Post-exposure Lopinavir-Ritonavir Prophylaxis versus Surveillance for Individuals Exposed to SARS-CoV-2: The COPEP Pragmatic Open-Label, Cluster Randomized Trial
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2021.101188, COPEP, NCT04364022, Dec 2021
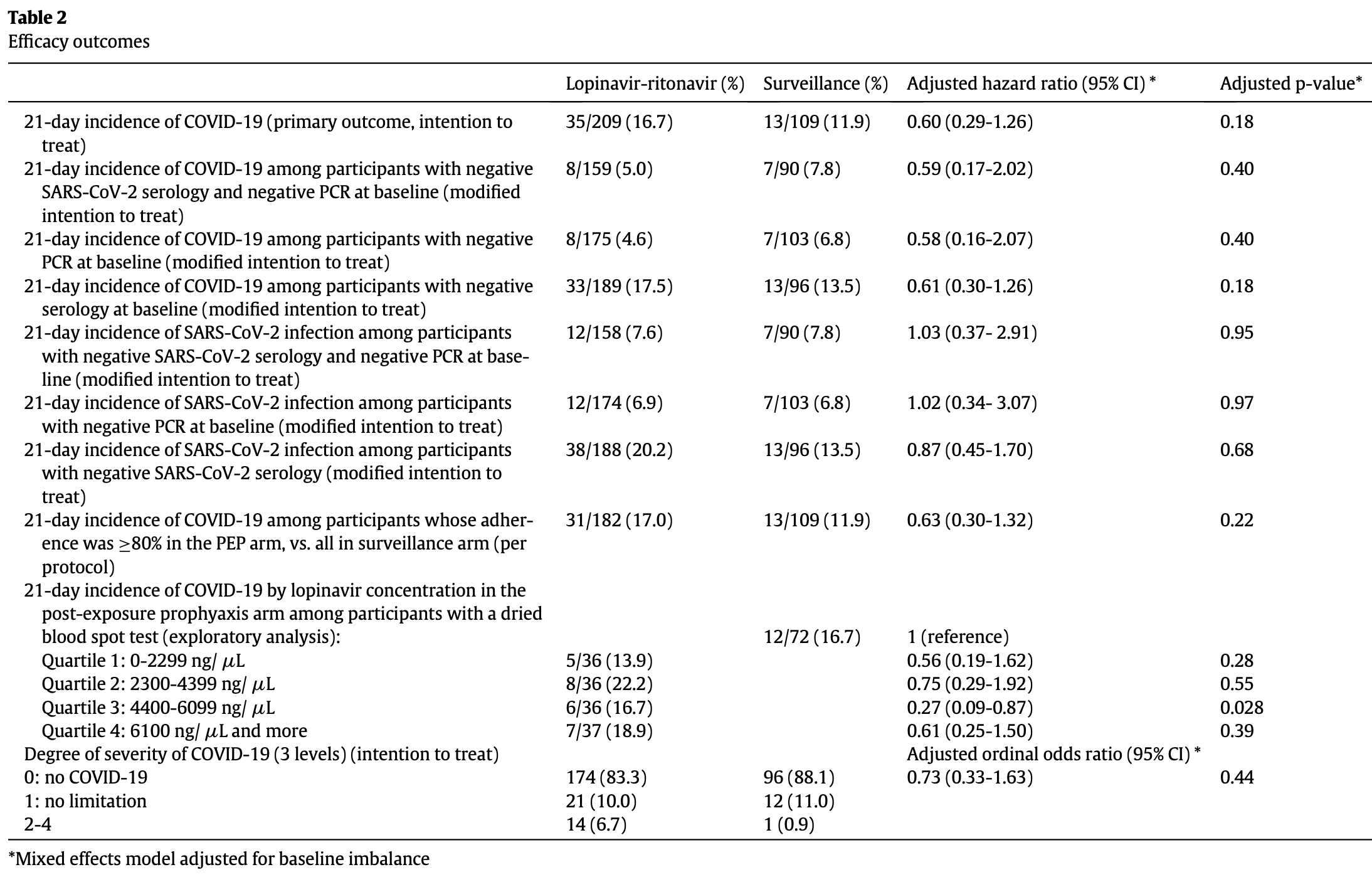
Open-label, cluster-randomized RCT 318 asymptomatic close contacts in Switzerland and Brazil showing no statistically significant difference in symptomatic COVID-19 at 21 days with LPV/r prophylaxis. The mid-trial changes in allocation and 10-month recruitment window introduces potential calendar-time confounding - e.g., if community incidence fell over time, this may over-represent lower-risk weeks in the LPV/r arm, thereby overestimating efficacy. Authors reports 2 hospitalizations but do not specify which group the patients were in.
This study is excluded in the after exclusion results of meta-analysis:
significant confounding by time possible.
|
risk of progression, 630.1% higher, RR 7.30, p = 0.02, treatment 14 of 209 (6.7%), control 1 of 109 (0.9%), level 2-4.
|
|
risk of symptomatic case, 40.0% lower, HR 0.60, p = 0.17, treatment 35 of 209 (16.7%), control 13 of 109 (11.9%), adjusted per study.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Labhardt et al., 31 Dec 2021, Randomized Controlled Trial, multiple countries, peer-reviewed, mean age 39.7, 26 authors, study period March 2020 - March 2021, trial NCT04364022 (history) (COPEP).
Contact: Alexandra.Calmy@hcuge.ch.
Post-exposure Lopinavir-Ritonavir Prophylaxis versus Surveillance for Individuals Exposed to SARS-CoV-2: The COPEP Pragmatic Open-Label, Cluster Randomized Trial
eClinicalMedicine, doi:10.1016/j.eclinm.2021.101188
which was 3-fold more frequent in the LPV/r arm (34/209 [16.3%] vs 6/109 [5.5%], respectively). During 21day follow-up, 48/318 (15.1%) participants developed in the LPV/r group and 13/ 109 (11.9%) in the surveillance group (unadjusted hazard ratio 1.44; 95% CI, 0.76-2.73). In the primary endpoint analysis, which was adjuted for baseline imbalance, the hazard ratio for developing COVID-19 in the LPV/r group vs surveillance was 0.60 (95% CI, 0.29-1.26; p =0.18). Interpretation: The role of LPV/r as PEP for COVID-19 remains unanswered. Although LPV/r over 5 days did not significantly reduce the incidence of COVID-19 in exposed individuals, we observed a change in the directionality of the effect in favour of LPV/r after adjusting for baseline imbalance. LPV/r for this indication merits further testing against SARS-CoV-2 in clinical trials.
Author contributions AC and TP had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Data sharing statement Anonymized data set with core-data from the trial will be made available on Zenodo upon publication of the manuscript. The full dataset will be available upon reasonable request to the corresponding author.
Declaration of Competing Interest Dr. Niklaus D Labhardt reports support for attending CROI conference 2020 and AIDS conference 2020 by Gilead Sciences, support for attending CROI Conference 2021 by ViiV healthcare, and participation on a Data Safety Monitoring Board for the C1 6201 PROTECT trial by Pharming Group NV. Dr. Mikaela Smit reports scientific grants at Imperial College London by CDRF (prime is PEPFAR) and NIH, and consulting fees for modeling consulting by Gilead Sciences, IAS, Maple Health. Dr. Enos Bernasconi reports grants from Merck & Co. and Swiss National Science Foundation, consulting fees and support for attending meeting by Gilead Sciences, Merck & Co., ViiV healthcare, Pfizer AG, Abbvie, paid to the Lugano Regional Hospital. Dr. Alexandra Calmy reports grant from MSD for qualitative research on women and clinical trials, and unrestricted grant from Gilead, ViiV Healthcare, MSD and SIDAIDE foundation related to the funding support to LIPO and metabolims Day Hospital at Geneva University Hospitals. All other authors have nothing to disclose.
..
References
Arabi, Asiri, Assiri, Interferon Beta-1b and LopinavirÀRitonavir for Middle East Respiratory Syndrome, New England Journal of Medicine
Bartoszko, Siemieniuk, Kum, Prophylaxis against covid-19: living systematic review and network meta-analysis, BMJ
Boulware, Pullen, Bangdiwala, A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19, N Engl J Med
Callaway, Fast-spreading COVID variant can elude immune responses, Nature
Cao, Wang, Wen, A Trial of LopinavirÀRitonavir in Adults Hospitalized with Severe Covid-19, New England Journal of Medicine
Choy, Wong, Kaewpreedee, Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro, Antiviral Res
Cohen, Burbelo, Reinfection with SARS-CoV-2: Implications for Vaccines, Clin Infect Dis, doi:10.1093/cid/ciaa1866
De Figueiredo, Simas, Karafillakis, Paterson, Larson, Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study, The Lancet
Eli, Company, Phase 3 Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Efficacy and Safety of LY3819253 Alone and in Combination With LY3832479 in Preventing SARS-CoV-2 Infection and COVID-19 in Skilled Nursing and Assisted Living Facility Residents and Staff; a NIAID and Lilly Collaborative Study
For, COVID-19 PREVEN-TION: INTERIM RESULTS
Hellwig, Lower incidence associated with prophylactic administration of ivermectin, International Journal of Antimicrobial Agents
Horby, Mafham, Bell, LopinavirÀritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial, The Lancet
Hung, Lung, Tso, Triple combination of interferon beta-1b, lopinavirÀritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial, The Lancet
Jing, Liu, Zhang, Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study, The Lancet Infectious Diseases
Karim, De Oliveira, New SARS-CoV-2 Variants -Clinical, Public Health, and Vaccine Implications, New England Journal of Medicine
Madewell, Yang, Longini, Halloran, Dean, Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis, JAMA Netw Open
Mahase, Covid-19: UK launches antivirals taskforce to deliver home treatments by autumn, BMJ
Mehra, Desai, Ruschitzka, Patel, RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis, The Lancet, doi:10.1016/S0140-6736(20)31180-6
Schwarzinger, Watson, Arwidson, Alla, Luchini, COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics, The Lancet Public Health, doi:10.1016/S2468-2667(21)00012-8
Siemieniuk, Bartoszko, Ge, Drug treatments for covid-19: living systematic review and network meta-analysis, BMJ
Smit, Marinosci, Agoritsas, Calmy, Prophylaxis for COVID-19: a systematic review, Clin Microbiol Infect, doi:10.1016/j.cmi.2021.01.013
Tan, Chan, Post-exposure prophylaxis against SARS-CoV-2 in close contacts of confirmed COVID-19 cases (CORIPREV): study protocol for a cluster-randomized trial, Trials
Thakur, Tan, Chan, Physiologically-Based Pharmacokinetic Modeling to Predict the Clinical Efficacy of the Coadministration of Lopinavir and Ritonavir against SARS-CoV-2, Clin Pharmacol Ther
Who, Pan, Peto, Repurposed Antiviral Drugs for Covid-19 -Interim WHO Solidarity Trial Results, N Engl J Med
DOI record:
{
"DOI": "10.1016/j.eclinm.2021.101188",
"ISSN": [
"2589-5370"
],
"URL": "http://dx.doi.org/10.1016/j.eclinm.2021.101188",
"alternative-id": [
"S2589537021004697"
],
"article-number": "101188",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Post-exposure Lopinavir-Ritonavir Prophylaxis versus Surveillance for Individuals Exposed to SARS-CoV-2: The COPEP Pragmatic Open-Label, Cluster Randomized Trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "eClinicalMedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.eclinm.2021.101188"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Labhardt",
"given": "Niklaus D",
"sequence": "first"
},
{
"affiliation": [],
"family": "Smit",
"given": "Mikaela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Petignat",
"given": "Ianis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perneger",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marinosci",
"given": "Annalisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ustero",
"given": "Pilar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Diniz Ribeiro",
"given": "Maria Pia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ragozzino",
"given": "Silvio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nicoletti",
"given": "Giovanni Jacopo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Faré",
"given": "Pietro Benedetto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Andrey",
"given": "Diego O",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jacquerioz",
"given": "Frederique",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lebowitz",
"given": "Dan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Agoritsas",
"given": "Thomas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meyer",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Spechbach",
"given": "Hervé",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salamun",
"given": "Julien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guessous",
"given": "Idris",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chappuis",
"given": "François",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kaiser",
"given": "Laurent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Decosterd",
"given": "Laurent Arthur",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grinsztejn",
"given": "Beatriz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bernasconi",
"given": "Enos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cardoso",
"given": "Sandra Wagner",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Calmy",
"given": "Alexandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Team",
"given": "for the COPEP Study",
"sequence": "additional"
}
],
"container-title": "eClinicalMedicine",
"container-title-short": "eClinicalMedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
11,
6
]
],
"date-time": "2021-11-06T03:47:34Z",
"timestamp": 1636170454000
},
"deposited": {
"date-parts": [
[
2023,
3,
21
]
],
"date-time": "2023-03-21T14:18:33Z",
"timestamp": 1679408313000
},
"indexed": {
"date-parts": [
[
2024,
8,
16
]
],
"date-time": "2024-08-16T00:51:04Z",
"timestamp": 1723769464803
},
"is-referenced-by-count": 4,
"issued": {
"date-parts": [
[
2021,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
21
]
],
"date-time": "2021-10-21T00:00:00Z",
"timestamp": 1634774400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537021004697?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537021004697?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "101188",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
12
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.eclinm.2021.101188_bib0001",
"unstructured": "A year without precedent: WHO's COVID-19 response. https://www.who.int/news-room/spotlight/a-year-without-precedent-who-s-covid-19-response (accessed March 16, 2021)."
},
{
"DOI": "10.1016/S1473-3099(20)30471-0",
"article-title": "Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study",
"author": "Jing",
"doi-asserted-by": "crossref",
"first-page": "1141",
"journal-title": "The Lancet Infectious Diseases",
"key": "10.1016/j.eclinm.2021.101188_bib0002",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2020.31756",
"article-title": "Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis",
"author": "Madewell",
"doi-asserted-by": "crossref",
"journal-title": "JAMA Netw Open",
"key": "10.1016/j.eclinm.2021.101188_bib0003",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1016/S2468-2667(21)00012-8",
"doi-asserted-by": "crossref",
"key": "10.1016/j.eclinm.2021.101188_bib0004",
"unstructured": "Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. The Lancet Public Health 2021; 0. DOI:10.1016/S2468-2667(21)00012-8."
},
{
"DOI": "10.1016/S0140-6736(20)31558-0",
"article-title": "Mapping global trends in vaccine confidence and investigating barriers to vaccine uptake: a large-scale retrospective temporal modelling study",
"author": "de",
"doi-asserted-by": "crossref",
"first-page": "898",
"journal-title": "The Lancet",
"key": "10.1016/j.eclinm.2021.101188_bib0005",
"volume": "396",
"year": "2020"
},
{
"article-title": "Reinfection with SARS-CoV-2: Implications for Vaccines",
"author": "Cohen",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.eclinm.2021.101188_bib0006",
"year": "2020"
},
{
"DOI": "10.1038/d41586-021-00121-z",
"article-title": "Fast-spreading COVID variant can elude immune responses",
"author": "Callaway",
"doi-asserted-by": "crossref",
"first-page": "500",
"journal-title": "Nature",
"key": "10.1016/j.eclinm.2021.101188_bib0007",
"volume": "589",
"year": "2021"
},
{
"article-title": "New SARS-CoV-2 Variants — Clinical, Public Health, and Vaccine Implications",
"author": "Abdool Karim",
"issue": "null",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.eclinm.2021.101188_bib0008",
"volume": "0",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n1077",
"article-title": "Covid-19: UK launches antivirals taskforce to deliver home treatments by autumn",
"author": "Mahase",
"doi-asserted-by": "crossref",
"first-page": "n1077",
"journal-title": "BMJ",
"key": "10.1016/j.eclinm.2021.101188_bib0009",
"volume": "373",
"year": "2021"
},
{
"article-title": "Regeneron Pharmaceuticals",
"key": "10.1016/j.eclinm.2021.101188_bib0010",
"year": "2021"
},
{
"key": "10.1016/j.eclinm.2021.101188_bib0011",
"unstructured": "Phase 3 Prevention Trial Showed 81% Reduced Risk of Symptomatic SARS-CoV-2 Infections with Subcutaneous Administration of REGEN-COVTM (casirivimab with imdevimab) | Regeneron Pharmaceuticals Inc. https://investor.regeneron.com/news-releases/news-release-details/phase-3-prevention-trial-showed-81-reduced-risk-symptomatic-sars/ (accessed April 19, 2021)."
},
{
"author": "Lilly",
"journal-title": "clinicaltrials.gov",
"key": "10.1016/j.eclinm.2021.101188_bib0012",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2016638",
"article-title": "A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19",
"author": "Boulware",
"doi-asserted-by": "crossref",
"first-page": "517",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.101188_bib0013",
"volume": "383",
"year": "2020"
},
{
"article-title": "Prevention of SARS-CoV-2 (COVID-19) Through Pre-Exposure Prophylaxis With Tenofovir Disoproxil Fumarate/Emtricitabine and Hydroxychloroquine in Healthcare Personnel: Randomized Clinical Trial Controlled With Placebo",
"journal-title": "clinicaltrials.gov",
"key": "10.1016/j.eclinm.2021.101188_bib0014",
"year": "2020"
},
{
"key": "10.1016/j.eclinm.2021.101188_bib0015",
"unstructured": "Control of COVID-19 Outbreaks in Long Term Care - Tabular View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/record/NCT04448119 (accessed March 22, 2021)."
},
{
"DOI": "10.1016/j.ijantimicag.2020.106248",
"article-title": "Lower incidence associated with prophylactic administration of ivermectin",
"author": "Hellwig",
"doi-asserted-by": "crossref",
"journal-title": "International Journal of Antimicrobial Agents",
"key": "10.1016/j.eclinm.2021.101188_bib0016",
"volume": "57",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m2980",
"article-title": "Drug treatments for covid-19: living systematic review and network meta-analysis",
"author": "Siemieniuk",
"doi-asserted-by": "crossref",
"first-page": "m2980",
"journal-title": "BMJ",
"key": "10.1016/j.eclinm.2021.101188_bib0017",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.1016/j.cmi.2021.01.013",
"article-title": "Prophylaxis for COVID-19: a systematic review",
"author": "Smit",
"doi-asserted-by": "crossref",
"journal-title": "Clin Microbiol Infect",
"key": "10.1016/j.eclinm.2021.101188_bib0018",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n949",
"article-title": "Prophylaxis against covid-19: living systematic review and network meta-analysis",
"author": "Bartoszko",
"doi-asserted-by": "crossref",
"first-page": "n949",
"journal-title": "BMJ",
"key": "10.1016/j.eclinm.2021.101188_bib0019",
"volume": "373",
"year": "2021"
},
{
"DOI": "10.1016/j.antiviral.2020.104786",
"article-title": "Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro",
"author": "Choy",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "10.1016/j.eclinm.2021.101188_bib0020",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2015294",
"article-title": "Interferon Beta-1b and Lopinavir–Ritonavir for Middle East Respiratory Syndrome",
"author": "Arabi",
"doi-asserted-by": "crossref",
"first-page": "1645",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.eclinm.2021.101188_bib0021",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)32013-4",
"article-title": "Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "1345",
"journal-title": "The Lancet",
"key": "10.1016/j.eclinm.2021.101188_bib0022",
"volume": "396",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2023184",
"article-title": "Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results",
"author": "Solidarity Trial Consortium",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.101188_bib0023",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "1787",
"journal-title": "New England Journal of Medicine",
"key": "10.1016/j.eclinm.2021.101188_bib0024",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31042-4",
"article-title": "Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial",
"author": "Hung",
"doi-asserted-by": "crossref",
"first-page": "1695",
"journal-title": "The Lancet",
"key": "10.1016/j.eclinm.2021.101188_bib0025",
"volume": "395",
"year": "2020"
},
{
"article-title": "RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis",
"author": "Mehra",
"journal-title": "The Lancet",
"key": "10.1016/j.eclinm.2021.101188_bib0026",
"volume": "0",
"year": "2020"
},
{
"key": "10.1016/j.eclinm.2021.101188_bib0027",
"unstructured": "BAMLANIVIMAB PREVENTS COVID-19 MORBIDITY AND MORTALITY IN NURSING-HOME SETTING. CROI Conference. https://www.croiconference.org/abstract/bamlanivimab-prevents-covid-19-morbidity-and-mortality-in-nursing-home-setting/ (accessed May 4, 2021)."
},
{
"key": "10.1016/j.eclinm.2021.101188_bib0028",
"unstructured": "CASIRIVIMAB WITH IMDEVIMAB ANTIBODY COCKTAIL FOR COVID-19 PREVENTION: INTERIM RESULTS. CROI Conference. https://www.croiconference.org/abstract/casirivimab-with-imdevimab-antibody-cocktail-for-covid-19-prevention-interim-results/ (accessed May 6, 2021)."
},
{
"key": "10.1016/j.eclinm.2021.101188_bib0029",
"unstructured": "REDUCTION IN INFECTIOUS SARS-CoV-2 IN TREATMENT STUDY OF COVID-19 WITH MOLNUPIRAVIR. https://www.croiconference.org/abstract/reduction-in-infectious-sars-cov-2-in-treatment-study-of-covid-19-with-molnupiravir/ (accessed May 6, 2021)."
},
{
"DOI": "10.1002/cpt.2014",
"article-title": "Physiologically-Based Pharmacokinetic Modeling to Predict the Clinical Efficacy of the Coadministration of Lopinavir and Ritonavir against SARS-CoV-2",
"author": "Thakur",
"doi-asserted-by": "crossref",
"first-page": "1176",
"journal-title": "Clin Pharmacol Ther",
"key": "10.1016/j.eclinm.2021.101188_bib0030",
"volume": "108",
"year": "2020"
},
{
"DOI": "10.1186/s13063-021-05134-7",
"article-title": "Post-exposure prophylaxis against SARS-CoV-2 in close contacts of confirmed COVID-19 cases (CORIPREV): study protocol for a cluster-randomized trial",
"author": "Tan",
"doi-asserted-by": "crossref",
"first-page": "224",
"journal-title": "Trials",
"key": "10.1016/j.eclinm.2021.101188_bib0031",
"volume": "22",
"year": "2021"
},
{
"key": "10.1016/j.eclinm.2021.101188_bib0032",
"unstructured": "Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort | PNAS. https://www.pnas.org/content/118/8/e2017962118 (accessed May 6, 2021)."
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589537021004697"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Post-exposure Lopinavir-Ritonavir Prophylaxis versus Surveillance for Individuals Exposed to SARS-CoV-2: The COPEP Pragmatic Open-Label, Cluster Randomized Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "42"
}