Dexamethasone in Hospitalized Patients with Covid-19
et al., New England Journal of Medicine, doi:10.1056/NEJMoa2021436, RECOVERY, NCT04381936, Feb 2021
RCT 6,425 hospitalized COVID-19 patients showing lower 28-day mortality with dexamethasone treatment. The benefit was most pronounced in patients who had symptoms for more than 7 days at randomization, suggesting dexamethasone is most effective when the disease is dominated by inflammatory processes rather than viral replication. 6-month results are from Horby et al.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments2.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
|
risk of death, 13.0% lower, RR 0.87, p = 0.005, treatment 596 of 2,104 (28.3%), control 1,297 of 4,321 (30.0%), NNT 59, adjusted per study, day 180.
|
|
risk of death, 17.0% lower, RR 0.83, p < 0.001, treatment 482 of 2,104 (22.9%), control 1,110 of 4,321 (25.7%), NNT 36, adjusted per study, day 28.
|
|
risk of mechanical ventilation, 21.0% lower, RR 0.79, p = 0.03, treatment 110 of 1,780 (6.2%), control 298 of 3,638 (8.2%), NNT 50, adjusted per study, day 28.
|
|
risk of no hospital discharge, 9.1% lower, RR 0.91, p = 0.003, treatment 2,104, control 4,321, adjusted per study, inverted to make RR<1 favor treatment, day 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Horby et al., 25 Feb 2021, Randomized Controlled Trial, United Kingdom, peer-reviewed, mean age 66.1, 26 authors, study period 19 March, 2020 - 8 June, 2020, trial NCT04381936 (history) (RECOVERY).
Dexamethasone in Hospitalized Patients with Covid-19
New England Journal of Medicine, doi:10.1056/nejmoa2021436
Coronavirus disease 2019 (Covid-19) is associated with diffuse lung damage. Glucocorticoids may modulate inflammation-mediated lung injury and thereby reduce progression to respiratory failure and death.
METHODS In this controlled, open-label trial comparing a range of possible treatments in patients who were hospitalized with Covid-19, we randomly assigned patients to receive oral or intravenous dexamethasone (at a dose of 6 mg once daily) for up to 10 days or to receive usual care alone. The primary outcome was 28-day mortality. Here, we report the final results of this assessment.
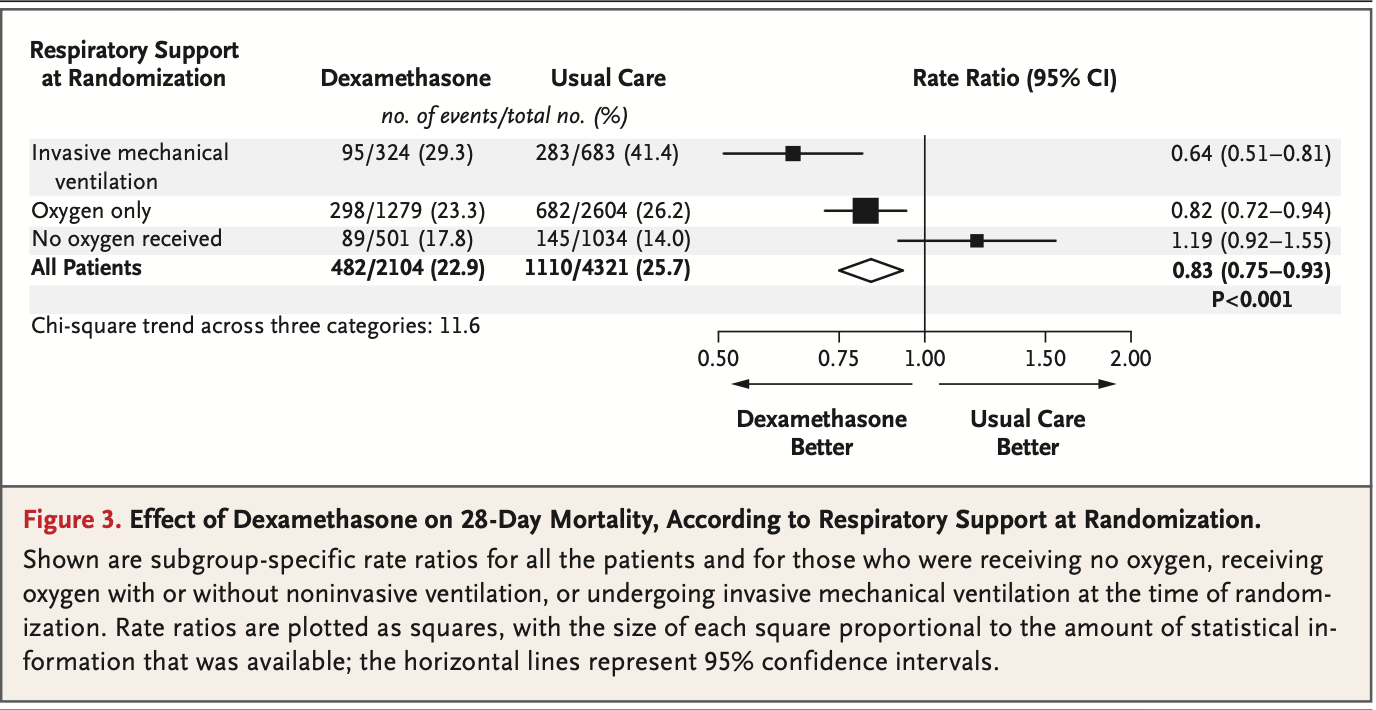
RESULTS A total of 2104 patients were assigned to receive dexamethasone and 4321 to receive usual care. Overall, 482 patients (22.9%) in the dexamethasone group and 1110 patients (25.7%) in the usual care group died within 28 days after randomization (age-adjusted rate ratio, 0.83; 95% confidence interval [CI], 0.75 to 0.93; P<0.001). The proportional and absolute between-group differences in mortality varied considerably according to the level of respiratory support that the patients were receiving at the time of randomization. In the dexamethasone group, the incidence of death was lower than that in the usual care group among patients receiving invasive mechanical ventilation (29.3% vs. 41.4%; rate ratio, 0.64; 95% CI, 0.51 to 0.81) and among those receiving oxygen without invasive mechanical ventilation (23.3% vs. 26.2%; rate ratio, 0.82; 95% CI, 0.72 to 0.94) but not among those who were receiving no respiratory support at randomization (17.8% vs. 14.0%; rate ratio, 1.19; 95% CI, 0.92 to 1.55).
CONCLUSIONS In patients hospitalized with Covid-19, the use of dexamethasone resulted in lower 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone at randomization but not among those receiving no respiratory support.
Appendix The affiliations of the members of the writing committee are as follows: the Nuffield Department of Medicine (P.H.), Nuffield Department of Population Health (J.R.E., M.M., J.L.B., L.L., N.S., E.J., R.H., M.J.L.), and MRC Population Health Research Unit (J.R.E., N.S., R.H., M.J.L.), University of Oxford, the Oxford University Hospitals NHS Foundation Trust (K.J.
References
Arabi, Mandourah, Hameed, Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome, Am J Respir Crit Care Med
Baillie, Digard, Influenza -time to target the host?, N Engl J Med
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -final report, N Engl J Med
Cao, Tu, Cheng, Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China, Clin Infect Dis
Carsana, Sonzogni, Nasr, The New England Journal of Medicine is produced by NEJM Group, a division of the Massachusetts Medical Society.
Chen, Zhou, Dong, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet
Cheng, Wong, Tong, Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome, Lancet
Collins, Bowman, Landray, Peto, The magic of randomization versus the myth of real-world evidence, N Engl J Med
Corral-Gudino, Bahamonde, Arnaiz-Revillas, GLUCOCOVID: a controlled trial of methylprednisolone in adults hospitalized with COVID-19 pneumonia, doi:10.1101/2020.06.17.20133579v1
Dagens, Sigfrid, Cai, Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review, BMJ
De Jong, Simmons, Thanh, Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia, Nat Med
Docherty, Harrison, Green, Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study, BMJ
He, Lau, Wu, Temporal dynamics in viral shedding and transmissibility of COVID-19, Nat Med
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Lansbury, Rodrigo, Leonardi-Bee, Nguyen-Van-Tam, Lim, Corticosteroids as adjunctive therapy in the treatment of influenza: an updated Cochrane systematic review and metaanalysis, Crit Care Med
Lee, Chan, Hui, Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients, J Clin Virol
Lee, Chan, Hui, Viral loads and duration of viral shedding in adult patients hospitalized with influenza, J Infect Dis
Moore, June, Cytokine release syndrome in severe COVID-19, Science
Rojek, Horby, Modernising epidemic science: enabling patient-centred research during epidemics, BMC Med
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med
Russell, Millar, Baillie, Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury, Lancet
Shang, Zhao, Hu, Du, Cao, On the use of corticosteroids for 2019-nCoV pneumonia, Lancet
Siemieniuk, Meade, Alonso-Coello, Corticosteroid therapy for patients hospitalized with communityacquired pneumonia: a systematic review and meta-analysis, Ann Intern Med
Siemieniuk, Rochwerg, Agoritsas, A living WHO guideline on drugs for Covid-19, BMJ
Sterne, Murthy, Diaz, Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a metaanalysis, JAMA
Stockman, Bellamy, Garner, SARS: systematic review of treatment effects, PLoS Med
To, Tsang, Leung, Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study, Lancet Infect Dis
Verity, Okell, Dorigatti, Estimates of the severity of coronavirus disease 2019: a model-based analysis, Lancet Infect Dis
Wang, Hu, Hu, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA
Whitty, Dexamethasone in the treatment of COVID-19: implementation and management of supply for treatment in hospitals
Wong, Lam, Wu, Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome, Clin Exp Immunol
Wölfel, Corman, Guggemos, Virological assessment of hospitalized patients with COVID-2019
Xu, Wu, Jiang, Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series, BMJ
Yusuf, Collins, Peto, Why do we need some large, simple randomized trials?, Stat Med
Zhao, Hu, Du, Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia, Zhonghua Jie He He Hu Xi Za Zhi
Zhou, Li, Chen, Viral dynamics in asymptomatic patients with COVID-19, Int J Infect Dis
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
Zhu, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, 2019, N Engl J Med
DOI record:
{
"DOI": "10.1056/nejmoa2021436",
"ISSN": [
"0028-4793",
"1533-4406"
],
"URL": "http://dx.doi.org/10.1056/NEJMoa2021436",
"alternative-id": [
"10.1056/NEJMoa2021436"
],
"author": [
{
"affiliation": [],
"name": "The RECOVERY Collaborative Group",
"sequence": "first"
}
],
"container-title": "New England Journal of Medicine",
"container-title-short": "N Engl J Med",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
7,
17
]
],
"date-time": "2020-07-17T16:04:22Z",
"timestamp": 1595001862000
},
"deposited": {
"date-parts": [
[
2024,
3,
27
]
],
"date-time": "2024-03-27T00:36:27Z",
"timestamp": 1711499787000
},
"funder": [
{
"DOI": "10.13039/100014013",
"award": [
"MC_PC_19056"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100014013",
"id-type": "DOI"
}
],
"name": "United Kingdom Research and Innovation"
},
{
"DOI": "10.13039/501100000272",
"award": [
"MC_PC_19056"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000272",
"id-type": "DOI"
}
],
"name": "National Institute for Health Research"
}
],
"indexed": {
"date-parts": [
[
2025,
6,
6
]
],
"date-time": "2025-06-06T16:35:04Z",
"timestamp": 1749227704949,
"version": "3.37.3"
},
"is-referenced-by-count": 7426,
"issue": "8",
"issued": {
"date-parts": [
[
2021,
2,
25
]
]
},
"journal-issue": {
"issue": "8",
"published-print": {
"date-parts": [
[
2021,
2,
25
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://www.nejmgroup.org/legal/terms-of-use.htm",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
25
]
],
"date-time": "2021-02-25T00:00:00Z",
"timestamp": 1614211200000
}
}
],
"link": [
{
"URL": "http://www.nejm.org/doi/pdf/10.1056/NEJMoa2021436",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "150",
"original-title": [],
"page": "693-704",
"prefix": "10.1056",
"published": {
"date-parts": [
[
2021,
2,
25
]
]
},
"published-print": {
"date-parts": [
[
2021,
2,
25
]
]
},
"publisher": "Massachusetts Medical Society",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A novel coronavirus from patients with pneumonia in China, 2019.",
"author": "Zhu N",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "N Engl J Med",
"key": "e_1_3_5_2_2",
"unstructured": "Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727-733.31978945",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30243-7",
"article-title": "Estimates of the severity of coronavirus disease 2019: a model-based analysis.",
"author": "Verity R",
"doi-asserted-by": "crossref",
"first-page": "669",
"journal-title": "Lancet Infect Dis",
"key": "e_1_3_5_3_2",
"unstructured": "Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis 2020;20:669-677.32240634",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study.",
"author": "Zhou F",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "e_1_3_5_4_2",
"unstructured": "Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-1062.32171076",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"article-title": "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study.",
"author": "Chen N",
"doi-asserted-by": "crossref",
"first-page": "507",
"journal-title": "Lancet",
"key": "e_1_3_5_5_2",
"unstructured": "Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-513.32007143",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa243",
"article-title": "Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China.",
"author": "Cao J",
"doi-asserted-by": "crossref",
"first-page": "748",
"journal-title": "Clin Infect Dis",
"key": "e_1_3_5_6_2",
"unstructured": "Cao J, Tu W-J, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis 2020;71:748-755.32239127",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-05991-x",
"article-title": "Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China.",
"author": "Ruan Q",
"doi-asserted-by": "crossref",
"first-page": "846",
"journal-title": "Intensive Care Med",
"key": "e_1_3_5_7_2",
"unstructured": "Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846-848.32125452",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m1985",
"article-title": "Features of 20133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study.",
"author": "Docherty AB",
"doi-asserted-by": "crossref",
"first-page": "m1985",
"journal-title": "BMJ",
"key": "e_1_3_5_8_2",
"unstructured": "Docherty AB, Harrison EM, Green CA, et al. Features of 20133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 2020;369:m1985-m1985.32444460",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19 — final report.",
"author": "Beigel JH",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "e_1_3_5_9_2",
"unstructured": "Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med 2020;383:1813-1826.32445440",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30434-5",
"article-title": "Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study.",
"author": "Carsana L",
"doi-asserted-by": "crossref",
"first-page": "1135",
"journal-title": "Lancet Infect Dis",
"key": "e_1_3_5_10_2",
"unstructured": "Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 2020;20:1135-1140.32526193",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1038/nm1477",
"article-title": "Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia.",
"author": "de Jong MD",
"doi-asserted-by": "crossref",
"first-page": "1203",
"journal-title": "Nat Med",
"key": "e_1_3_5_11_2",
"unstructured": "de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006;12:1203-1207.16964257",
"volume": "12",
"year": "2006"
},
{
"DOI": "10.1111/j.1365-2249.2004.02415.x",
"article-title": "Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome.",
"author": "Wong CK",
"doi-asserted-by": "crossref",
"first-page": "95",
"journal-title": "Clin Exp Immunol",
"key": "e_1_3_5_12_2",
"unstructured": "Wong CK, Lam CWK, Wu AKL, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 2004;136:95-103.15030519",
"volume": "136",
"year": "2004"
},
{
"DOI": "10.1056/NEJMcibr1304414",
"article-title": "Influenza — time to target the host?",
"author": "Baillie JK",
"doi-asserted-by": "crossref",
"first-page": "191",
"journal-title": "N Engl J Med",
"key": "e_1_3_5_13_2",
"unstructured": "Baillie JK, Digard P. Influenza — time to target the host? N Engl J Med 2013;369:191-193.23841736",
"volume": "369",
"year": "2013"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China.",
"author": "Huang C",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "Lancet",
"key": "e_1_3_5_14_2",
"unstructured": "Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506.31986264",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1126/science.abb8925",
"article-title": "Cytokine release syndrome in severe COVID-19.",
"author": "Moore JB",
"doi-asserted-by": "crossref",
"first-page": "473",
"journal-title": "Science",
"key": "e_1_3_5_15_2",
"unstructured": "Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020;368:473-474.32303591",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30361-5",
"article-title": "On the use of corticosteroids for 2019-nCoV pneumonia.",
"author": "Shang L",
"doi-asserted-by": "crossref",
"first-page": "683",
"journal-title": "Lancet",
"key": "e_1_3_5_16_2",
"unstructured": "Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 2020;395:683-684.32122468",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30317-2",
"article-title": "Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury.",
"author": "Russell CD",
"doi-asserted-by": "crossref",
"first-page": "473",
"journal-title": "Lancet",
"key": "e_1_3_5_17_2",
"unstructured": "Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 2020;395:473-475.32043983",
"volume": "395",
"year": "2020"
},
{
"key": "e_1_3_5_18_2",
"unstructured": "Corral-Gudino L Bahamonde A Arnaiz-Revillas F et al. GLUCOCOVID: a controlled trial of methylprednisolone in adults hospitalized with COVID-19 pneumonia. June 18 2020 (https://www.medrxiv.org/content/10.1101/2020.06.17.20133579v1). preprint."
},
{
"DOI": "10.1136/bmj.m1936",
"article-title": "Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review.",
"author": "Dagens A",
"doi-asserted-by": "crossref",
"first-page": "m1936",
"journal-title": "BMJ",
"key": "e_1_3_5_19_2",
"unstructured": "Dagens A, Sigfrid L, Cai E, et al. Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review. BMJ 2020;369:m1936-m1936.32457027",
"volume": "369",
"year": "2020"
},
{
"article-title": "Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia",
"author": "Zhao JP",
"first-page": "183",
"journal-title": "Zhonghua Jie He He Hu Xi Za Zhi",
"key": "e_1_3_5_20_2",
"unstructured": "Zhao JP, Hu Y, Du RH, et al. Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:183-184. (In Chinese.)32164084",
"volume": "43",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"article-title": "Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China.",
"author": "Wang D",
"doi-asserted-by": "crossref",
"first-page": "1061",
"journal-title": "JAMA",
"key": "e_1_3_5_21_2",
"unstructured": "Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061-1069.32031570",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m606",
"article-title": "Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series.",
"author": "Xu XW",
"doi-asserted-by": "crossref",
"first-page": "m606",
"journal-title": "BMJ",
"key": "e_1_3_5_22_2",
"unstructured": "Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020;368:m606-m606.32075786",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.17023",
"article-title": "Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis.",
"author": "WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group",
"doi-asserted-by": "crossref",
"first-page": "1330",
"journal-title": "JAMA",
"key": "e_1_3_5_23_2",
"unstructured": "WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA 2020;324:1330-1341.32876694",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1002/sim.4780030421",
"article-title": "Why do we need some large, simple randomized trials?",
"author": "Yusuf S",
"doi-asserted-by": "crossref",
"first-page": "409",
"journal-title": "Stat Med",
"key": "e_1_3_5_24_2",
"unstructured": "Yusuf S, Collins R, Peto R. Why do we need some large, simple randomized trials? Stat Med 1984;3:409-422.6528136",
"volume": "3",
"year": "1984"
},
{
"article-title": "Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group.",
"author": "ISIS-2 (Second International Study of Infarct Survival) Collaborative Group",
"first-page": "349",
"journal-title": "Lancet",
"key": "e_1_3_5_25_2",
"unstructured": "ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet 1988;2:349-360.2899772",
"volume": "2",
"year": "1988"
},
{
"DOI": "10.1056/NEJMsb1901642",
"article-title": "The magic of randomization versus the myth of real-world evidence.",
"author": "Collins R",
"doi-asserted-by": "crossref",
"first-page": "674",
"journal-title": "N Engl J Med",
"key": "e_1_3_5_26_2",
"unstructured": "Collins R, Bowman L, Landray M, Peto R. The magic of randomization versus the myth of real-world evidence. N Engl J Med 2020;382:674-678.32053307",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1186/s12916-016-0760-x",
"article-title": "Modernising epidemic science: enabling patient-centred research during epidemics.",
"author": "Rojek AM",
"doi-asserted-by": "crossref",
"first-page": "212",
"journal-title": "BMC Med",
"key": "e_1_3_5_27_2",
"unstructured": "Rojek AM, Horby PW. Modernising epidemic science: enabling patient-centred research during epidemics. BMC Med 2016;14:212-212.27989237",
"volume": "14",
"year": "2016"
},
{
"key": "e_1_3_5_28_2",
"unstructured": "Whitty C. Dexamethasone in the treatment of COVID-19: implementation and management of supply for treatment in hospitals. London: Medicines and Healthcare Products Regulatory Agency June 16 2020 (https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAlert.aspx?AlertID=103054)."
},
{
"DOI": "10.1371/journal.pmed.0030343",
"article-title": "SARS: systematic review of treatment effects.",
"author": "Stockman LJ",
"doi-asserted-by": "crossref",
"first-page": "e343",
"issue": "9",
"journal-title": "PLoS Med",
"key": "e_1_3_5_29_2",
"unstructured": "Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med 2006;3(9):e343-e343.16968120",
"volume": "3",
"year": "2006"
},
{
"DOI": "10.1164/rccm.201706-1172OC",
"article-title": "Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome.",
"author": "Arabi YM",
"doi-asserted-by": "crossref",
"first-page": "757",
"journal-title": "Am J Respir Crit Care Med",
"key": "e_1_3_5_30_2",
"unstructured": "Arabi YM, Mandourah Y, Al-Hameed F, et al. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018;197:757-767.29161116",
"volume": "197",
"year": "2018"
},
{
"DOI": "10.1097/CCM.0000000000004093",
"article-title": "Corticosteroids as adjunctive therapy in the treatment of influenza: an updated Cochrane systematic review and meta-analysis.",
"author": "Lansbury LE",
"doi-asserted-by": "crossref",
"first-page": "e98",
"issue": "2",
"journal-title": "Crit Care Med",
"key": "e_1_3_5_31_2",
"unstructured": "Lansbury LE, Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Shen Lim W. Corticosteroids as adjunctive therapy in the treatment of influenza: an updated Cochrane systematic review and meta-analysis. Crit Care Med 2020;48(2):e98-e106.31939808",
"volume": "48",
"year": "2020"
},
{
"DOI": "10.7326/M15-0715",
"article-title": "Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis.",
"author": "Siemieniuk RA",
"doi-asserted-by": "crossref",
"first-page": "519",
"journal-title": "Ann Intern Med",
"key": "e_1_3_5_32_2",
"unstructured": "Siemieniuk RA, Meade MO, Alonso-Coello P, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med 2015;163:519-528.26258555",
"volume": "163",
"year": "2015"
},
{
"DOI": "10.1016/j.jcv.2004.07.006",
"article-title": "Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients.",
"author": "Lee N",
"doi-asserted-by": "crossref",
"first-page": "304",
"journal-title": "J Clin Virol",
"key": "e_1_3_5_33_2",
"unstructured": "Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol 2004;31:304-309.15494274",
"volume": "31",
"year": "2004"
},
{
"DOI": "10.1086/600383",
"article-title": "Viral loads and duration of viral shedding in adult patients hospitalized with influenza.",
"author": "Lee N",
"doi-asserted-by": "crossref",
"first-page": "492",
"journal-title": "J Infect Dis",
"key": "e_1_3_5_34_2",
"unstructured": "Lee N, Chan PKS, Hui DSC, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis 2009;200:492-500.19591575",
"volume": "200",
"year": "2009"
},
{
"DOI": "10.1016/S0140-6736(04)16255-7",
"article-title": "Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome.",
"author": "Cheng PKC",
"doi-asserted-by": "crossref",
"first-page": "1699",
"journal-title": "Lancet",
"key": "e_1_3_5_35_2",
"unstructured": "Cheng PKC, Wong DA, Tong LKL, et al. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet 2004;363:1699-1700.15158632",
"volume": "363",
"year": "2004"
},
{
"DOI": "10.1016/S1473-3099(20)30196-1",
"article-title": "Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study.",
"author": "To KK-W",
"doi-asserted-by": "crossref",
"first-page": "565",
"journal-title": "Lancet Infect Dis",
"key": "e_1_3_5_36_2",
"unstructured": "To KK-W, Tsang OT-T, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020;20:565-574.32213337",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.05.030",
"article-title": "Viral dynamics in asymptomatic patients with COVID-19.",
"author": "Zhou R",
"doi-asserted-by": "crossref",
"first-page": "288",
"journal-title": "Int J Infect Dis",
"key": "e_1_3_5_37_2",
"unstructured": "Zhou R, Li F, Chen F, et al. Viral dynamics in asymptomatic patients with COVID-19. Int J Infect Dis 2020;96:288-290.32437933",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.1038/s41591-020-0869-5",
"article-title": "Temporal dynamics in viral shedding and transmissibility of COVID-19.",
"author": "He X",
"doi-asserted-by": "crossref",
"first-page": "672",
"journal-title": "Nat Med",
"key": "e_1_3_5_38_2",
"unstructured": "He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020;26:672-675.32296168",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2196-x",
"article-title": "Virological assessment of hospitalized patients with COVID-2019.",
"author": "Wölfel R",
"doi-asserted-by": "crossref",
"first-page": "465",
"journal-title": "Nature",
"key": "e_1_3_5_39_2",
"unstructured": "Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020;581:465-469.32235945",
"volume": "581",
"year": "2020"
},
{
"key": "e_1_3_5_40_2",
"unstructured": "COVID-19 treatment guidelines: corticosteroids. Bethesda MD: National Institutes of Health 2020 (https://www.covid19treatmentguidelines.nih.gov/dexamethasone/)."
},
{
"article-title": "A living WHO guideline on drugs for Covid-19.",
"author": "Siemieniuk R",
"first-page": "m3379",
"journal-title": "BMJ",
"key": "e_1_3_5_41_2",
"unstructured": "Siemieniuk R, Rochwerg B, Agoritsas T, et al. A living WHO guideline on drugs for Covid-19. BMJ 2020;370:m3379-m3379.32887691",
"volume": "370",
"year": "2020"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2020.06.22.20137273",
"id-type": "doi"
}
],
"has-review": [
{
"asserted-by": "object",
"id": "10.3410/f.738341916.793577274",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "http://www.nejm.org/doi/10.1056/NEJMoa2021436"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Dexamethasone in Hospitalized Patients with Covid-19",
"type": "journal-article",
"volume": "384"
}