Ursodeoxycholic acid and COVID-19 outcomes: a cohort study and data synthesis of state-of-art evidence
et al., Expert Review of Anti-infective Therapy, doi:10.1080/14787210.2024.2376153, Jul 2024
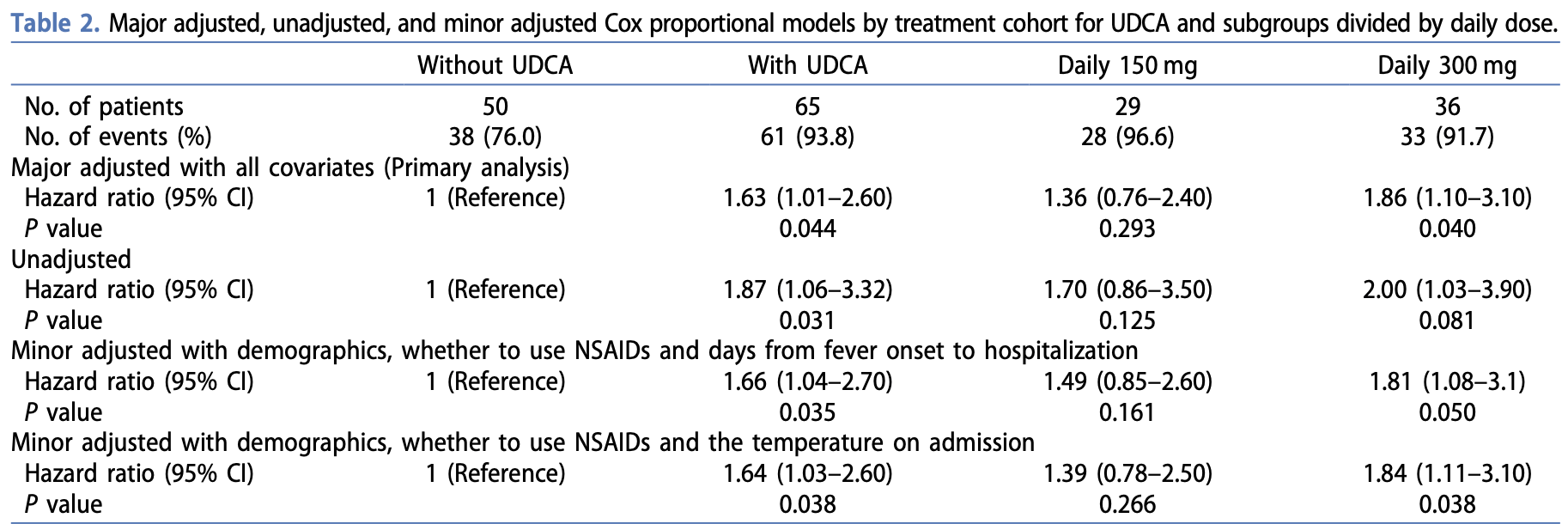
Retrospective 115 hospitalized COVID-19 patients in China showing faster time to body temperature recovery with ursodeoxycholic acid (UDCA) treatment. Results were better for higher dose treatment (300mg vs. 150mg).
Authors also perform a meta analysis showing lower risk of severe/critical COVID-19 with UDCA, which is listed separately1.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments2.
|
time to recovery, 38.7% lower, HR 0.61, p = 0.04, treatment 65, control 50, adjusted per study, inverted to make HR<1 favor treatment, multivariable, Cox proportional hazards.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Yu et al., 8 Jul 2024, retrospective, China, peer-reviewed, 12 authors, study period 10 December, 2022 - 30 December, 2022.
Contact: zhandc@nju.edu.cn, tangshaoqiu@nju.edu.cn, guoyu@cpu.edu.cn.
Ursodeoxycholic acid and COVID-19 outcomes: a cohort study and data synthesis of state-of-art evidence
Expert Review of Anti-infective Therapy, doi:10.1080/14787210.2024.2376153
Background: The potential of ursodeoxycholic acid (UDCA) in inhibiting angiotensin-converting enzyme 2 was demonstrated. However, conflicting evidence emerged regarding the association between UDCA and COVID-19 outcomes, prompting the need for a comprehensive investigation. Research design and methods: Patients diagnosed with COVID-19 infection were retrospectively analyzed and divided into two groups: the UDCA-treated group and the control group. Kaplan-Meier recovery analysis and Cox proportional hazards models were used to evaluate the recovery time and hazard ratios. Additionally, study-level pooled analyses for multiple clinical outcomes were performed. Results: In the 115-patient cohort, UDCA treatment was significantly associated with a reduced recovery time. The subgroup analysis suggests that the 300 mg subgroup had a significant (adjusted hazard ratio: 1.63 [95% CI, 1.01 to 2.60]) benefit with a shorter duration of fever. The results of pooled analyses also show that UDCA treatment can significantly reduce the incidence of severe/critical diseases in COVID-19 (adjusted odds ratio: 0.68 [95% CI, 0.50 to 0.94]). Conclusions: UDCA treatment notably improves the recovery time following an Omicron strain infection without observed safety concerns. These promising results advocate for UDCA as a viable treatment for COVID-19, paving the way for further large-scale and prospective research to explore the full potential of UDCA.
Abbreviations
Author contributions Guo YU, Yang Yu, De-Chuan Zhan: conception of cohort study; Guo YU, Guo-Fu Li: design of the study; Jian Li, Shao-Qiu Tang, Lu-Yao Han: Resources and data curation for cohort study; Yang Yu, Guo Yu, Lu-Yao Han, Zhi-Long Zhang, Tian-Shuo Liu: Investigation of cohort study; Guo-Fu Li: Conception of evidence synthesis and meta-analytical methodology; Lu-Yao Han, Shu-Xin Jiao, Yu-Wei Qiao, Na Zhang: Systematic review and meta-analysis; Lu-Yao Han, Guo-Fu Li, Guo Yu: interpretation of the results; Lu-Yao Han, Guo-Fu Li, Guo Yu: drafting of the manuscript; Guo-Fu Li, Guo Yu: critical revision of the manuscript for important intellectual content.
Declaration of interest The following financial interests may be considered potential competing interests: Y. Yu is the controlling shareholder of Polixir Technologies Co., Ltd. Zhi-Long Zhang and T.S. Liu declare receipt of employment by Polixir Technologies Co., Ltd. The research sponsor, Polixir Technologies Co., Ltd., did not play any role in research design, data collection, analysis and interpretation, report writing, and the decision to submit this paper for publication. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures Peer reviewers on this manuscript have no relevant..
References
Abenavoli, Aquila, Sacco, Liver damage and impaired coagulation in COVID-19 patients: a case series, Diseases, doi:10.3390/diseases11040141
Bosch, Dumortier, Maucort-Boulch, Preventive administration of UDCA after liver transplantation for primary biliary cirrhosis is associated with a lower risk of disease recurrence, J Hepatol, doi:10.1016/j.jhep.2015.07.038
Brevini, Maes, Webb, FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2, Nature, doi:10.1038/s41586-022-05594-0
Brusasco, Corradi, Dazzi, The use of continuous positive airway pressure during the second and third waves of the COVID-19 pandemic, ERJ Open Res, doi:10.1183/23120541.00365-2022
Burkard, Von Eckardstein, Rentsch, Differentiated quantification of human bile acids in serum by high-performance liquid chromatography-tandem mass spectrometry, J Chromatogr B Analyt Technol Biomed Life Sci, doi:10.1016/j.jchromb.2005.08.016
Chiang, Ferrell, Discovery of farnesoid X receptor and its role in bile acid metabolism, Mol Cell Endocrinol, doi:10.1016/j.mce.2022.111618
Colapietro, Angelotti, Masetti, Ursodeoxycholic acid does not improve COVID-19 outcome in hospitalized patients, Viruses-Basel, doi:10.3390/v15081738
Costello, Waller, Smith, Ursodeoxycholic acid and severe COVID-19 outcomes in people with liver disease: a cohort study using the OpenSAFELY platform, medRxiv
Dilger, Hohenester, Winkler-Budenhofer, Effect of ursodeoxycholic acid on bile acid profiles and intestinal detoxification machinery in primary biliary cirrhosis and health, J Hepatol, doi:10.1016/j.jhep.2012.02.014
Dyson, Hirschfield, Adams, Novel therapeutic targets in primary biliary cirrhosis, Nat Rev Gastro Hepat, doi:10.1038/nrgastro.2015.12
Gaziano, Giambartolomei, Pereira, Actionable druggable genome-wide Mendelian randomization identifies repurposing opportunities for COVID-19, Nat Med, doi:10.1038/s41591-021-01310-z
Glatz, Trauner, The importance of serum bile acid level analysis and treatment with ursodeoxycholic acid in intrahepatic cholestasis of pregnancy: a case series from central Europe, Arch Dermatol, doi:10.1001/archderm.143.6.757
Gomez-Ospina, Potter, Xiao, Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis, Nat Commun, doi:10.1038/ncomms10713
Grant, Geoghegan, Arbyn, The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): a systematic review and meta-analysis of 148 studies from 9 countries, PLOS ONE, doi:10.1371/journal.pone.0234765
Henriksen, Hansen, Lycas, Cholestasis alters brain lipid and bile acid composition and compromises motor function in neonatal piglets, Physiol Rep, doi:10.14814/phy2.15368
Hu, Zhang, Huang, Effect of ursodeoxycholic acid on preventing SARS-CoV-2 infection in patients with liver transplantation: a multicenter retrospective cohort study, QJM Int J Med, doi:10.1093/qjmed/hcad254
John, Bastaich, Webb, Ursodeoxycholic acid is associated with a reduction in SARS-CoV-2 infection and reduced severity of COVID-19 in patients with cirrhosis, J Intern Med, doi:10.1111/joim.13630
Kragholm, Torp-Pedersen, Fosbol, Non-steroidal anti-inflammatory drug use in COVID-19, Lancet Rheumatol, doi:10.1016/S2665-9913(21)00144-2
Li, An, Yu, Do proton pump inhibitors influence SARS-CoV-2 related outcomes? A meta-analysis, Gut, doi:10.1136/gutjnl-2020-323366
Li, Qiao, Yu, Helicobacter pylori therapy and risk of gastric cancer after endoscopic resection of dysplasia: a sensitivity analysis assessing impact of unmeasured confounding, Gastroenterology, doi:10.1053/j.gastro.2024.03.010
Li, Yu, Drug-induced liver injury with ritonavir-boosted nirmatrelvir: evidence from coronavirus disease 2019 emergency use authorization adverse event reporting system, Gastroenterology, doi:10.1053/j.gastro.2023.02.008
Li, Zhu, Cui, Protective effect of ursodeoxycholic acid on COVID-19 in patients with chronic liver disease, Front Cell Infect Microbiol, doi:10.3389/fcimb.2023.1178590
Luo, Wan, Liu, Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range, Stat Methods Med Res, doi:10.1177/0962280216669183
Marrone, Covino, Merra, Ursodeoxycholic acid does not affect the clinical outcome of SARS-CoV-2 infection: a retrospective study of propensity score-matched cohorts, Liver Int, doi:10.1111/liv.15736
Monteil, Kwon, Prado, Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2, Cell, doi:10.1016/j.cell.2020.04.004
Pan, Zhang, Meng, Can ursodeoxycholic acid prevent SARS-CoV-2 infection or reduce the COVID-19 severity? Current knowledge and unresolved issues, Infect Immun
Passamonti, Cattaneo, Arcaini, Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study, Lancet Haematol, doi:10.1016/S2352-3026(20)30251-9
Pedersen, Greenan, Arora, Ursodeoxycholic acid decreases incidence of primary biliary cholangitis and biliary complications after liver transplantation: a meta-analysis, Liver Transplant, doi:10.1002/lt.25935
Qiao, Yu, Li, Overall Survival Benefit with Sacituzumab Govitecan in Metastatic Breast Cancer: A Post Hoc Interaction Analyses of a Randomized Controlled Trail, Clin Drug Investig, doi:10.1007/s40261-024-01367-x
Ramos, Rattis, Ottaviani, ACE2 down-regulation may act as a transient molecular disease causing RAAS dysregulation and tissue damage in the microcirculatory environment among COVID-19 patients, Am J Pathol, doi:10.1016/j.ajpath.2021.04.010
Rizk, Wenziger, Tran, Angiotensin-converting enzyme inhibitor and angiotensin receptor blocker use associated with reduced mortality and other disease outcomes in US veterans with COVID-19, Drugs, doi:10.1007/s40265-021-01639-2
Tianjiao, Huatao, Bibo, Clinical efficacy of ursodeoxycholic acid in the treatment of COVID-19, Chin J Clin Infect Dis
Vanderweele, Ding, Sensitivity analysis in observational research: introducing the E-value, Ann Intern Med, doi:10.7326/M16-2607
Vanderweele, On a square-root transformation of the odds ratio for a common outcome, Epidemiology, doi:10.1097/EDE.0000000000000733
Verdecchia, Cavallini, Spanevello, The pivotal link between ACE2 deficiency and SARS-CoV-2 infection, Eur J Internal Med, doi:10.1016/j.ejim.2020.04.037
Wells, Shea, Connell, The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses
Wu, Guan, Li, Data reproducibility issues on evidence synthesis of adverse events associated with HER2-targeted antibody-drug conjugates, EClinicalMedicine, doi:10.1016/j.eclinm.2023.101904
Wu, Yu, Li, Validity of meta-analytical data on cutaneous adverse events with phosphoinositide 3-kinase inhibitors, JAMA Oncol, doi:10.1001/jamaoncol.2023.3384
Xiao, Cai, Li, Data extraction and handling issues on evidence synthesis of risk of immunoglobulin e-mediated food allergy, JAMA Pediatr, doi:10.1001/jamapediatrics.2023.2465
Yu, Zhang, Guan, Validity of meta-analytical estimates and hazard ratios quantifying survival benefit of immunochemotherapy in low PD-L1-Expressing esophageal squamous cell carcinoma, J Clin Oncol, doi:10.1200/JCO.23.00057
Zhu, Zhang, Chen, Analysis of human C24 bile acids metabolome in serum and urine based on enzyme digestion of conjugated bile acids and LC-MS determination of unconjugated bile acids, Anal Bioanal Chem, doi:10.1007/s00216-018-1183-7
Zhu, Zhu, The effect of self-limiting on the prevention and control of the diffuse COVID-19 epidemic with delayed and temporal-spatial heterogeneous, BMC Infect Dis, doi:10.1186/s12879-021-06670-y
DOI record:
{
"DOI": "10.1080/14787210.2024.2376153",
"ISSN": [
"1478-7210",
"1744-8336"
],
"URL": "http://dx.doi.org/10.1080/14787210.2024.2376153",
"alternative-id": [
"10.1080/14787210.2024.2376153"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=ierz20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=ierz20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2024-03-26"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2024-06-10"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2024-07-08"
}
],
"author": [
{
"affiliation": [
{
"name": "National Key Laboratory for Novel Software Technology, Nanjing University, China"
},
{
"name": "Polixir.ai"
}
],
"family": "Yu",
"given": "Yang",
"sequence": "first"
},
{
"affiliation": [
{
"name": "School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, China"
}
],
"family": "Li",
"given": "Guo-Fu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital of Nanjing University, Nanjing University, China"
}
],
"family": "Li",
"given": "Jian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, China"
}
],
"family": "Han",
"given": "Lu-Yao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Key Laboratory for Novel Software Technology, Nanjing University, China"
},
{
"name": "Polixir.ai"
}
],
"family": "Zhang",
"given": "Zhi-Long",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Key Laboratory for Novel Software Technology, Nanjing University, China"
},
{
"name": "Polixir.ai"
}
],
"family": "Liu",
"given": "Tian-Shuo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, China"
}
],
"family": "Jiao",
"given": "Shu-Xin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, China"
}
],
"family": "Qiao",
"given": "Yu-Wei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, China"
}
],
"family": "Zhang",
"given": "Na",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Key Laboratory for Novel Software Technology, Nanjing University, China"
}
],
"family": "Zhan",
"given": "De-Chuan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital of Nanjing University, Nanjing University, China"
}
],
"family": "Tang",
"given": "Shao-Qiu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, China"
}
],
"family": "Yu",
"given": "Guo",
"sequence": "additional"
}
],
"container-title": "Expert Review of Anti-infective Therapy",
"container-title-short": "Expert Review of Anti-infective Therapy",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2024,
7,
8
]
],
"date-time": "2024-07-08T11:53:11Z",
"timestamp": 1720439591000
},
"deposited": {
"date-parts": [
[
2024,
7,
8
]
],
"date-time": "2024-07-08T11:53:24Z",
"timestamp": 1720439604000
},
"funder": [
{
"DOI": "10.13039/501100004608",
"doi-asserted-by": "publisher",
"name": "Natural Science Foundation of Jiangsu Province"
},
{
"DOI": "10.13039/501100010035",
"award": [
"BK20200005"
],
"doi-asserted-by": "publisher",
"name": "Outstanding Youth Foundation of Jiangsu Province"
}
],
"indexed": {
"date-parts": [
[
2024,
7,
9
]
],
"date-time": "2024-07-09T00:24:43Z",
"timestamp": 1720484683068
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
7,
8
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/14787210.2024.2376153",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"prefix": "10.1080",
"published": {
"date-parts": [
[
2024,
7,
8
]
]
},
"published-online": {
"date-parts": [
[
2024,
7,
8
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1002/lt.25935",
"article-title": "Ursodeoxycholic Acid Decreases Incidence of Primary Biliary Cholangitis and Biliary Complications After Liver Transplantation: A Meta-Analysis",
"author": "Pedersen MR",
"doi-asserted-by": "crossref",
"first-page": "866",
"issue": "6",
"journal-title": "Liver transplantation",
"key": "e_1_3_8_2_1",
"unstructured": "Pedersen MR, Greenan G, Arora S, et al. Ursodeoxycholic Acid Decreases Incidence of Primary Biliary Cholangitis and Biliary Complications After Liver Transplantation: A Meta-Analysis. Liver transplantation. 2021 Jun;27(6):866–875.",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1016/j.jhep.2015.07.038",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_3_1"
},
{
"DOI": "10.1038/s41586-022-05594-0",
"article-title": "FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2",
"author": "Brevini T",
"doi-asserted-by": "crossref",
"issue": "7950",
"journal-title": "Nature",
"key": "e_1_3_8_4_1",
"unstructured": "Brevini T, Maes M, Webb GJ, et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature. 2023 Mar 2;615(7950):134±.",
"volume": "615"
},
{
"DOI": "10.3390/v15081738",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_5_1"
},
{
"article-title": "Effect of Ursodeoxycholic Acid on Preventing SARS-CoV-2 Infection in Patients With Liver Transplantation: a multicenter retrospective cohort study",
"author": "Hu L",
"journal-title": "QJM-INT J MED",
"key": "e_1_3_8_7_1",
"unstructured": "Hu L, Zhang H, Huang C, et al. Effect of Ursodeoxycholic Acid on Preventing SARS-CoV-2 Infection in Patients With Liver Transplantation: a multicenter retrospective cohort study. QJM-INT J MED. 2023 2023-Nov-09.",
"year": "2023"
},
{
"DOI": "10.1111/joim.13630",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_8_1"
},
{
"DOI": "10.3389/fcimb.2023.1178590",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_9_1"
},
{
"article-title": "Ursodeoxycholic acid does not affect the clinical outcome of SARS-CoV-2 infection: A retrospective study of propensity score-matched cohorts",
"author": "Marrone G",
"journal-title": "Liver Int",
"key": "e_1_3_8_10_1",
"unstructured": "Marrone G, Covino M, Merra G, et al. Ursodeoxycholic acid does not affect the clinical outcome of SARS-CoV-2 infection: A retrospective study of propensity score-matched cohorts. Liver Int. 2023.",
"year": "2023"
},
{
"article-title": "Clinical efficacy of ursodeoxycholic acid in the treatment of COVID-19",
"author": "Tianjiao T",
"first-page": "289",
"issue": "4",
"journal-title": "Chin J Clin Infect Dis",
"key": "e_1_3_8_11_1",
"unstructured": "Tianjiao T, Huatao L, Bibo M, et al. Clinical efficacy of ursodeoxycholic acid in the treatment of COVID-19. Chin J Clin Infect Dis. 2023;16(4):289–292 and 298.",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.1186/s12879-021-06670-y",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_12_1"
},
{
"DOI": "10.1200/JCO.23.00057",
"article-title": "Validity of Meta-Analytical Estimates and Hazard Ratios Quantifying Survival Benefit of Immunochemotherapy in Low PD-L1-Expressing Esophageal Squamous Cell Carcinoma",
"author": "Yu G",
"doi-asserted-by": "crossref",
"first-page": "2862",
"issue": "15",
"journal-title": "Journal of clinical oncology : official journal of the American Society of Clinical Oncology",
"key": "e_1_3_8_13_1",
"unstructured": "Yu G, Zhang N, Guan AJ, et al. Validity of Meta-Analytical Estimates and Hazard Ratios Quantifying Survival Benefit of Immunochemotherapy in Low PD-L1-Expressing Esophageal Squamous Cell Carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2023 May 20;41(15):2862–2864.",
"volume": "41",
"year": "2023"
},
{
"key": "e_1_3_8_14_1",
"unstructured": "Wells GA Shea B O’Connell D et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000."
},
{
"DOI": "10.1097/EDE.0000000000000733",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_15_1"
},
{
"DOI": "10.7326/M16-2607",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_16_1"
},
{
"DOI": "10.1177/0962280216669183",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_17_1"
},
{
"DOI": "10.1136/gutjnl-2020-323366",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_18_1"
},
{
"DOI": "10.1016/j.mce.2022.111618",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_19_1"
},
{
"DOI": "10.1038/ncomms10713",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_20_1"
},
{
"DOI": "10.1007/s00216-018-1183-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_21_1"
},
{
"DOI": "10.1001/archderm.143.6.757",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_22_1"
},
{
"DOI": "10.1016/j.jchromb.2005.08.016",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_23_1"
},
{
"DOI": "10.14814/phy2.15368",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_24_1"
},
{
"DOI": "10.1016/j.jhep.2012.02.014",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_25_1"
},
{
"DOI": "10.1038/nrgastro.2015.12",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_26_1"
},
{
"DOI": "10.1371/journal.pone.0234765",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_27_1"
},
{
"key": "e_1_3_8_28_1",
"unstructured": "China updates COVID-19 diagnosis treatment protocol [Internet]. 2023 [cited 2023]. Available from: http://english.nmpa.gov.cn/2023-01/10/c_861471.htm"
},
{
"article-title": "FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2",
"author": "Brevini T",
"journal-title": "Nature",
"key": "e_1_3_8_29_1",
"unstructured": "Brevini T, Maes M, Webb GJ, et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature. 2022 Dec 5.",
"year": "2022"
},
{
"DOI": "10.1038/s41591-021-01310-z",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_30_1"
},
{
"DOI": "10.1016/j.cell.2020.04.004",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_31_1"
},
{
"DOI": "10.1007/s40265-021-01639-2",
"article-title": "Angiotensin-Converting Enzyme Inhibitor and Angiotensin Receptor Blocker Use Associated with Reduced Mortality and Other Disease Outcomes in US Veterans with COVID-19",
"author": "Rizk JG",
"doi-asserted-by": "crossref",
"first-page": "43",
"issue": "1",
"journal-title": "Drugs",
"key": "e_1_3_8_32_1",
"unstructured": "Rizk JG, Wenziger C, Tran D, et al. Angiotensin-Converting Enzyme Inhibitor and Angiotensin Receptor Blocker Use Associated with Reduced Mortality and Other Disease Outcomes in US Veterans with COVID-19. Drugs. 2022 01 01;82(1):43–54.",
"volume": "82",
"year": "2022"
},
{
"DOI": "10.1016/S2665-9913(21)00144-2",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_33_1"
},
{
"DOI": "10.1053/j.gastro.2024.03.010",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_34_1"
},
{
"DOI": "10.1001/jamaoncol.2023.3384",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_35_1"
},
{
"DOI": "10.1001/jamapediatrics.2023.2465",
"article-title": "Data Extraction and Handling Issues on Evidence Synthesis of Risk of Immunoglobulin E-Mediated Food Allergy",
"author": "Xiao YT",
"doi-asserted-by": "crossref",
"first-page": "983",
"issue": "9",
"journal-title": "JAMA pediatrics",
"key": "e_1_3_8_36_1",
"unstructured": "Xiao YT, Cai J, Li GF. Data Extraction and Handling Issues on Evidence Synthesis of Risk of Immunoglobulin E-Mediated Food Allergy. JAMA pediatrics. 2023 Sep 1;177(9):983.",
"volume": "177",
"year": "2023"
},
{
"DOI": "10.1016/j.eclinm.2023.101904",
"article-title": "Data reproducibility issues on evidence synthesis of adverse events associated with HER2-targeted antibody-drug conjugates",
"author": "Wu DN",
"doi-asserted-by": "crossref",
"first-page": "101904",
"journal-title": "EClinicalMedicine",
"key": "e_1_3_8_37_1",
"unstructured": "Wu DN, Guan AJ, Li GF. Data reproducibility issues on evidence synthesis of adverse events associated with HER2-targeted antibody-drug conjugates. EClinicalMedicine. 2023 Apr;58:101904.",
"volume": "58",
"year": "2023"
},
{
"DOI": "10.1016/j.ejim.2020.04.037",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_38_1"
},
{
"article-title": "Liver Damage and Impaired Coagulation in COVID-19 Patients: A Case Series",
"author": "Abenavoli L",
"issue": "4",
"journal-title": "Diseases (Basel, Switzerland)",
"key": "e_1_3_8_39_1",
"unstructured": "Abenavoli L, Aquila I, Sacco MA, et al. Liver Damage and Impaired Coagulation in COVID-19 Patients: A Case Series. Diseases (Basel, Switzerland). 2023 Oct 13;11(4).",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1016/j.ajpath.2021.04.010",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_40_1"
},
{
"article-title": "Can Ursodeoxycholic Acid Prevent SARS-CoV-2 Infection or Reduce the COVID-19 Severity? Current Knowledge and Unresolved Issues",
"author": "Pan S",
"first-page": "114",
"issue": "3",
"journal-title": "Infect Immun",
"key": "e_1_3_8_41_1",
"unstructured": "Pan S, Zhang Y, Meng F, et al. Can Ursodeoxycholic Acid Prevent SARS-CoV-2 Infection or Reduce the COVID-19 Severity? Current Knowledge and Unresolved Issues. Infect Immun. 2023;3(3):114–119.",
"volume": "3",
"year": "2023"
},
{
"DOI": "10.1053/j.gastro.2023.02.008",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_42_1"
},
{
"DOI": "10.1016/S2352-3026(20)30251-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_43_1"
},
{
"DOI": "10.1183/23120541.00365-2022",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_44_1"
},
{
"key": "e_1_3_8_45_1",
"unstructured": "Clinical Spectrum of SARS-CoV-2 Infection 2022. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/"
}
],
"reference-count": 43,
"references-count": 43,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/14787210.2024.2376153"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Ursodeoxycholic acid and COVID-19 outcomes: a cohort study and data synthesis of state-of-art evidence",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01"
}