.",
"key": "ref_4",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2015301",
"article-title": "Remdesivir for 5 or 10 Days in Patients with Severe COVID-19",
"author": "Goldman",
"doi-asserted-by": "crossref",
"first-page": "1827",
"journal-title": "N. Engl. J. Med.",
"key": "ref_5",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1569",
"journal-title": "Lancet",
"key": "ref_6",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1007/s00134-021-06507-x",
"article-title": "Tocilizumab and remdesivir in hospitalized patients with severe COVID-19 pneumonia: A randomized clinical trial",
"author": "Rosas",
"doi-asserted-by": "crossref",
"first-page": "1258",
"journal-title": "Intensive Care Med.",
"key": "ref_7",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2023184",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "WHO Solidarity Trial Consortium, Pan, H., Peto, R., Henao-Restrepo, A.-M., Preziosi, M.-P., Sathiyamoorthy, V., Abdool Karim, Q., Alejandria, M.M., Hernández García, C., and Kieny, M.-P.. (2021). Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med., 384, 497–511."
},
{
"DOI": "10.3389/fimmu.2022.1015355",
"article-title": "Oral GS-441524 derivatives: Next-generation inhibitors of SARS-CoV-2 RNA-dependent RNA polymerase",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "1015355",
"journal-title": "Front. Immunol.",
"key": "ref_9",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00430-3",
"article-title": "Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "1279",
"journal-title": "Lancet Infect. Dis.",
"key": "ref_10",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(22)00192-8",
"article-title": "The future of Paxlovid for COVID-19",
"author": "Burki",
"doi-asserted-by": "crossref",
"first-page": "e68",
"journal-title": "Lancet Respir. Med.",
"key": "ref_11",
"volume": "10",
"year": "2022"
},
{
"key": "ref_12",
"unstructured": "(2023, July 28). Multiple Pathways for SARS-CoV-2 Resistance to Nirmatrelvir|Nature. Available online: https://www.nature.com/articles/s41586-022-05514-2."
},
{
"DOI": "10.1345/aph.1K615",
"article-title": "Induction effects of ritonavir: Implications for drug interactions",
"author": "Foisy",
"doi-asserted-by": "crossref",
"first-page": "1048",
"journal-title": "Ann. Pharmacother.",
"key": "ref_13",
"volume": "42",
"year": "2008"
},
{
"DOI": "10.1016/j.jbc.2021.100867",
"article-title": "Decoding molnupiravir-induced mutagenesis in SARS-CoV-2",
"doi-asserted-by": "crossref",
"first-page": "100867",
"journal-title": "J. Biol. Chem.",
"key": "ref_14",
"volume": "297",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)31607-X",
"article-title": "Primary biliary cholangitis",
"author": "Lleo",
"doi-asserted-by": "crossref",
"first-page": "1915",
"journal-title": "Lancet",
"key": "ref_15",
"volume": "396",
"year": "2020"
},
{
"DOI": "10.1038/bmt.2015.52",
"article-title": "Sinusoidal obstruction syndrome/veno-occlusive disease: Current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT)",
"author": "Mohty",
"doi-asserted-by": "crossref",
"first-page": "781",
"journal-title": "Bone Marrow Transplant.",
"key": "ref_16",
"volume": "50",
"year": "2015"
},
{
"DOI": "10.1038/s41586-022-05594-0",
"article-title": "FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2",
"author": "Brevini",
"doi-asserted-by": "crossref",
"first-page": "134",
"journal-title": "Nature",
"key": "ref_17",
"volume": "615",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-04385-3",
"article-title": "Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "657",
"journal-title": "Nature",
"key": "ref_18",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.2165/00003495-198427020-00001",
"article-title": "Ursodeoxycholic acid: A review of its pharmacological properties and therapeutic efficacy",
"author": "Ward",
"doi-asserted-by": "crossref",
"first-page": "95",
"journal-title": "Drugs",
"key": "ref_19",
"volume": "27",
"year": "1984"
},
{
"DOI": "10.3109/00365529409103618",
"article-title": "Pharmacology of ursodeoxycholic acid, an enterohepatic drug",
"author": "Hofmann",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Scand. J. Gastroenterol. Suppl.",
"key": "ref_20",
"volume": "204",
"year": "1994"
},
{
"DOI": "10.1097/00007890-198712000-00011",
"article-title": "Venoocclusive disease of the liver following bone marrow transplantation",
"author": "Jones",
"doi-asserted-by": "crossref",
"first-page": "778",
"journal-title": "Transplantation",
"key": "ref_21",
"volume": "44",
"year": "1987"
},
{
"DOI": "10.1038/s41409-019-0705-z",
"article-title": "Prophylactic, preemptive, and curative treatment for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: A position statement from an international expert group",
"author": "Mohty",
"doi-asserted-by": "crossref",
"first-page": "485",
"journal-title": "Bone Marrow Transplant.",
"key": "ref_22",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1111/liv.15660",
"article-title": "Ursodeoxycholic acid administration did not reduce susceptibility to SARS-CoV-2 infection in children",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "1950",
"journal-title": "Liver Int.",
"key": "ref_23",
"volume": "43",
"year": "1950"
},
{
"DOI": "10.1111/joim.13630",
"article-title": "Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis",
"author": "John",
"doi-asserted-by": "crossref",
"first-page": "636",
"journal-title": "J. Intern. Med.",
"key": "ref_24",
"volume": "293",
"year": "2023"
},
{
"DOI": "10.3389/fcimb.2023.1178590",
"article-title": "Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1178590",
"journal-title": "Front. Cell. Infect. Microbiol.",
"key": "ref_25",
"volume": "13",
"year": "2023"
},
{
"key": "ref_26",
"unstructured": "(2023, August 08). Liverpool COVID-19 Interactions. Available online: https://www.covid19-druginteractions.org/checker."
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4915/15/8/1738"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Virology",
"Infectious Diseases"
],
"subtitle": [],
"title": "Ursodeoxycholic Acid Does Not Improve COVID-19 Outcome in Hospitalized Patients",
"type": "journal-article",
"volume": "15"
}