Association Between Ursodeoxycholic Acid and Clinical Outcomes in Patients With COVID-19 Infection: Population-Based Cohort Study
et al., JMIR Public Health and Surveillance, doi:10.2196/59274, Aug 2024
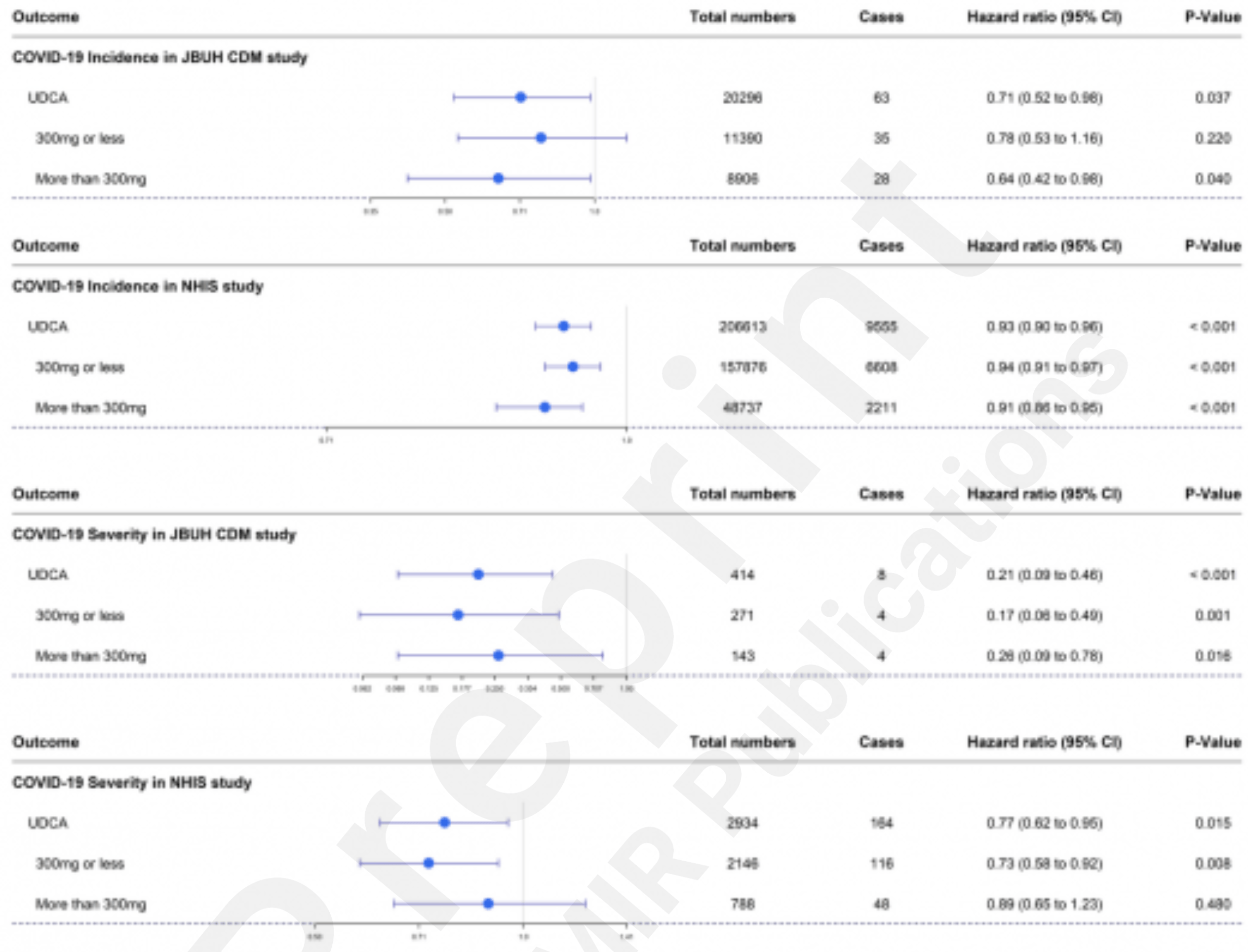
Retrospective 1,675,593 patients in the Jeonbuk CDM cohort and 8,528,533 patients in the NHIS cohort, showing ursodeoxycholic acid (UDCA) intake associated with significantly lower risk of COVID-19 infection and severe COVID-19.
|
risk of severe case, 57.2% lower, HR 0.43, p = 0.19, treatment 2,934, control 2,934, adjusted per study, combined.
|
|
risk of severe case, 79.0% lower, HR 0.21, p < 0.001, treatment 414, control 414, adjusted per study, propensity score matching, multivariable, Cox proportional hazards.
|
|
risk of severe case, 23.0% lower, HR 0.77, p = 0.02, treatment 2,934, control 2,934, adjusted per study, propensity score matching, multivariable, Cox proportional hazards.
|
|
risk of case, 14.8% lower, HR 0.85, p = 0.21, adjusted per study, combined.
|
|
risk of case, 29.0% lower, HR 0.71, p = 0.03, treatment 20,296, control 20,296, adjusted per study, propensity score matching, multivariable, Cox proportional hazards.
|
|
risk of case, 7.0% lower, HR 0.93, p < 0.001, adjusted per study, propensity score matching, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Lee et al., 14 Aug 2024, retrospective, South Korea, peer-reviewed, 14 authors.
Contact: kjsjdk@gmail.com.
Ursodeoxycholic acid is associated with better clinical outcomes in COVID-19 patients: A population-based cohort study
doi:10.2196/preprints.59274
Background: Several studies have investigated the relationship between ursodeoxycholic acid (UDCA) and coronavirus disease 2019 . However, complex and conflicting results have caused confusion in the application of these results.
Objective: We aimed to investigate whether the association between UDCA and COVID can also be demonstrated through analysis of a large-scale cohort. Methods: This retrospective cohort study used internal and external validation cohorts: the Jeonbuk common data model (CDM) cohort (JBUH-CDM) and the Korean National Health Insurance claim-based database (NHIS), respectively. We investigated UDCA intake and its relationship with COVID-19 susceptibility and severity using validated propensity score (PS) matching. Results: Regarding the COVID-19 susceptibility UDCA intake is associated with being significantly lowered to 0.71 in JBUH-CDM (hazard ratio; HR) (95% confidence interval (CI): 0.52-0.98) value was significantly lowered to 0.93 (95% CI: 0.90-0.96) in the NHIS. Regarding the COVID-19 severity, UDCA intake was analyzed to be significantly lowered to 0.21 (95% CI: 0.09-0.46) in JBUH-CDM. It was also found that the HR value was significantly lowered to 0.77 in NHIS (95% CI: 0.62-0.95). Conclusions: Using a large-scale local cohort and an external validation cohort, we confirmed that UDCA intake was significantly associated with the reduction of COVID-19 susceptibility and severity. These trends remained consistent regardless of UDCA dosage. This suggests the potential of UDCA as a preventive and therapeutic agent for COVID-19.
(n = 421) were excluded. In the matched case, patients who died before the matched date (n = 421) were excluded. After PS matching, the number of patients in (each of) the UDCA and matched cases were 2,934 patients. The procedure regarding the population of the NHIS study is shown in Figure 2 .
Validation of PS Matching Supplementary Tables 1 and 2 show the frequencies and SMDs between UDCA and matched cases after PS matching in COVID-19 susceptibility and severity, respectively. All SMDs did not exceed the value of 0.2. There were no significant differences between the two groups (all SMDs < 0.2).
COVID-19 Susceptibility and Severity Analysis Supplementary Table 3 shows details of the analysis of COVID-19 susceptibility in JBUH CDM and NHIS data. In the JBUH CDM study, it was found that the aHR value was significantly lowered to 0.71 (95% CI: 0.52-0.98, P=.031) (Figure 3A ). In the case of the UDCA group in the NHIS study, the aHR value for COVID-19 susceptibility was analyzed and was found to be significantly lowered to 0.93 (95% CI: 0.90-0.96, P<.001) (Figure 3B ). In this study, we investigated the presence or absence of UDCA and the occurrence of COVID according to the UDCA dose. In the JBUH CDM study, the aHR value of 0.64 (95% CI: 0.42-0.98, P=.038) for doses > 300 mg was significant, but the aHR value of 0.78 (95% CI: 0.53-1.16, P=.208) for doses ≤ 300 mg was insignificant.(Figure 3C ). In the NHIS study, the aHR value of 0.94 (95% CI: 0.91-0.97,..
References
Abdulrab, Al-Maweri, Halboub, Ursodeoxycholic acid as a candidate therapeutic to alleviate and/or prevent COVID-19-associated cytokine storm, Med Hypotheses, doi:10.1016/j.mehy.2020.109897
Benedetto, Head, Angelini, Blackstone, Statistical primer: propensity score matching and its alternatives, Eur J Cardiothorac Surg, doi:10.1093/ejcts/ezy167
Brevini, Maes, Webb, John, Fuchs et al., FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2, Nature, doi:10.1038/s41586-022-05594-0
Colapietro, Angelotti, Masetti, Shiffer, Pugliese et al., Ursodeoxycholic Acid Does Not Improve COVID-19 Outcome in Hospitalized Patients, Viruses, doi:10.3390/v15081738
Corpechot, Verdoux, Frank-Soltysiak, Vallee, Grimaldi, Exploring the impact of ursodeoxycholic acid therapy on COVID-19 in a real-word setting, J Med Virol, doi:10.1002/jmv.29418
Cox, Regression models and life-tables, Journal of the Royal Statistical Society. Series B (Methodological)
Granger, Watkins, Sergeant, Lunt, A review of the use of propensity score diagnostics in papers published in high-ranking medical journals, BMC Med Res Methodol, doi:10.1186/s12874-020-00994-0
Hirayama, Nishiwaki, Hamaguchi, Ito, Ueyama et al., Intestinal Collinsella may mitigate infection and exacerbation of COVID-19 by producing ursodeoxycholate, PLoS One, doi:10.1371/journal.pone.0260451
Hu, Zhang, Huang, Shen, Feng et al., Effect of ursodeoxycholic acid on preventing SARS-CoV-2 infection in patients with liver transplantation: a multicenter retrospective cohort study, QJM, doi:10.1093/qjmed/hcad254
Hwang, Kim, Im, Moon, Kim et al., Treatment outcomes of oral doxycycline versus intravenous azithromycin in adults hospitalized with scrub typhus: A retrospective study using inverse probability treatment weighting (IPTW) propensity analysis, Travel Med Infect Dis, doi:10.1016/j.tmaid.2022.102525
Hwang, You, Yeom, Lee, Lee et al., Influenza viral infection is a risk factor for severe illness in COVID-19 patients: a nationwide population-based cohort study, Emerg Microbes Infect, doi:10.1080/22221751.2022.2164215
Jeong, Choi, Kim, Park, Kim et al., SARS-CoV-2 infection in severe asthma is associated with worsening of COVID-19 through respiratory NLRP3 inflammasome activation, Allergy, doi:10.1111/all.15452
John, Bastaich, Ferreira, Doshi, Taddei et al., COVID-19 vaccine effectiveness and community prevalence of Alpha, Delta and Omicron variants in patients with cirrhosis, Gut, doi:10.1136/gutjnl-2022-327799
John, Bastaich, Webb, Brevini, Moon et al., Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis, J Intern Med, doi:10.1111/joim.13630
John, Deng, Scheinberg, Mahmud, Taddei et al., Association of BNT162b2 mRNA and mRNA-1273 Vaccines With COVID-19 Infection and Hospitalization Among Patients With Cirrhosis, JAMA Intern Med, doi:10.1001/jamainternmed.2021.4325
Kim, Kang, Lee, Yang, Yeom et al., Periodontitis is associated with the development of fungal sinusitis: A nationwide 12-year follow-up study, J Clin Periodontol, doi:10.1111/jcpe.13753
Ko, Lee, Kim, Jo, Kumar et al., Anti-inflammatory effects of ursodeoxycholic acid by lipopolysaccharide-stimulated inflammatory responses in RAW 264.7 macrophages, PLoS One, doi:10.1371/journal.pone.0180673
Kotb, Molecular mechanisms of ursodeoxycholic acid toxicity & side effects: ursodeoxycholic acid freezes regeneration & induces hibernation mode, Int J Mol Sci, doi:10.3390/ijms13078882
Li, Wang, Chen, Zhu, Chen, A high-dose of ursodeoxycholic acid treatment alleviates liver inflammation by remodeling gut microbiota and bile acid profile in a mouse model of non-alcoholic steatohepatitis, Biomed Pharmacother, doi:10.1016/j.biopha.2024.116617
Li, Zhu, Cui, Lin, Li, Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease, Front Cell Infect Microbiol, doi:10.3389/fcimb.2023.1178590
Liu, Wang, Ursodeoxycholic acid administration did not reduce susceptibility to SARS-CoV-2 infection in children, Liver Int, doi:10.1111/liv.15660
Mao, Lin, Zhang, Zhang, Zhang et al., Understanding the role of ursodeoxycholic acid and gut microbiome in non-alcoholic fatty liver disease: current evidence and perspectives, Front Pharmacol, doi:10.3389/fphar.2024.1371574
Marrone, Covino, Merra, Piccioni, Amodeo et al., Ursodeoxycholic acid does not affect the clinical outcome of SARS CoV 2 infection: A retrospective study of -propensity score matched cohorts
Mistry, Barmania, Mellet, Peta, Strydom et al., Cumulative incidence plot of A. COVID-19 susceptibility according to UDCA usage in JBUH CDM database, B. COVID-19 susceptibility according to UDCA usage in NHIS database, C. COVID-19 susceptibility according to UDCA dosage (?300mg, >300mg) in JBUH CDM database, D. COVID-19 susceptibility according to UDCA dosage (?300mg, >300mg) in NHIS database. Cumulative incidence plot of A. COVID-19 severity according to UDCA usage in JBUH CDM database, B. COVID-19 severity according to UDCA usage in NHIS database, C. COVID-19 severity according to UDCA dosage (?300mg, >300mg) in JBUH CDM database, D. COVID-19 severity according to UDCA dosage (?300mg, Front Immunol, doi:10.3389/fimmu.2021.809244
Niu, Xu, Zhang, Sun, Gan et al., Ursodeoxycholic acid stimulates alveolar fluid clearance in LPS-induced pulmonary edema via ALX/cAMP/PI3K pathway, J Cell Physiol, doi:10.1002/jcp.28602
Oostveen, Reeskamp, Kaiser, Hartgers, Meessen et al., Can human trans intestinal cholesterol excretion be stimulated using ursodeoxycholic acid and ezetimibe: A randomized placebo controlled cross-over study
Poochi, Easwaran, Balasubramanian, Anbuselvam, Meyyazhagan et al., Employing bioactive compounds derived from Ipomoea obscura (L.) to evaluate potential inhibitor for SARS-CoV-2 main protease and ACE2 protein, Food Front, doi:10.1002/fft2.29
Subramanian, Iles, Ikramuddin, Steer, Merit of an Ursodeoxycholic Acid Clinical Trial in COVID-19 Patients, Vaccines, doi:10.3390/vaccines8020320
Thuy, Bao, Moon, Ursodeoxycholic acid ameliorates cell migration retarded by the SARS-CoV-2 spike protein in BEAS-2B human bronchial epithelial cells, Biomed Pharmacother, doi:10.1016/j.biopha.2022.113021
Tonin, Arends, Latest development in the synthesis of ursodeoxycholic acid (UDCA): a critical review, Beilstein J Org Chem, doi:10.3762/bjoc.14.33
Verma, Jazrawi, Ahmed, Davis, Bland et al., Optimum dose of ursodeoxycholic acid in primary biliary cirrhosis, Eur J Gastroenterol Hepatol, doi:10.1097/00042737-199910000-00001
Wong, Hui, Yip, Lui, Hui et al., Minimal Risk of Drug-Induced Liver Injury With Molnupiravir and Ritonavir-Boosted Nirmatrelvir, Gastroenterology, doi:10.1053/j.gastro.2022.09.008
Wu, Zhao, Guo, Yu, Wu et al., Ursodeoxycholic acid alleviates nonalcoholic fatty liver disease by inhibiting apoptosis and improving autophagy via activating AMPK, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2020.05.128
You, Kim, Jeong, Yeom, Kim et al., Septal deviation could be associated with the development of bronchial asthma: A nationwide cohort study, J Allergy Clin Immunol Pract, doi:10.1016/j.jaip.2021.11.002
Zampino, Mele, Florio, Bertolino, Andini et al., Liver injury in remdesivir-treated COVID-19 patients, Hepatol Int, doi:10.1007/s12072-020-10077-3
Zhang, Qi, Herbal medicines exhibit a high affinity for ACE2 in treating COVID-19, Biosci Trends, doi:10.5582/bst.2022.01534
DOI record:
{
"DOI": "10.2196/59274",
"ISSN": [
"2369-2960"
],
"URL": "http://dx.doi.org/10.2196/59274",
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0808-2376",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lee",
"given": "Hyunjun",
"sequence": "first"
},
{
"affiliation": [],
"family": "Kim",
"given": "Min Gul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yeom",
"given": "Sang Woo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Noh",
"given": "Sang Jae",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jeong",
"given": "Cho Yun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Min Ji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kang",
"given": "Min Gu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ko",
"given": "Ji Hoon",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Park",
"given": "Su Cheol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kweon",
"given": "Hyeok Tae",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sim",
"given": "Sang Il",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lee",
"given": "Hyun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "You",
"given": "Yeon Seok",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Jong Seung",
"sequence": "additional"
}
],
"container-title": "JMIR Public Health and Surveillance",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
8,
14
]
],
"date-time": "2024-08-14T07:09:44Z",
"timestamp": 1723619384000
},
"deposited": {
"date-parts": [
[
2024,
8,
14
]
],
"date-time": "2024-08-14T07:09:44Z",
"timestamp": 1723619384000
},
"indexed": {
"date-parts": [
[
2024,
8,
15
]
],
"date-time": "2024-08-15T00:22:51Z",
"timestamp": 1723681371121
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
4,
8
]
]
},
"language": "en",
"member": "1010",
"original-title": [],
"prefix": "10.2196",
"published": {
"date-parts": [
[
2024,
4,
8
]
]
},
"published-online": {
"date-parts": [
[
2024,
4,
8
]
]
},
"publisher": "JMIR Publications Inc.",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "http://preprints.jmir.org/preprint/59274/accepted"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Ursodeoxycholic acid is associated with better clinical outcomes in COVID-19 patients: A population-based cohort study (Preprint)",
"type": "journal-article"
}