Ursodeoxycholic acid and COVID-19 outcomes: a cohort study and data synthesis of state-of-art evidence
et al., Expert Review of Anti-infective Therapy, doi:10.1080/14787210.2024.2376153, PROSPERO CRD42023495522, Jul 2024
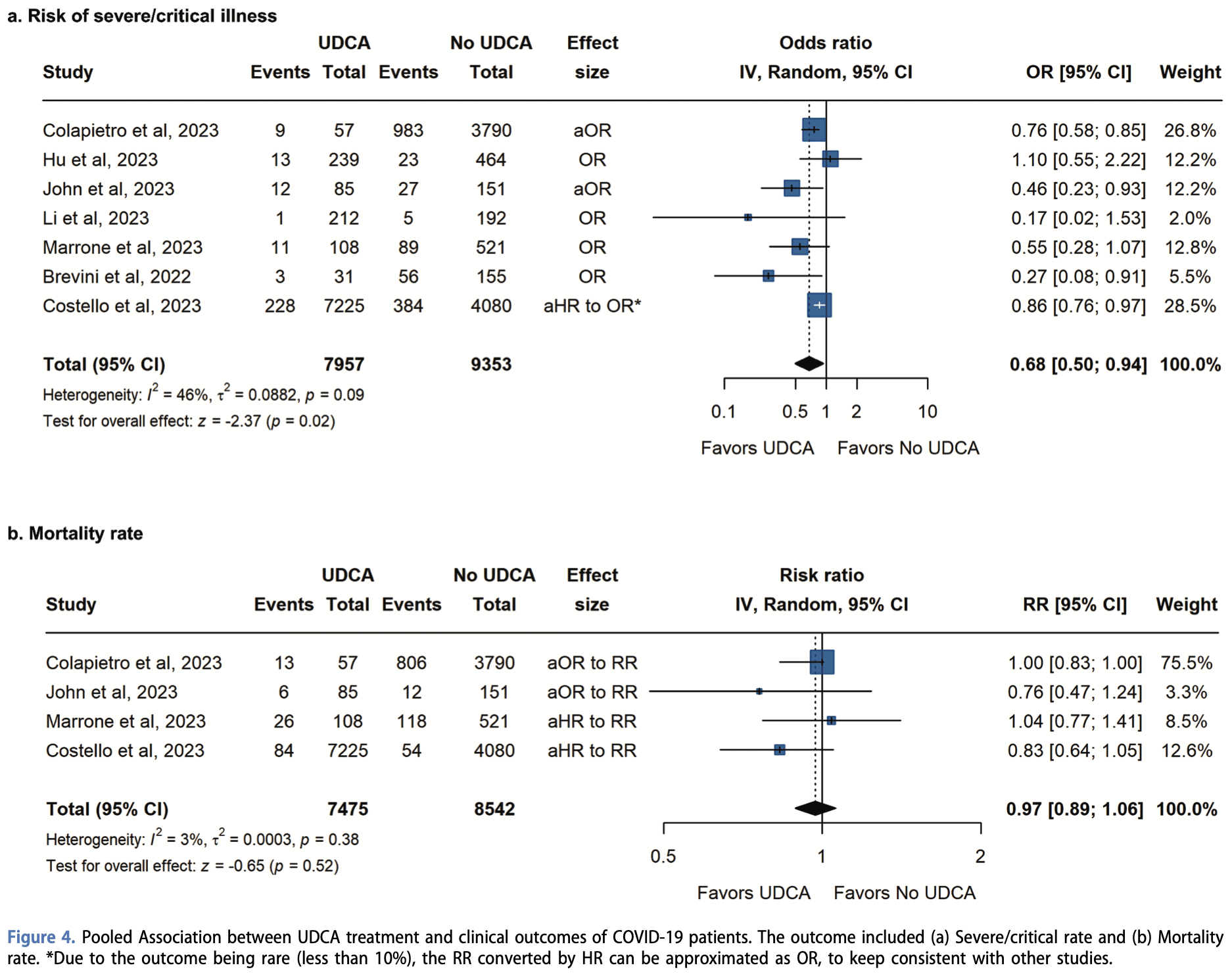
Meta analysis of 9 studies showing lower risk of severe/critical COVID-19 with UDCA. There was no significant difference for mortality.
Authors also perform a retrospective study which is listed separately1.
Currently there are 21 ursodeoxycholic acid studies and meta-analysis shows:
| Outcome | Improvement |
|---|---|
| Mortality | 7% lower [-27‑33%] |
| Ventilation | 6% lower [-293‑78%] |
| ICU admission | 27% lower [-52‑65%] |
| Hospitalization | 10% lower [-7‑24%] |
| Cases | 17% fewer [11‑23%] |
|
risk of death, 3.0% lower, OR 0.97, p = 0.50, RR approximated with OR.
|
|
risk of severe case, 32.0% lower, OR 0.68, p = 0.02, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Yu et al., 8 Jul 2024, peer-reviewed, 12 authors, trial PROSPERO CRD42023495522.
DOI record:
{
"DOI": "10.1080/14787210.2024.2376153",
"ISSN": [
"1478-7210",
"1744-8336"
],
"URL": "http://dx.doi.org/10.1080/14787210.2024.2376153",
"alternative-id": [
"10.1080/14787210.2024.2376153"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=ierz20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=ierz20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2024-03-26"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2024-06-10"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2024-07-08"
}
],
"author": [
{
"affiliation": [
{
"name": "National Key Laboratory for Novel Software Technology, Nanjing University, China"
},
{
"name": "Polixir.ai"
}
],
"family": "Yu",
"given": "Yang",
"sequence": "first"
},
{
"affiliation": [
{
"name": "School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, China"
}
],
"family": "Li",
"given": "Guo-Fu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital of Nanjing University, Nanjing University, China"
}
],
"family": "Li",
"given": "Jian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, China"
}
],
"family": "Han",
"given": "Lu-Yao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Key Laboratory for Novel Software Technology, Nanjing University, China"
},
{
"name": "Polixir.ai"
}
],
"family": "Zhang",
"given": "Zhi-Long",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Key Laboratory for Novel Software Technology, Nanjing University, China"
},
{
"name": "Polixir.ai"
}
],
"family": "Liu",
"given": "Tian-Shuo",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, China"
}
],
"family": "Jiao",
"given": "Shu-Xin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, China"
}
],
"family": "Qiao",
"given": "Yu-Wei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, China"
}
],
"family": "Zhang",
"given": "Na",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Key Laboratory for Novel Software Technology, Nanjing University, China"
}
],
"family": "Zhan",
"given": "De-Chuan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Hospital of Nanjing University, Nanjing University, China"
}
],
"family": "Tang",
"given": "Shao-Qiu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, China"
}
],
"family": "Yu",
"given": "Guo",
"sequence": "additional"
}
],
"container-title": "Expert Review of Anti-infective Therapy",
"container-title-short": "Expert Review of Anti-infective Therapy",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2024,
7,
8
]
],
"date-time": "2024-07-08T11:53:11Z",
"timestamp": 1720439591000
},
"deposited": {
"date-parts": [
[
2024,
7,
8
]
],
"date-time": "2024-07-08T11:53:24Z",
"timestamp": 1720439604000
},
"funder": [
{
"DOI": "10.13039/501100004608",
"doi-asserted-by": "publisher",
"name": "Natural Science Foundation of Jiangsu Province"
},
{
"DOI": "10.13039/501100010035",
"award": [
"BK20200005"
],
"doi-asserted-by": "publisher",
"name": "Outstanding Youth Foundation of Jiangsu Province"
}
],
"indexed": {
"date-parts": [
[
2024,
7,
9
]
],
"date-time": "2024-07-09T00:24:43Z",
"timestamp": 1720484683068
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
7,
8
]
]
},
"language": "en",
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/14787210.2024.2376153",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"prefix": "10.1080",
"published": {
"date-parts": [
[
2024,
7,
8
]
]
},
"published-online": {
"date-parts": [
[
2024,
7,
8
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1002/lt.25935",
"article-title": "Ursodeoxycholic Acid Decreases Incidence of Primary Biliary Cholangitis and Biliary Complications After Liver Transplantation: A Meta-Analysis",
"author": "Pedersen MR",
"doi-asserted-by": "crossref",
"first-page": "866",
"issue": "6",
"journal-title": "Liver transplantation",
"key": "e_1_3_8_2_1",
"unstructured": "Pedersen MR, Greenan G, Arora S, et al. Ursodeoxycholic Acid Decreases Incidence of Primary Biliary Cholangitis and Biliary Complications After Liver Transplantation: A Meta-Analysis. Liver transplantation. 2021 Jun;27(6):866–875.",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1016/j.jhep.2015.07.038",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_3_1"
},
{
"DOI": "10.1038/s41586-022-05594-0",
"article-title": "FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2",
"author": "Brevini T",
"doi-asserted-by": "crossref",
"issue": "7950",
"journal-title": "Nature",
"key": "e_1_3_8_4_1",
"unstructured": "Brevini T, Maes M, Webb GJ, et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature. 2023 Mar 2;615(7950):134±.",
"volume": "615"
},
{
"DOI": "10.3390/v15081738",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_5_1"
},
{
"article-title": "Effect of Ursodeoxycholic Acid on Preventing SARS-CoV-2 Infection in Patients With Liver Transplantation: a multicenter retrospective cohort study",
"author": "Hu L",
"journal-title": "QJM-INT J MED",
"key": "e_1_3_8_7_1",
"unstructured": "Hu L, Zhang H, Huang C, et al. Effect of Ursodeoxycholic Acid on Preventing SARS-CoV-2 Infection in Patients With Liver Transplantation: a multicenter retrospective cohort study. QJM-INT J MED. 2023 2023-Nov-09.",
"year": "2023"
},
{
"DOI": "10.1111/joim.13630",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_8_1"
},
{
"DOI": "10.3389/fcimb.2023.1178590",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_9_1"
},
{
"article-title": "Ursodeoxycholic acid does not affect the clinical outcome of SARS-CoV-2 infection: A retrospective study of propensity score-matched cohorts",
"author": "Marrone G",
"journal-title": "Liver Int",
"key": "e_1_3_8_10_1",
"unstructured": "Marrone G, Covino M, Merra G, et al. Ursodeoxycholic acid does not affect the clinical outcome of SARS-CoV-2 infection: A retrospective study of propensity score-matched cohorts. Liver Int. 2023.",
"year": "2023"
},
{
"article-title": "Clinical efficacy of ursodeoxycholic acid in the treatment of COVID-19",
"author": "Tianjiao T",
"first-page": "289",
"issue": "4",
"journal-title": "Chin J Clin Infect Dis",
"key": "e_1_3_8_11_1",
"unstructured": "Tianjiao T, Huatao L, Bibo M, et al. Clinical efficacy of ursodeoxycholic acid in the treatment of COVID-19. Chin J Clin Infect Dis. 2023;16(4):289–292 and 298.",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.1186/s12879-021-06670-y",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_12_1"
},
{
"DOI": "10.1200/JCO.23.00057",
"article-title": "Validity of Meta-Analytical Estimates and Hazard Ratios Quantifying Survival Benefit of Immunochemotherapy in Low PD-L1-Expressing Esophageal Squamous Cell Carcinoma",
"author": "Yu G",
"doi-asserted-by": "crossref",
"first-page": "2862",
"issue": "15",
"journal-title": "Journal of clinical oncology : official journal of the American Society of Clinical Oncology",
"key": "e_1_3_8_13_1",
"unstructured": "Yu G, Zhang N, Guan AJ, et al. Validity of Meta-Analytical Estimates and Hazard Ratios Quantifying Survival Benefit of Immunochemotherapy in Low PD-L1-Expressing Esophageal Squamous Cell Carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2023 May 20;41(15):2862–2864.",
"volume": "41",
"year": "2023"
},
{
"key": "e_1_3_8_14_1",
"unstructured": "Wells GA Shea B O’Connell D et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000."
},
{
"DOI": "10.1097/EDE.0000000000000733",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_15_1"
},
{
"DOI": "10.7326/M16-2607",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_16_1"
},
{
"DOI": "10.1177/0962280216669183",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_17_1"
},
{
"DOI": "10.1136/gutjnl-2020-323366",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_18_1"
},
{
"DOI": "10.1016/j.mce.2022.111618",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_19_1"
},
{
"DOI": "10.1038/ncomms10713",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_20_1"
},
{
"DOI": "10.1007/s00216-018-1183-7",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_21_1"
},
{
"DOI": "10.1001/archderm.143.6.757",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_22_1"
},
{
"DOI": "10.1016/j.jchromb.2005.08.016",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_23_1"
},
{
"DOI": "10.14814/phy2.15368",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_24_1"
},
{
"DOI": "10.1016/j.jhep.2012.02.014",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_25_1"
},
{
"DOI": "10.1038/nrgastro.2015.12",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_26_1"
},
{
"DOI": "10.1371/journal.pone.0234765",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_27_1"
},
{
"key": "e_1_3_8_28_1",
"unstructured": "China updates COVID-19 diagnosis treatment protocol [Internet]. 2023 [cited 2023]. Available from: http://english.nmpa.gov.cn/2023-01/10/c_861471.htm"
},
{
"article-title": "FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2",
"author": "Brevini T",
"journal-title": "Nature",
"key": "e_1_3_8_29_1",
"unstructured": "Brevini T, Maes M, Webb GJ, et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature. 2022 Dec 5.",
"year": "2022"
},
{
"DOI": "10.1038/s41591-021-01310-z",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_30_1"
},
{
"DOI": "10.1016/j.cell.2020.04.004",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_31_1"
},
{
"DOI": "10.1007/s40265-021-01639-2",
"article-title": "Angiotensin-Converting Enzyme Inhibitor and Angiotensin Receptor Blocker Use Associated with Reduced Mortality and Other Disease Outcomes in US Veterans with COVID-19",
"author": "Rizk JG",
"doi-asserted-by": "crossref",
"first-page": "43",
"issue": "1",
"journal-title": "Drugs",
"key": "e_1_3_8_32_1",
"unstructured": "Rizk JG, Wenziger C, Tran D, et al. Angiotensin-Converting Enzyme Inhibitor and Angiotensin Receptor Blocker Use Associated with Reduced Mortality and Other Disease Outcomes in US Veterans with COVID-19. Drugs. 2022 01 01;82(1):43–54.",
"volume": "82",
"year": "2022"
},
{
"DOI": "10.1016/S2665-9913(21)00144-2",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_33_1"
},
{
"DOI": "10.1053/j.gastro.2024.03.010",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_34_1"
},
{
"DOI": "10.1001/jamaoncol.2023.3384",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_35_1"
},
{
"DOI": "10.1001/jamapediatrics.2023.2465",
"article-title": "Data Extraction and Handling Issues on Evidence Synthesis of Risk of Immunoglobulin E-Mediated Food Allergy",
"author": "Xiao YT",
"doi-asserted-by": "crossref",
"first-page": "983",
"issue": "9",
"journal-title": "JAMA pediatrics",
"key": "e_1_3_8_36_1",
"unstructured": "Xiao YT, Cai J, Li GF. Data Extraction and Handling Issues on Evidence Synthesis of Risk of Immunoglobulin E-Mediated Food Allergy. JAMA pediatrics. 2023 Sep 1;177(9):983.",
"volume": "177",
"year": "2023"
},
{
"DOI": "10.1016/j.eclinm.2023.101904",
"article-title": "Data reproducibility issues on evidence synthesis of adverse events associated with HER2-targeted antibody-drug conjugates",
"author": "Wu DN",
"doi-asserted-by": "crossref",
"first-page": "101904",
"journal-title": "EClinicalMedicine",
"key": "e_1_3_8_37_1",
"unstructured": "Wu DN, Guan AJ, Li GF. Data reproducibility issues on evidence synthesis of adverse events associated with HER2-targeted antibody-drug conjugates. EClinicalMedicine. 2023 Apr;58:101904.",
"volume": "58",
"year": "2023"
},
{
"DOI": "10.1016/j.ejim.2020.04.037",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_38_1"
},
{
"article-title": "Liver Damage and Impaired Coagulation in COVID-19 Patients: A Case Series",
"author": "Abenavoli L",
"issue": "4",
"journal-title": "Diseases (Basel, Switzerland)",
"key": "e_1_3_8_39_1",
"unstructured": "Abenavoli L, Aquila I, Sacco MA, et al. Liver Damage and Impaired Coagulation in COVID-19 Patients: A Case Series. Diseases (Basel, Switzerland). 2023 Oct 13;11(4).",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1016/j.ajpath.2021.04.010",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_40_1"
},
{
"article-title": "Can Ursodeoxycholic Acid Prevent SARS-CoV-2 Infection or Reduce the COVID-19 Severity? Current Knowledge and Unresolved Issues",
"author": "Pan S",
"first-page": "114",
"issue": "3",
"journal-title": "Infect Immun",
"key": "e_1_3_8_41_1",
"unstructured": "Pan S, Zhang Y, Meng F, et al. Can Ursodeoxycholic Acid Prevent SARS-CoV-2 Infection or Reduce the COVID-19 Severity? Current Knowledge and Unresolved Issues. Infect Immun. 2023;3(3):114–119.",
"volume": "3",
"year": "2023"
},
{
"DOI": "10.1053/j.gastro.2023.02.008",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_42_1"
},
{
"DOI": "10.1016/S2352-3026(20)30251-9",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_43_1"
},
{
"DOI": "10.1183/23120541.00365-2022",
"doi-asserted-by": "publisher",
"key": "e_1_3_8_44_1"
},
{
"key": "e_1_3_8_45_1",
"unstructured": "Clinical Spectrum of SARS-CoV-2 Infection 2022. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/"
}
],
"reference-count": 43,
"references-count": 43,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/14787210.2024.2376153"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Ursodeoxycholic acid and COVID-19 outcomes: a cohort study and data synthesis of state-of-art evidence",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01"
}