Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial
et al., Med, doi:10.1016/j.medj.2020.04.001, ELACOI, NCT04252885, Dec 2020
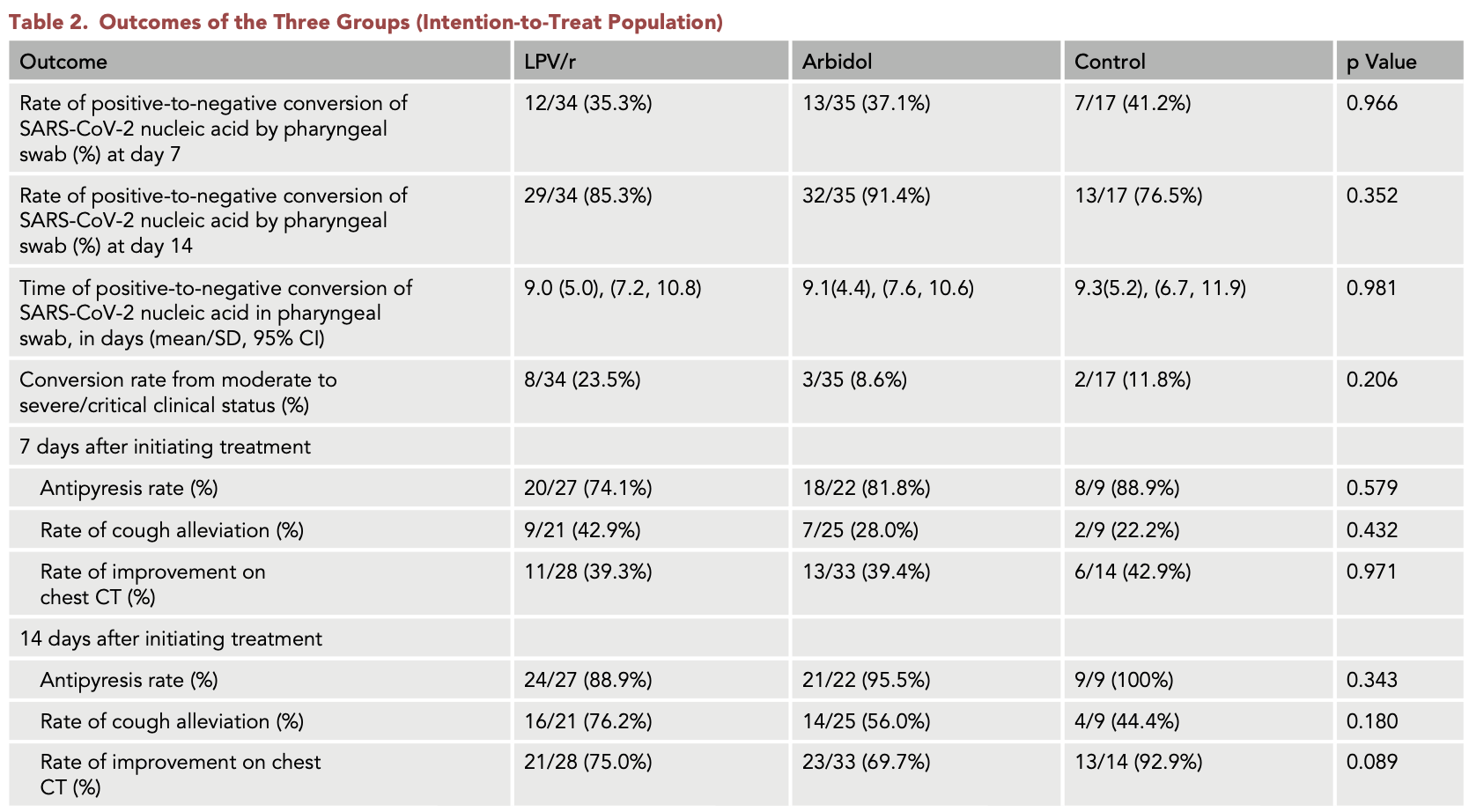
RCT 86 mild/moderate COVID-19 patients showing no significant difference in outcomes with lopinavir/ritonavir or arbidol compared to control.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
risk of progression, 100% higher, RR 2.00, p = 0.46, treatment 8 of 34 (23.5%), control 2 of 17 (11.8%), progression to severe/critical.
|
|
risk of no recovery, 400.0% higher, RR 5.00, p = 0.56, treatment 3 of 27 (11.1%), control 0 of 9 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm), day 14, fever.
|
|
risk of no recovery, 57.1% lower, RR 0.43, p = 0.12, treatment 5 of 21 (23.8%), control 5 of 9 (55.6%), NNT 3.1, day 14, cough.
|
|
chest CT improvement, 250.0% higher, RR 3.50, p = 0.23, treatment 7 of 28 (25.0%), control 1 of 14 (7.1%), day 14.
|
|
time to viral-, 3.2% lower, relative time 0.97, p = 0.84, treatment mean 9.0 (±5.0) n=34, control mean 9.3 (±5.2) n=17.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Li et al., 31 Dec 2020, Randomized Controlled Trial, China, peer-reviewed, mean age 49.4, 20 authors, study period 1 February, 2020 - 28 March, 2020, average treatment delay 4.8 days, trial NCT04252885 (history) (ELACOI).
Contact: llheliza@126.com (corresponding author), llheliza@126.com (corresponding author), gz8hdxl@126.com, gz8hzfc@126.com.
Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial
Med, doi:10.1016/j.medj.2020.04.001
Effective therapies against COVID-19 are urgently needed Lopinavir/ritonavir and arbidol were tested in
AUTHOR CONTRIBUTIONS L.L., X.D., and Y.L. conceived the study and designed the protocol. W.C. and F.Z. provided oversight. C.W. contributed to statistical analysis and interpretation of data. L.L. and W.L. drafted the manuscript. F.L. and F.H. conducted nuclear acid RT-PCR. Z.X. and Y.G. reviewed the data independently. J.L. and L.Z. reviewed all radiologic images independently. X.M., J.W., Y.W., P.P., X.C., W.H., and G.X. conducted the study and collected data.
DECLARATION OF INTERESTS The authors declare no competing interests.
STAR + METHODS
KEY RESOURCE TABLE LEAD CONTACT Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Linghua Li (llheliza@126.com).
Data and Code Availability This study did not generate new unique reagents and did not generate new datasets.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Procedures A standardized protocol was developed for collecting clinical data for all participants. The following data were collected: 1) important dates, including fever onset, admission, progression to severe clinical status, positive-to-negative conversion of SARS-CoV-2 nucleic acid, improvement of chest computed tomography [CT] scan, discharge, or death; 2) presence of predefined comorbidities (hypertension, diabetes mellitus, etc.); 3) daily observation of clinical parameters (temperature, pulse, respiratory rate, oxygen saturation, Inhaled oxygen concentration if needed); 4) The..
References
Brooks, Burtseva, Ellery, Marsh, Lew et al., Antiviral activity of arbidol, a broad-spectrum drug for use against respiratory viruses, varies according to test conditions, J. Med. Virol
Cao, Wang, Wen, Liu, Wang et al., A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19, N. Engl. J. Med
Chan, Lai, Chu, Tsui, Tam et al., Treatment of severe acute respiratory syndrome with lopinavir/ ritonavir: a multicentre retrospective matched cohort study, Hong Kong Med. J
Chen, Lan, Yuan, Deng, Li et al., Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity, Emerg. Microbes Infect
Chen, Ling, Xi, Liu, Li et al., Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia, Chin J Epidemiol, doi:10.3760/cma.j.cn311365-20200210-00050
Jin, Cai, Cheng, Cheng, Deng et al., for the Zhongnan Hospital of Wuhan University Novel Coronavirus Management and Research Team, Evidence-Based Medicine Chapter of China International Exchange and Promotive Association for Medical and Health Care (CPAM) (2020). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version), Mil. Med. Res
Khamitov, Loginova, Ia, Shchukina, Borisevich et al., Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures, Vopr. Virusol
Lai, Shih, Ko, Tang, Hsueh, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges, Int. J. Antimicrob. Agents
Leneva, Falynskova, Makhmudova, Poromov, Yatsyshina et al., Umifenovir susceptibility monitoring and characterization of influenza viruses isolated during ARBITR clinical study, J. Med. Virol
Lu, Zhao, Li, Niu, Yang et al., Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding, Lancet
Mangum, Graham, Lopinavir-Ritonavir: a new protease inhibitor, Pharmacotherapy
Pasquau, Hidalgo-Tenorio, Montes, Romero-Palacios, Vergas et al., High quality of life, treatment tolerability, safety and efficacy in HIV patients switching from triple therapy to lopinavir/ritonavir monotherapy: A randomized clinical trial, PLoS ONE
Popov, Simakova, Dmitrenko, Shchelkanov, Time course of changes in cytokines (IFN-g, IFN-a, IL-18, TNFa) in the treatment of moderate influenza A (H1N1) pdm09 (2013-2016) with oseltamivir (Tamiflu) and umifenovir (Arbidol) alone and in combination with Kagocel, Ter. Arkh
Sheahan, Sims, Leist, Scha ¨fer, Won et al., Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV, Nat. Commun
Wang, Hu, Hu, Zhu, Liu et al., Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China, JAMA, doi:10.1001/jama.2020.1585
Wang, Yang, Li, Wen, Zhang, Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China, Clin. Infect. Dis, doi:10.1093/cid/ciaa272
Yang, Yu, Xu, Shu, Xia et al., Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study, Lancet Respir. Med, doi:10.1016/S2213-2600(20)30079-5
Zhang, Wang, Qi, Shen, Li, Clinical trial analysis of 2019-nCoV therapy registered in China, J. Med. Virol, doi:10.1002/jmv.25733
DOI record:
{
"DOI": "10.1016/j.medj.2020.04.001",
"ISSN": [
"2666-6340"
],
"URL": "http://dx.doi.org/10.1016/j.medj.2020.04.001",
"alternative-id": [
"S2666634020300015"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Med"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.medj.2020.04.001"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 Published by Elsevier Inc."
}
],
"author": [
{
"affiliation": [],
"family": "Li",
"given": "Yueping",
"sequence": "first"
},
{
"affiliation": [],
"family": "Xie",
"given": "Zhiwei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lin",
"given": "Weiyin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cai",
"given": "Weiping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wen",
"given": "Chunyan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guan",
"given": "Yujuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mo",
"given": "Xiaoneng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Jian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Yaping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peng",
"given": "Ping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Xudan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hong",
"given": "Wenxin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xiao",
"given": "Guangming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Jinxin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Lieguang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hu",
"given": "Fengyu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Feng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Fuchun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deng",
"given": "Xilong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Linghua",
"sequence": "additional"
}
],
"container-title": "Med",
"container-title-short": "Med",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"cell.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
5,
19
]
],
"date-time": "2020-05-19T09:36:41Z",
"timestamp": 1589881001000
},
"deposited": {
"date-parts": [
[
2023,
10,
1
]
],
"date-time": "2023-10-01T08:09:59Z",
"timestamp": 1696147799000
},
"funder": [
{
"award": [
"2018ZX10302103-002",
"2017ZX10202102-003-004"
],
"name": "Chinese 13th Five-Year National Science and technology major project"
},
{
"award": [
"2019-2021"
],
"name": "Infectious Disease Specialty of Guangzhou High-level Clinical Key Specialty"
}
],
"indexed": {
"date-parts": [
[
2025,
7,
15
]
],
"date-time": "2025-07-15T03:13:28Z",
"timestamp": 1752549208239
},
"is-referenced-by-count": 159,
"issue": "1",
"issued": {
"date-parts": [
[
2020,
12
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2020,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
12,
1
]
],
"date-time": "2020-12-01T00:00:00Z",
"timestamp": 1606780800000
}
},
{
"URL": "http://www.elsevier.com/open-access/userlicense/1.0/",
"content-version": "vor",
"delay-in-days": 382,
"start": {
"date-parts": [
[
2021,
12,
18
]
],
"date-time": "2021-12-18T00:00:00Z",
"timestamp": 1639785600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2666634020300015?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2666634020300015?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "105-113.e4",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
12
]
]
},
"published-print": {
"date-parts": [
[
2020,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "Clinical Features of 69 Cases with Coronavirus Disease 2019 in Wuhan, China",
"author": "Wang",
"journal-title": "Clin. Infect. Dis.",
"key": "10.1016/j.medj.2020.04.001_bib1",
"year": "2020"
},
{
"key": "10.1016/j.medj.2020.04.001_bib2",
"series-title": "Coronavirus disease 2019 (COVID-19) Situation Report –71",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30251-8",
"article-title": "Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "565",
"journal-title": "Lancet",
"key": "10.1016/j.medj.2020.04.001_bib3",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30079-5",
"article-title": "Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "475",
"journal-title": "Lancet Respir. Med.",
"key": "10.1016/j.medj.2020.04.001_bib4",
"volume": "8",
"year": "2020"
},
{
"key": "10.1016/j.medj.2020.04.001_bib5",
"unstructured": "National Health Commission of China. (2020). Diagnosis and treatment of pneumonia caused by new coronavirus (trial version 6). http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml."
},
{
"DOI": "10.1592/phco.21.17.1352.34419",
"article-title": "Lopinavir-Ritonavir: a new protease inhibitor",
"author": "Mangum",
"doi-asserted-by": "crossref",
"first-page": "1352",
"journal-title": "Pharmacotherapy",
"key": "10.1016/j.medj.2020.04.001_bib6",
"volume": "21",
"year": "2001"
},
{
"DOI": "10.1371/journal.pone.0195068",
"article-title": "High quality of life, treatment tolerability, safety and efficacy in HIV patients switching from triple therapy to lopinavir/ritonavir monotherapy: A randomized clinical trial",
"author": "Pasquau",
"doi-asserted-by": "crossref",
"first-page": "e0195068",
"journal-title": "PLoS ONE",
"key": "10.1016/j.medj.2020.04.001_bib7",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.1038/s41467-019-13940-6",
"article-title": "Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV",
"author": "Sheahan",
"doi-asserted-by": "crossref",
"first-page": "222",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.medj.2020.04.001_bib8",
"volume": "11",
"year": "2020"
},
{
"article-title": "Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study",
"author": "Chan",
"first-page": "399",
"journal-title": "Hong Kong Med. J.",
"key": "10.1016/j.medj.2020.04.001_bib9",
"volume": "9",
"year": "2003"
},
{
"DOI": "10.1002/jmv.22234",
"article-title": "Antiviral activity of arbidol, a broad-spectrum drug for use against respiratory viruses, varies according to test conditions",
"author": "Brooks",
"doi-asserted-by": "crossref",
"first-page": "170",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.medj.2020.04.001_bib10",
"volume": "84",
"year": "2012"
},
{
"article-title": "[Time course of changes in cytokines (IFN-γ, IFN-α, IL-18, TNF-α) in the treatment of moderate influenza A (H1N1) pdm09 (2013-2016) with oseltamivir (Tamiflu) and umifenovir (Arbidol) alone and in combination with Kagocel]",
"author": "Popov",
"first-page": "66",
"journal-title": "Ter. Arkh.",
"key": "10.1016/j.medj.2020.04.001_bib11",
"volume": "89",
"year": "2017"
},
{
"DOI": "10.1002/jmv.25358",
"article-title": "Umifenovir susceptibility monitoring and characterization of influenza viruses isolated during ARBITR clinical study",
"author": "Leneva",
"doi-asserted-by": "crossref",
"first-page": "588",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.medj.2020.04.001_bib12",
"volume": "91",
"year": "2019"
},
{
"article-title": "[Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures]",
"author": "Khamitov",
"first-page": "9",
"journal-title": "Vopr. Virusol.",
"key": "10.1016/j.medj.2020.04.001_bib13",
"volume": "53",
"year": "2008"
},
{
"article-title": "Clinical trial analysis of 2019-nCoV therapy registered in China",
"author": "Zhang",
"journal-title": "J. Med. Virol.",
"key": "10.1016/j.medj.2020.04.001_bib15",
"year": "2020"
},
{
"article-title": "A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version)",
"author": "Jin",
"first-page": "4",
"journal-title": "Mil. Med. Res.",
"key": "10.1016/j.medj.2020.04.001_bib16",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"article-title": "Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China",
"author": "Wang",
"doi-asserted-by": "crossref",
"journal-title": "JAMA",
"key": "10.1016/j.medj.2020.04.001_bib17",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105924",
"article-title": "Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges",
"author": "Lai",
"doi-asserted-by": "crossref",
"first-page": "105924",
"journal-title": "Int. J. Antimicrob. Agents",
"key": "10.1016/j.medj.2020.04.001_bib18",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "1787",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.medj.2020.04.001_bib19",
"volume": "382",
"year": "2020"
},
{
"article-title": "Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia",
"author": "Chen",
"journal-title": "Chin J Epidemiol",
"key": "10.1016/j.medj.2020.04.001_bib20",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1080/22221751.2020.1732837",
"article-title": "Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "469",
"journal-title": "Emerg. Microbes Infect.",
"key": "10.1016/j.medj.2020.04.001_bib14",
"volume": "9",
"year": "2020"
}
],
"reference-count": 20,
"references-count": 20,
"relation": {
"has-preprint": [
{
"asserted-by": "object",
"id": "10.1101/2020.03.19.20038984",
"id-type": "doi"
}
]
},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2666634020300015"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy and Safety of Lopinavir/Ritonavir or Arbidol in Adult Patients with Mild/Moderate COVID-19: An Exploratory Randomized Controlled Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "1"
}