Lopinavir/ritonavir for treatment of non-hospitalized patients with COVID-19: a randomized clinical trial
et al., International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.12.028, TREATNOW, NCT04372628, Mar 2023
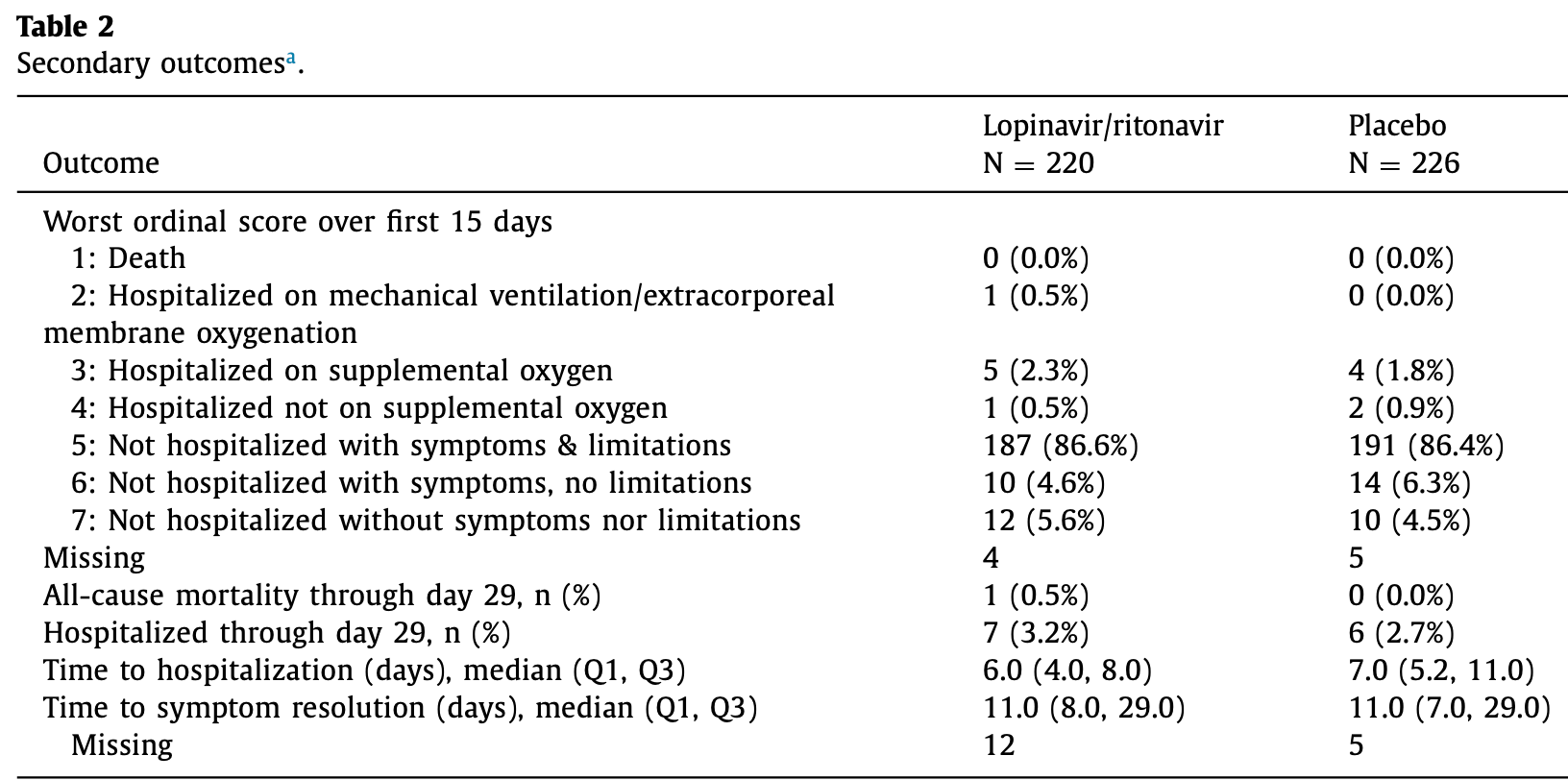
RCT 437 non-hospitalized COVID-19 patients showing no significant differences with lopinavir/ritonavir (LPV/r) treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 202.7% higher, RR 3.03, p = 0.49, treatment 1 of 220 (0.5%), control 0 of 226 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 19.8% higher, RR 1.20, p = 0.78, treatment 7 of 220 (3.2%), control 6 of 226 (2.7%).
|
|
risk of no recovery, 3.1% higher, HR 1.03, p = 0.88, treatment 220, control 226, inverted to make HR<1 favor treatment, ordinal category, day 15.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kaizer et al., 31 Mar 2023, Double Blind Randomized Controlled Trial, placebo-controlled, USA, peer-reviewed, mean age 41.0, 16 authors, study period June 2020 - December 2021, trial NCT04372628 (history) (TREATNOW).
Contact: adit.ginde@cuanschutz.edu.
Lopinavir/ritonavir for treatment of non-hospitalized patients with COVID-19: a randomized clinical trial
International Journal of Infectious Diseases, doi:10.1016/j.ijid.2022.12.028
Objectives: Effective and widely available therapies are still needed for outpatients with COVID-19. We aimed to evaluate the efficacy and safety of lopinavir/ritonavir (LPV/r) for early treatment of nonhospitalized individuals diagnosed with COVID-19. Methods: This randomized, placebo (Plb)-controlled, double-blind, multi-site decentralized clinical trial enrolled non-hospitalized adults with confirmed SARS-CoV-2 infection and six or fewer days of acute respiratory infection symptoms who were randomized to either twice-daily oral LPV/r (400 mg/100 mg) or Plb for 14 days. Daily surveys on study days 1 through 16 and again on study day 28 evaluated symptoms, daily activities, and hospitalization status. The primary outcome was longitudinal change in an ordinal scale based on a combination of symptoms, activity, and hospitalization status through day 15 and was analyzed by use of a Bayesian longitudinal proportional odds logistic regression model for estimating the probability of a superior recovery for LPV/r over Plb (odds ratio > 1). Results: Between June 2020 and December 2021, 448 participants were randomized to receive either LPV/r (n = 216) or Plb (n = 221). The mean symptom duration before randomization was 4.3 days (SD 1.3). There were no differences between treatment groups through the first 15 days for the ordinal primary outcome (odds ratio 0.96; 95% credible interval: 0.66 to 1.41). There were 3.2% (n = 7) of LPV/r and 2.7% (n = 6) of Plb participants hospitalized by day 28. Serious adverse events did not differ between groups. Conclusion: LPV/r did not significantly improve symptom resolution or reduce hospitalization in nonhospitalized participants with COVID-19.
Author contributions Funding was obtained by NIS, TWR, and AAG. The study was designed by AMK, NIS, TWR, CJL, and AAG. The underlying data were verified by AMK, JW, and KWH, and data analyses were done by AMK. AMK wrote the first draft of the manuscript. All authors interpreted data, provided critical review and revision of the text, and approved the final version of the manuscript. AAG takes responsibility for the manuscript as a whole.
Disclaimer The views expressed are those of the author(s) and do not reflect the official views or policy of the Department of Defense or its Components. The voluntary, fully informed consent of the subjects used in this research was obtained as required by 32 CFR 219 and DODI 3216.02_AFI 40-402.
Data sharing statement Deidentified data from the Trial of Early Antiviral Therapies during Non-hospitalized Outpatient Window (TREAT NOW) trial will be made available 1 year after publication of final results from the platform by written request, contingent on approval from the trial steering committee. Supporting documents will be made available, including the protocol, statistical analysis plan, informed consent document, and data dictionary. Data will be made available to researchers after approval of a proposal for use of the data.
Supplementary materials Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.ijid.2022.12.028 .
References
Baldelli, Corbellino, Clementi, Cattaneo, Gervasoni, Lopinavir/ritonavir in COVID-19 patients: maybe yes, but at what dose?, J Antimicrob Chemother, doi:10.1093/jac/dkaa190
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19 -Final Report, N Engl J Med, doi:10.1056/NEJMoa2007764
Cao, Wang, Wen, Liu, Wang et al., A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19, N Engl J Med, doi:10.1056/NEJMoa2001282
Chan, Yao, Yeung, Deng, Bao et al., Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset, J Infect Dis, doi:10.1093/infdis/jiv392
Gottlieb, Nirula, Chen, Boscia, Heller et al., Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial, JAMA, doi:10.1001/jama.2021.0202
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early remdesivir to prevent progression to severe Covid-19 in outpatients, N Engl J Med, doi:10.1056/NEJMoa2116846
Group, Horby, Lim, Emberson, Mafham et al., Dexamethasone in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Harrell, rmsb: Bayesian Regression Modeling Strategies
Hope, Understanding and improving recovery from COVID-19, Ann Intern Med, doi:10.7326/M22-1492
Kaizer, Wild, Lindsell, Rice, Self et al., Trial of Early antiviral Therapies during Non-hospitalized Outpatient Window (TREAT NOW) for COVID-19: a summary of the protocol and analysis plan for a decentralized randomized controlled trial, Trials, doi:10.1186/s13063-022-06213-z
Kalil, Patterson, Mehta, Tomashek, Wolfe et al., Baricitinib plus remdesivir for hospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2031994
Karolyi, Omid, Pawelka, Jilma, Stimpfl et al., High dose lopinavir/ritonavir does not lead to sufficient plasma levels to inhibit SARS-CoV-2 in hospitalized patients with COVID-19, Front Pharmacol, doi:10.3389/fphar.2021.704767
Kim, Won, Kee, Jung, Jang, Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for Middle East respiratory syndrome, Antivir Ther, doi:10.3851/IMP3002
León, Dorabawila, Nelson, Lutterloh, Bauer et al., COVID-19 cases and hospitalizations by COVID-19 vaccination status and previous COVID-19 diagnosis-California and New York, May-November 2021, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7104e1
Li, Xie, Lin, Cai, Wen et al., Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial, Med (N Y), doi:10.1016/j.medj.2020.04.001
Lozano, Santibáñez, Severino, Saldias, Vera et al., How far are we from predicting multi-drug interactions during treatment for COVID-19 infection?, Br J Pharmacol, doi:10.1111/bph.penalty-@M15819
Magro, Zanella, Pescarolo, Castelli, Quiros-Roldan, Lopinavir/ritonavir: repurposing an old drug for HIV infection in COVID-19 treatment, Biomed J, doi:10.1016/j.bj.2020.11.005
Martinez, Compounds with therapeutic potential against novel respiratory 2019 coronavirus, Antimicrob Agents Chemother, doi:10.1128/AAC.00399-20
Marzolini, Stader, Stoeckle, Franzeck, Egli et al., Effect of systemic inflammatory response to SARS-CoV-2 on lopinavir and hydroxychloroquine plasma concentrations, Antimicrob Agents Chemother, doi:10.1128/AAC.01177-20
Nutho, Mahalapbutr, Hengphasatporn, Pattaranggoon, Simanon et al., Why are lopinavir and ritonavir effective against the newly emerged coronavirus 2019? Atomistic insights into the inhibitory mechanisms, Biochemistry, doi:10.1021/acs.biochem.0c00160
Pillaiyar, Manickam, Namasivayam, Hayashi, Jung, An overview of severe acute respiratory syndrome-coronavirus (SARS-CoV) 3CL protease inhibitors: peptidomimetics and small molecule chemotherapy, J Med Chem, doi:10.1021/acs.jmedchem.5b01461
Reis, Silva, Silva, Thabane, Singh et al., Effect of early treatment with hydroxychloroquine or lopinavir and ritonavir on risk of hospitalization among patients with COVID-19: the TOGETHER randomized clinical trial, JAMA Netw Open, doi:10.1001/jamanetworkopen.2021.6468
Saravolatz, Depcinski, Sharma, Molnupiravir and nirmatrelvir-ritonavir: oral Coronavirus Disease 2019 antiviral drugs, Clin Infect Dis, doi:10.1093/cid/ciac180
Sneller, Liang, Marques, Chung, Shanbhag et al., A longitudinal study of COVID-19 sequelae and immunity: baseline findings, Ann Intern Med, doi:10.7326/M21-4905
Takashita, Kinoshita, Yamayoshi, Sakai-Tagawa, Fujisaki et al., Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2, N Engl J Med, doi:10.1056/NEJMc2201933
Vangeel, Chiu, Jonghe, Maes, Slechten et al., Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antiviral Res, doi:10.1016/j.antiviral.2022.105252
Wang, Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study, J Chem Inf Model, doi:10.1021/acs.jcim.0c00179
Waxman, Makov-Assif, Reis, Netzer, Balicer et al., Comparing COVID-19-related hospitalization rates among individuals with infection-induced and vaccine-induced immunity in Israel, Nat Commun, doi:10.1038/s41467-022-29858-5
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2035002
Wen, Chen, Tang, Wang, Zhou et al., Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis, Ann Med, doi:10.1080/07853890.2022.2034936
Zumla, Chan, Azhar, Hui, Yuen, Coronaviruses-drug discovery and therapeutic options, Nat Rev Drug Discov, doi:10.1038/nrd.2015.37
DOI record:
{
"DOI": "10.1016/j.ijid.2022.12.028",
"ISSN": [
"1201-9712"
],
"URL": "http://dx.doi.org/10.1016/j.ijid.2022.12.028",
"alternative-id": [
"S1201971222006658"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Lopinavir/ritonavir for treatment of non-hospitalized patients with COVID-19: a randomized clinical trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Infectious Diseases"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijid.2022.12.028"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Authors. Published by Elsevier Ltd on behalf of International Society for Infectious Diseases."
}
],
"author": [
{
"ORCID": "https://orcid.org/0000-0003-2334-5514",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kaizer",
"given": "Alexander M.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Shapiro",
"given": "Nathan I.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-2927-8239",
"affiliation": [],
"authenticated-orcid": false,
"family": "Wild",
"given": "Jessica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brown",
"given": "Samuel M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cwik",
"given": "B. Jessica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hart",
"given": "Kimberly W.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jones",
"given": "Alan E.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-5809-4060",
"affiliation": [],
"authenticated-orcid": false,
"family": "Pulia",
"given": "Michael S.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-9300-3045",
"affiliation": [],
"authenticated-orcid": false,
"family": "Self",
"given": "Wesley H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Clay",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Smith",
"given": "Stephanie A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ng",
"given": "Patrick C.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6391-0448",
"affiliation": [],
"authenticated-orcid": false,
"family": "Thompson",
"given": "B. Taylor",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7136-5408",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rice",
"given": "Todd W.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3297-2811",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lindsell",
"given": "Christopher J.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ginde",
"given": "Adit A.",
"sequence": "additional"
}
],
"container-title": "International Journal of Infectious Diseases",
"container-title-short": "International Journal of Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"ijidonline.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
12,
27
]
],
"date-time": "2022-12-27T00:44:50Z",
"timestamp": 1672101890000
},
"deposited": {
"date-parts": [
[
2024,
5,
11
]
],
"date-time": "2024-05-11T14:46:17Z",
"timestamp": 1715438777000
},
"indexed": {
"date-parts": [
[
2025,
5,
29
]
],
"date-time": "2025-05-29T18:23:23Z",
"timestamp": 1748543003537,
"version": "3.40.5"
},
"is-referenced-by-count": 16,
"issued": {
"date-parts": [
[
2023,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
1
]
],
"date-time": "2023-03-01T00:00:00Z",
"timestamp": 1677628800000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
1
]
],
"date-time": "2023-03-01T00:00:00Z",
"timestamp": 1677628800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
12,
21
]
],
"date-time": "2022-12-21T00:00:00Z",
"timestamp": 1671580800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971222006658?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1201971222006658?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "223-229",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
3
]
]
},
"published-print": {
"date-parts": [
[
2023,
3
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19 – Final Report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.12.028_bib0001",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early remdesivir to prevent progression to severe Covid-19 in outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.12.028_bib0002",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1001/jama.2021.0202",
"article-title": "Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "632",
"journal-title": "JAMA",
"key": "10.1016/j.ijid.2022.12.028_bib0003",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19",
"author": "Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.12.028_bib0004",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus remdesivir for hospitalized adults with Covid-19",
"author": "Kalil",
"doi-asserted-by": "crossref",
"first-page": "795",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.12.028_bib0005",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2035002",
"article-title": "REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"first-page": "238",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.12.028_bib0006",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2022.105252",
"article-title": "Remdesivir, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern",
"author": "Vangeel",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "10.1016/j.ijid.2022.12.028_bib0007",
"volume": "198",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2201933",
"article-title": "Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "1475",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.12.028_bib0008",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(21)00751-9",
"article-title": "Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial",
"doi-asserted-by": "crossref",
"first-page": "622",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.ijid.2022.12.028_bib0009",
"volume": "22",
"year": "2022"
},
{
"article-title": "Molnupiravir and nirmatrelvir-ritonavir: oral Coronavirus Disease 2019 antiviral drugs",
"author": "Saravolatz",
"first-page": "ciac180",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.ijid.2022.12.028_bib0010",
"year": "2022"
},
{
"DOI": "10.1111/bph.15819",
"article-title": "How far are we from predicting multi-drug interactions during treatment for COVID-19 infection?",
"author": "Lozano",
"doi-asserted-by": "crossref",
"first-page": "3831",
"journal-title": "Br J Pharmacol",
"key": "10.1016/j.ijid.2022.12.028_bib0011",
"volume": "179",
"year": "2022"
},
{
"DOI": "10.1038/s41467-022-29858-5",
"article-title": "Comparing COVID-19-related hospitalization rates among individuals with infection-induced and vaccine-induced immunity in Israel",
"author": "Waxman",
"doi-asserted-by": "crossref",
"first-page": "2202",
"journal-title": "Nat Commun",
"key": "10.1016/j.ijid.2022.12.028_bib0012",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1080/07853890.2022.2034936",
"article-title": "Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis",
"author": "Wen",
"doi-asserted-by": "crossref",
"first-page": "516",
"journal-title": "Ann Med",
"key": "10.1016/j.ijid.2022.12.028_bib0013",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7104e1",
"article-title": "COVID-19 cases and hospitalizations by COVID-19 vaccination status and previous COVID-19 diagnosis—California and New York, May–November 2021",
"author": "León",
"doi-asserted-by": "crossref",
"first-page": "125",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "10.1016/j.ijid.2022.12.028_bib0014",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.7326/M21-4905",
"article-title": "A longitudinal study of COVID-19 sequelae and immunity: baseline findings",
"author": "Sneller",
"doi-asserted-by": "crossref",
"first-page": "969",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.ijid.2022.12.028_bib0015",
"volume": "175",
"year": "2022"
},
{
"DOI": "10.7326/M22-1492",
"article-title": "Understanding and improving recovery from COVID-19",
"author": "Hope",
"doi-asserted-by": "crossref",
"first-page": "1041",
"journal-title": "Ann Intern Med",
"key": "10.1016/j.ijid.2022.12.028_bib0016",
"volume": "175",
"year": "2022"
},
{
"DOI": "10.1038/nrd.2015.37",
"article-title": "Coronaviruses—drug discovery and therapeutic options",
"author": "Zumla",
"doi-asserted-by": "crossref",
"first-page": "327",
"journal-title": "Nat Rev Drug Discov",
"key": "10.1016/j.ijid.2022.12.028_bib0017",
"volume": "15",
"year": "2016"
},
{
"DOI": "10.1093/infdis/jiv392",
"article-title": "Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "1904",
"journal-title": "J Infect Dis",
"key": "10.1016/j.ijid.2022.12.028_bib0018",
"volume": "212",
"year": "2015"
},
{
"DOI": "10.1021/acs.jcim.0c00179",
"article-title": "Fast identification of possible drug treatment of coronavirus disease-19 (COVID-19) through computational drug repurposing study",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "3277",
"journal-title": "J Chem Inf Model",
"key": "10.1016/j.ijid.2022.12.028_bib0019",
"volume": "60",
"year": "2020"
},
{
"DOI": "10.1128/AAC.00399-20",
"article-title": "Compounds with therapeutic potential against novel respiratory 2019 coronavirus",
"author": "Martinez",
"doi-asserted-by": "crossref",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.ijid.2022.12.028_bib0020",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.3851/IMP3002",
"article-title": "Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for Middle East respiratory syndrome",
"author": "Kim",
"doi-asserted-by": "crossref",
"first-page": "455",
"journal-title": "Antivir Ther",
"key": "10.1016/j.ijid.2022.12.028_bib0021",
"volume": "21",
"year": "2016"
},
{
"DOI": "10.1016/j.bj.2020.11.005",
"article-title": "Lopinavir/ritonavir: repurposing an old drug for HIV infection in COVID-19 treatment",
"author": "Magro",
"doi-asserted-by": "crossref",
"first-page": "43",
"journal-title": "Biomed J",
"key": "10.1016/j.ijid.2022.12.028_bib0022",
"volume": "44",
"year": "2021"
},
{
"DOI": "10.1021/acs.biochem.0c00160",
"article-title": "Why are lopinavir and ritonavir effective against the newly emerged coronavirus 2019? Atomistic insights into the inhibitory mechanisms",
"author": "Nutho",
"doi-asserted-by": "crossref",
"first-page": "1769",
"journal-title": "Biochemistry",
"key": "10.1016/j.ijid.2022.12.028_bib0023",
"volume": "59",
"year": "2020"
},
{
"DOI": "10.1021/acs.jmedchem.5b01461",
"article-title": "An overview of severe acute respiratory syndrome–coronavirus (SARS-CoV) 3CL protease inhibitors: peptidomimetics and small molecule chemotherapy",
"author": "Pillaiyar",
"doi-asserted-by": "crossref",
"first-page": "6595",
"journal-title": "J Med Chem",
"key": "10.1016/j.ijid.2022.12.028_bib0024",
"volume": "59",
"year": "2016"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.12.028_bib0025",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "1787",
"journal-title": "N Engl J Med",
"key": "10.1016/j.ijid.2022.12.028_bib0026",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)32013-4",
"article-title": "Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"doi-asserted-by": "crossref",
"first-page": "1345",
"journal-title": "Lancet",
"key": "10.1016/j.ijid.2022.12.028_bib0027",
"volume": "396",
"year": "2020"
},
{
"article-title": "Efficacy and safety of lopinavir/ritonavir or arbidol in adult patients with mild/moderate COVID-19: an exploratory randomized controlled trial",
"author": "Li",
"journal-title": "Med (N Y)",
"key": "10.1016/j.ijid.2022.12.028_bib0028",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2021.6468",
"article-title": "Effect of early treatment with hydroxychloroquine or lopinavir and ritonavir on risk of hospitalization among patients with COVID-19: the TOGETHER randomized clinical trial",
"author": "Reis",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.ijid.2022.12.028_bib0029",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1186/s13063-022-06213-z",
"article-title": "Trial of Early antiviral Therapies during Non-hospitalized Outpatient Window (TREAT NOW) for COVID-19: a summary of the protocol and analysis plan for a decentralized randomized controlled trial",
"author": "Kaizer",
"doi-asserted-by": "crossref",
"first-page": "273",
"journal-title": "Trials",
"key": "10.1016/j.ijid.2022.12.028_bib0030",
"volume": "23",
"year": "2022"
},
{
"key": "10.1016/j.ijid.2022.12.028_bib0031",
"unstructured": "World health organization. WHO R&D Blueprint: informal consultation on prioritization of candidate therapeutic agents for use in novel coronavirus 2019 infection, Geneva, Switzerland, 24 January 2020. Geneva: World Health Organization; 2020."
},
{
"key": "10.1016/j.ijid.2022.12.028_bib0032",
"unstructured": "Harrell F. rmsb: Bayesian Regression Modeling Strategies. R package version 0.1.0 ed 2022. https://cran.r-project.org/web/packages/rmsb/index.html."
},
{
"DOI": "10.1093/jac/dkaa190",
"article-title": "Lopinavir/ritonavir in COVID-19 patients: maybe yes, but at what dose?",
"author": "Baldelli",
"doi-asserted-by": "crossref",
"first-page": "2704",
"journal-title": "J Antimicrob Chemother",
"key": "10.1016/j.ijid.2022.12.028_bib0033",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2021.704767",
"article-title": "High dose lopinavir/ritonavir does not lead to sufficient plasma levels to inhibit SARS-CoV-2 in hospitalized patients with COVID-19",
"author": "Karolyi",
"doi-asserted-by": "crossref",
"journal-title": "Front Pharmacol",
"key": "10.1016/j.ijid.2022.12.028_bib0034",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1128/AAC.01177-20",
"article-title": "Effect of systemic inflammatory response to SARS-CoV-2 on lopinavir and hydroxychloroquine plasma concentrations",
"author": "Marzolini",
"doi-asserted-by": "crossref",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.ijid.2022.12.028_bib0035",
"volume": "64",
"year": "2020"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1201971222006658"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Lopinavir/ritonavir for treatment of non-hospitalized patients with COVID-19: a randomized clinical trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "128"
}