Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening
et al., International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.07.235, Dec 2020
Quercetin for COVID-19
36th treatment shown to reduce risk in
January 2022, now with p = 0.0018 from 9 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
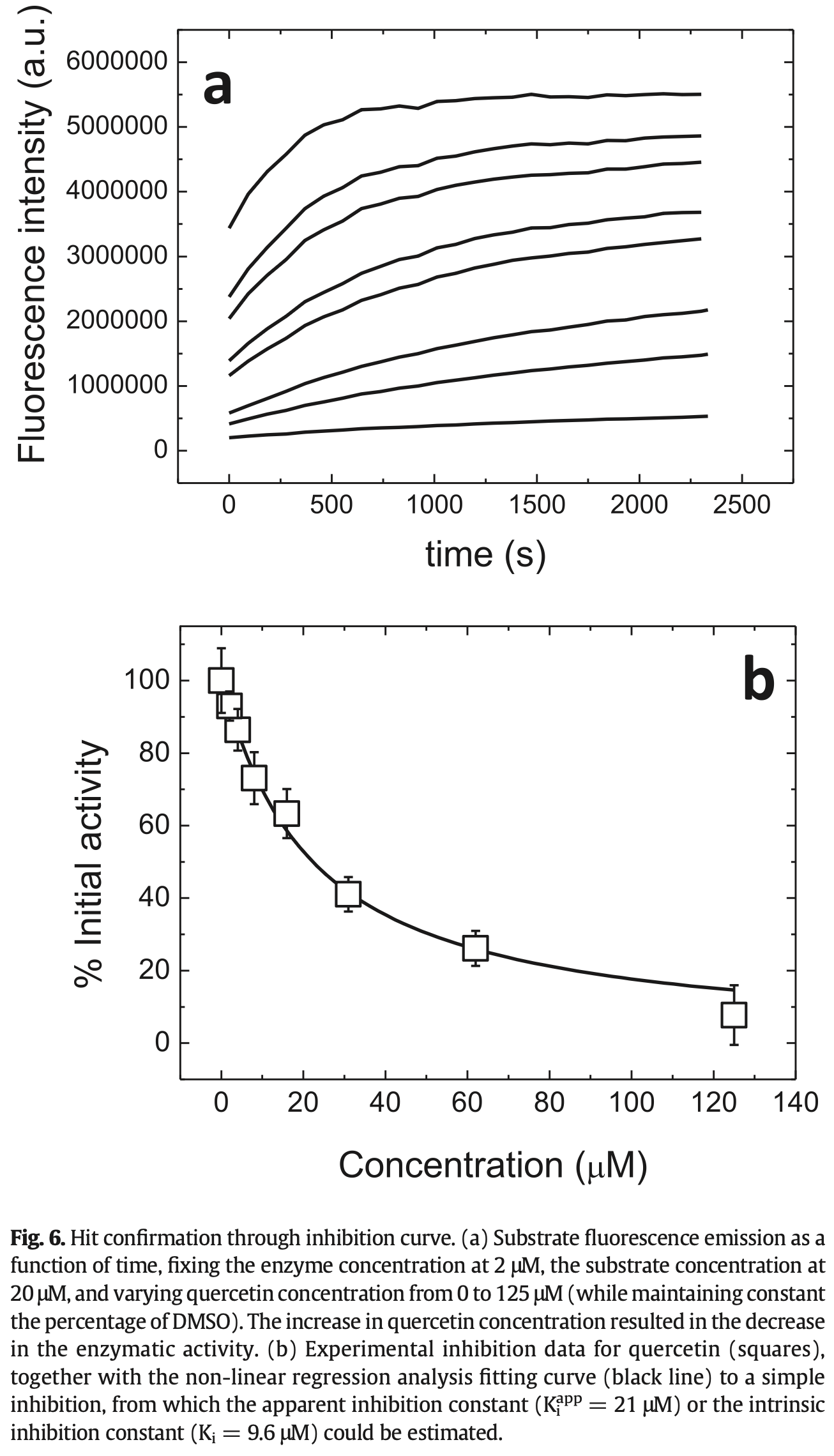
In vitro study showing that quercetin inhibits the SARS-CoV-2 main protease (3CLpro). Authors performed biophysical characterization of 3CLpro structural stability and catalytic activity, designed a molecular screening procedure, and identified quercetin as a reasonably potent inhibitor from a 150-compound library. Multiple experimental techniques confirmed quercetin binds to 3CLpro's active site: thermal shift assays showed quercetin destabilizes the protein in a concentration-dependent manner, isothermal titration calorimetry demonstrated binding with favorable enthalpic and entropic contributions, and molecular docking simulations indicated binding modes in the active site with predicted affinities matching experimental values. Quercetin's binding location overlapped with that of a known α-ketoamide inhibitor.
Bioavailability. Quercetin has low bioavailability and studies typically use advanced formulations to improve bioavailability which may be required to reach therapeutic concentrations.
91 preclinical studies support the efficacy of quercetin for COVID-19:
In silico studies predict inhibition of SARS-CoV-2, or minimization of side effects, with quercetin or metabolites via binding to the spikeA,11,12,18,19,32,34,35,37,40,48,49,51,52,75 (and specifically the receptor binding domainB,8), MproC,7,8,11,12,16,18,20,22,24,26,28,30,33,34,37,40,44,46-48,52-55,72 , RNA-dependent RNA polymeraseD,8,10-12,18,42 , PLproE,12,47,55 , ACE2F,27,32,33,37,38,47,51 , TMPRSS2G,32, nucleocapsidH,12, helicaseI,12,39,44 , endoribonucleaseJ,49, NSP16/10K,15, cathepsin LL,36, Wnt-3M,32, FZDN,32, LRP6O,32, ezrinP,50, ADRPQ,48, NRP1R,51, EP300S,25, PTGS2T,33, HSP90AA1U,25,33 , matrix metalloproteinase 9V,41, IL-6W,31,45 , IL-10X,31, VEGFAY,45, and RELAZ,45 proteins, and inhibition of spike-ACE2 interactionAA,9.
In vitro studies demonstrate inhibition of the MproC,24,58,63,71 protein, and inhibition of spike-ACE2 interactionAA,59.
In vitro studies demonstrate efficacy in Calu-3AB,62, A549AC,31, HEK293-ACE2+AD,70, Huh-7AE,35, Caco-2AF,61, Vero E6AG,29,52,61 , mTECAH,64, RAW264.7AI,64, and HLMECAJ,9 cells.
Animal studies demonstrate efficacy in K18-hACE2 miceAK,67, db/db miceAL,64,74 , BALB/c miceAM,73, and rats29.
Quercetin reduced proinflammatory cytokines and protected lung and kidney tissue against LPS-induced damage in mice73, inhibits LPS-induced cytokine storm by modulating key inflammatory and antioxidant pathways in macrophages14, may block ACE2-spike interaction and NLRP3 inflammasome, limiting viral entry and inflammation5, upregulates the SIRT1/AMPK axis to inhibit oxidative injury and accelerate viral clearance76, inhibits SARS-CoV-2 ORF3a ion channel activity, which contributes to viral pathogenicity and cytotoxicity66, may alleviate COVID-19 ARDS via inhibition of EGFR and JAK2 inflammatory targets1, may destabilize the Spike protein, IL-6R, and integrins via conserved residues, blocking viral entry, hyperinflammation, and platelet aggregation77, and may reduce COVID-19 neuroinflammation and cognitive dysfunction through anti-inflammatory mechanisms and neuroprotective effects78.
1.
Gupta et al., Harnessing phytoconstituents to treat COVID-19 triggered acute respiratory distress syndrome: Insights from network pharmacology, and molecular modeling, Phytochemistry Letters, doi:10.1016/j.phytol.2025.104105.
2.
Sun et al., Feasibility of the inhibitor development for SARS-CoV-2: a systematic approach for drug design, Journal of Molecular Modeling, doi:10.1007/s00894-025-06541-2.
3.
Torabfam et al., Improving quercetin solubility via structural modification enhances dual-target coronavirus entry: an integrated in-vitro and in-silico study, Scientific Reports, doi:10.1038/s41598-025-27374-2.
4.
Abdelhameed et al., Phytochemical and antiviral investigation of Cynanchum acutum L. extract and derived semi-synthetic analogs targeting SARS-CoV-2 main protease, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-025-00907-2.
5.
Manikyam et al., INP-Guided Network Pharmacology Discloses Multi-Target Therapeutic Strategy Against Cytokine and IgE Storms in the SARS-CoV-2 NB.1.8.1 Variant, Research Square, doi:10.21203/rs.3.rs-6819274/v1.
6.
Makoana et al., Integration of metabolomics and chemometrics with in-silico and in-vitro approaches to unravel SARS-Cov-2 inhibitors from South African plants, PLOS ONE, doi:10.1371/journal.pone.0320415.
7.
Bano et al., Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability, Viruses, doi:10.3390/v17030402.
8.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
9.
Moharram et al., Secondary metabolites of Alternaria alternate appraisal of their SARS-CoV-2 inhibitory and anti-inflammatory potentials, PLOS ONE, doi:10.1371/journal.pone.0313616.
10.
Metwaly et al., Integrated study of Quercetin as a potent SARS-CoV-2 RdRp inhibitor: Binding interactions, MD simulations, and In vitro assays, PLOS ONE, doi:10.1371/journal.pone.0312866.
11.
Al balawi et al., Assessing multi-target antiviral and antioxidant activities of natural compounds against SARS-CoV-2: an integrated in vitro and in silico study, Bioresources and Bioprocessing, doi:10.1186/s40643-024-00822-z.
12.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
13.
Pan et al., Decoding the mechanism of Qingjie formula in the prevention of COVID-19 based on network pharmacology and molecular docking, Heliyon, doi:10.1016/j.heliyon.2024.e39167.
14.
Xu et al., Quercetin inhibited LPS-induced cytokine storm by interacting with the AKT1-FoxO1 and Keap1-Nrf2 signaling pathway in macrophages, Scientific Reports, doi:10.1038/s41598-024-71569-y.
15.
Tamil Selvan et al., Computational Investigations to Identify Potent Natural Flavonoid Inhibitors of the Nonstructural Protein (NSP) 16/10 Complex Against Coronavirus, Cureus, doi:10.7759/cureus.68098.
16.
Sunita et al., Characterization of Phytochemical Inhibitors of the COVID-19 Primary Protease Using Molecular Modelling Approach, Asian Journal of Microbiology and Biotechnology, doi:10.56557/ajmab/2024/v9i28800.
17.
Wu et al., Biomarkers Prediction and Immune Landscape in Covid-19 and “Brain Fog”, Elsevier BV, doi:10.2139/ssrn.4897774.
18.
Raman et al., Phytoconstituents of Citrus limon (Lemon) as Potential Inhibitors Against Multi Targets of SARS‐CoV‐2 by Use of Molecular Modelling and In Vitro Determination Approaches, ChemistryOpen, doi:10.1002/open.202300198.
19.
Asad et al., Exploring the antiviral activity of Adhatoda beddomei bioactive compounds in interaction with coronavirus spike protein, Archives of Medical Reports, 1:1, archmedrep.com/index.php/amr/article/view/3.
20.
Irfan et al., Phytoconstituents of Artemisia Annua as potential inhibitors of SARS CoV2 main protease: an in silico study, BMC Infectious Diseases, doi:10.1186/s12879-024-09387-w.
21.
Yuan et al., Network pharmacology and molecular docking reveal the mechanisms of action of Panax notoginseng against post-COVID-19 thromboembolism, Review of Clinical Pharmacology and Pharmacokinetics - International Edition, doi:10.61873/DTFA3974.
22.
Nalban et al., Targeting COVID-19 (SARS-CoV-2) main protease through phytochemicals of Albizia lebbeck: molecular docking, molecular dynamics simulation, MM–PBSA free energy calculations, and DFT analysis, Journal of Proteins and Proteomics, doi:10.1007/s42485-024-00136-w.
23.
Zhou et al., Bioinformatics and system biology approaches to determine the connection of SARS-CoV-2 infection and intrahepatic cholangiocarcinoma, PLOS ONE, doi:10.1371/journal.pone.0300441.
24.
Waqas et al., Discovery of Novel Natural Inhibitors Against SARS-CoV-2 Main Protease: A Rational Approach to Antiviral Therapeutics, Current Medicinal Chemistry, doi:10.2174/0109298673292839240329081008.
25.
Hasanah et al., Decoding the therapeutic potential of empon-empon: a bioinformatics expedition unraveling mechanisms against COVID-19 and atherosclerosis, International Journal of Applied Pharmaceutics, doi:10.22159/ijap.2024v16i2.50128.
26.
Shaik et al., Computational identification of selected bioactive compounds from Cedrus deodara as inhibitors against SARS-CoV-2 main protease: a pharmacoinformatics study, Indian Drugs, doi:10.53879/id.61.02.13859.
27.
Wang et al., Investigating the Mechanism of Qu Du Qiang Fei 1 Hao Fang Formula against Coronavirus Disease 2019 Based on Network Pharmacology Method, World Journal of Traditional Chinese Medicine, doi:10.4103/2311-8571.395061.
28.
Singh et al., Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 M Pro protein - An in-silico and cell-based approach, Research Square, doi:10.21203/rs.3.rs-3888947/v1.
29.
El-Megharbel et al., Chemical and spectroscopic characterization of (Artemisinin/Quercetin/ Zinc) novel mixed ligand complex with assessment of its potent high antiviral activity against SARS-CoV-2 and antioxidant capacity against toxicity induced by acrylamide in male rats, PeerJ, doi:10.7717/peerj.15638.
30.
Akinwumi et al., Evaluation of therapeutic potentials of some bioactive compounds in selected African plants targeting main protease (Mpro) in SARS-CoV-2: a molecular docking study, Egyptian Journal of Medical Human Genetics, doi:10.1186/s43042-023-00456-4.
31.
Yang et al., Active ingredient and mechanistic analysis of traditional Chinese medicine formulas for the prevention and treatment of COVID-19: Insights from bioinformatics and in vitro experiments, Medicine, doi:10.1097/MD.0000000000036238.
32.
Chandran et al., Molecular docking analysis of quercetin with known CoVid-19 targets, Bioinformation, doi:10.6026/973206300191081.
33.
Qin et al., Exploring the bioactive compounds of Feiduqing formula for the prevention and management of COVID-19 through network pharmacology and molecular docking, Medical Data Mining, doi:10.53388/MDM202407003.
34.
Moschovou et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, International Journal of Molecular Sciences, doi:10.3390/ijms242115894.
35.
Pan (B) et al., Quercetin: A promising drug candidate against the potential SARS-CoV-2-Spike mutants with high viral infectivity, Computational and Structural Biotechnology Journal, doi:10.1016/j.csbj.2023.10.029.
36.
Ahmed et al., Evaluation of the Effect of Zinc, Quercetin, Bromelain and Vitamin C on COVID-19 Patients, International Journal of Diabetes Management, doi:10.61797/ijdm.v2i2.259.
37.
Thapa et al., In-silico Approach for Predicting the Inhibitory Effect of Home Remedies on Severe Acute Respiratory Syndrome Coronavirus-2, Makara Journal of Science, doi:10.7454/mss.v27i3.1609.
38.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
39.
Singh (B) et al., Flavonoids as Potent Inhibitor of SARS-CoV-2 Nsp13 Helicase: Grid Based Docking Approach, Middle East Research Journal of Pharmaceutical Sciences, doi:10.36348/merjps.2023.v03i04.001.
40.
Mandal et al., In silico anti-viral assessment of phytoconstituents in a traditional (Siddha Medicine) polyherbal formulation – Targeting Mpro and pan-coronavirus post-fusion Spike protein, Journal of Traditional and Complementary Medicine, doi:10.1016/j.jtcme.2023.07.004.
41.
Sai Ramesh et al., Computational analysis of the phytocompounds of Mimusops elengi against spike protein of SARS CoV2 – An Insilico model, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2023.125553.
42.
Corbo et al., Inhibitory potential of phytochemicals on five SARS-CoV-2 proteins: in silico evaluation of endemic plants of Bosnia and Herzegovina, Biotechnology & Biotechnological Equipment, doi:10.1080/13102818.2023.2222196.
43.
Azmi et al., Utilization of quercetin flavonoid compounds in onion (Allium cepa L.) as an inhibitor of SARS-CoV-2 spike protein against ACE2 receptors, 11th International Seminar on New Paradigm and Innovation on Natural Sciences and its Application, doi:10.1063/5.0140285.
44.
Alanzi et al., Structure-based virtual identification of natural inhibitors of SARS-CoV-2 and its Delta and Omicron variant proteins, Future Virology, doi:10.2217/fvl-2022-0184.
45.
Yang (B) et al., In silico evidence implicating novel mechanisms of Prunella vulgaris L. as a potential botanical drug against COVID-19-associated acute kidney injury, Frontiers in Pharmacology, doi:10.3389/fphar.2023.1188086.
46.
Wang (B) et al., Computational Analysis of Lianhua Qingwen as an Adjuvant Treatment in Patients with COVID-19, Society of Toxicology Conference, 2023, www.researchgate.net/publication/370491709_Y_Wang_A_E_Tan_O_Chew_A_Hsueh_and_D_E_Johnson_2023_Computational_Analysis_of_Lianhua_Qingwen_as_an_Adjuvant_Treatment_in_Patients_with_COVID-19_Toxicologist_1921_507.
47.
Ibeh et al., Computational studies of potential antiviral compounds from some selected Nigerian medicinal plants against SARS-CoV-2 proteins, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2023.101230.
48.
Nguyen et al., The Potential of Ameliorating COVID-19 and Sequelae From Andrographis paniculata via Bioinformatics, Bioinformatics and Biology Insights, doi:10.1177/11779322221149622.
49.
Alavi et al., Interaction of Epigallocatechin Gallate and Quercetin with Spike Glycoprotein (S-Glycoprotein) of SARS-CoV-2: In Silico Study, Biomedicines, doi:10.3390/biomedicines10123074.
50.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
51.
Şimşek et al., In silico identification of SARS-CoV-2 cell entry inhibitors from selected natural antivirals, Journal of Molecular Graphics and Modelling, doi:10.1016/j.jmgm.2021.108038.
52.
Kandeil et al., Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2, Pathogens, doi:10.3390/pathogens10060758.
53.
Rehman et al., Natural Compounds as Inhibitors of SARS-CoV-2 Main Protease (3CLpro): A Molecular Docking and Simulation Approach to Combat COVID-19, Current Pharmaceutical Design, doi:10.2174/1381612826999201116195851.
54.
Sekiou et al., In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2, ChemRxiv, doi:10.26434/chemrxiv.12181404.v1.
55.
Zhang et al., In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus, Journal of Integrative Medicine, doi:10.1016/j.joim.2020.02.005.
56.
Sisti et al., Evaluation of respiratory virus transmissibility and resilience from fomites: the case of 11 SARS-CoV-2 clinical isolates, Applied and Environmental Microbiology, doi:10.1128/aem.00774-25.
57.
Spinelli et al., Amphibian‐Derived Peptides as Natural Inhibitors of SARS‐CoV‐2 Main Protease (Mpro): A Combined In Vitro and In Silico Approach, Chemistry & Biodiversity, doi:10.1002/cbdv.202403202.
58.
Aguilera-Rodriguez et al., Inhibition of SARS-CoV-2 3CLpro by chemically modified tyrosinase from Agaricus bisporus, RSC Medicinal Chemistry, doi:10.1039/D4MD00289J.
59.
Emam et al., Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors, AMB Express, doi:10.1186/s13568-024-01739-8.
60.
Fang et al., Development of nanoparticles incorporated with quercetin and ACE2-membrane as a novel therapy for COVID-19, Journal of Nanobiotechnology, doi:10.1186/s12951-024-02435-2.
61.
Roy et al., Quercetin inhibits SARS-CoV-2 infection and prevents syncytium formation by cells co-expressing the viral spike protein and human ACE2, Virology Journal, doi:10.1186/s12985-024-02299-w.
62.
DiGuilio et al., Quercetin improves and protects Calu-3 airway epithelial barrier function, Frontiers in Cell and Developmental Biology, doi:10.3389/fcell.2023.1271201.
63.
Zhang (B) et al., Discovery of the covalent SARS‐CoV‐2 Mpro inhibitors from antiviral herbs via integrating target‐based high‐throughput screening and chemoproteomic approaches, Journal of Medical Virology, doi:10.1002/jmv.29208.
64.
Wu (B) et al., SARS-CoV-2 N protein induced acute kidney injury in diabetic db/db mice is associated with a Mincle-dependent M1 macrophage activation, Frontiers in Immunology, doi:10.3389/fimmu.2023.1264447.
65.
Xu (B) et al., Bioactive compounds from Huashi Baidu decoction possess both antiviral and anti-inflammatory effects against COVID-19, Proceedings of the National Academy of Sciences, doi:10.1073/pnas.2301775120.
66.
Fam et al., Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites, Scientific Reports, doi:10.1038/s41598-023-31764-9.
67.
Aguado et al., Senolytic therapy alleviates physiological human brain aging and COVID-19 neuropathology, bioRxiv, doi:10.1101/2023.01.17.524329.
68.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
69.
Munafò et al., Quercetin and Luteolin Are Single-digit Micromolar Inhibitors of the SARS-CoV-2 RNA-dependent RNA Polymerase, Research Square, doi:10.21203/rs.3.rs-1149846/v1.
70.
Singh (C) et al., The spike protein of SARS-CoV-2 virus induces heme oxygenase-1: Pathophysiologic implications, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, doi:10.1016/j.bbadis.2021.166322.
71.
Bahun et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594.
72.
Abian et al., Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.07.235.
73.
Shaker et al., Anti-cytokine Storm Activity of Fraxin, Quercetin, and their Combination on Lipopolysaccharide-Induced Cytokine Storm in Mice: Implications in COVID-19, Iranian Journal of Medical Sciences, doi:10.30476/ijms.2023.98947.3102.
74.
Wu (C) et al., Treatment with Quercetin inhibits SARS-CoV-2 N protein-induced acute kidney injury by blocking Smad3-dependent G1 cell cycle arrest, Molecular Therapy, doi:10.1016/j.ymthe.2022.12.002.
75.
Azmi (B) et al., The role of vitamin D receptor and IL‐6 in COVID‐19, Molecular Genetics & Genomic Medicine, doi:10.1002/mgg3.2172.
76.
Shokri-Afra et al., Targeting SIRT1: A Potential Strategy for Combating Severe COVID‐19, BioMed Research International, doi:10.1155/bmri/9507417.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The receptor binding domain is a specific region of the spike protein that binds ACE2 and is a major target of neutralizing antibodies. Focusing on the precise binding site allows highly specific disruption of viral attachment with reduced potential for off-target effects.
c.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
d.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
e.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
f.
The angiotensin converting enzyme 2 (ACE2) protein is a host cell transmembrane protein that serves as the cellular receptor for the SARS-CoV-2 spike protein. ACE2 is expressed on many cell types, including epithelial cells in the lungs, and allows the virus to enter and infect host cells. Inhibition may affect ACE2's physiological function in blood pressure control.
g.
Transmembrane protease serine 2 (TMPRSS2) is a host cell protease that primes the spike protein, facilitating cellular entry. TMPRSS2 activity helps enable cleavage of the spike protein required for membrane fusion and virus entry. Inhibition may especially protect respiratory epithelial cells, buy may have physiological effects.
h.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
i.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
j.
The endoribonuclease, also known as NendoU or nsp15, cleaves specific sequences in viral RNA which may help the virus evade detection by the host immune system. Inhibition may hinder the virus's ability to mask itself from the immune system, facilitating a stronger immune response.
k.
The NSP16/10 complex consists of non-structural proteins 16 and 10, forming a 2'-O-methyltransferase that modifies the viral RNA cap structure. This modification helps the virus evade host immune detection by mimicking host mRNA, making NSP16/10 a promising antiviral target.
l.
Cathepsin L is a host lysosomal cysteine protease that can prime the spike protein through an alternative pathway when TMPRSS2 is unavailable. Dual targeting of cathepsin L and TMPRSS2 may maximize disruption of alternative pathways for virus entry.
m.
Wingless-related integration site (Wnt) ligand 3 is a host signaling molecule that activates the Wnt signaling pathway, which is important in development, cell growth, and tissue repair. Some studies suggest that SARS-CoV-2 infection may interfere with the Wnt signaling pathway, and that Wnt3a is involved in SARS-CoV-2 entry.
n.
The frizzled (FZD) receptor is a host transmembrane receptor that binds Wnt ligands, initiating the Wnt signaling cascade. FZD serves as a co-receptor, along with ACE2, in some proposed mechanisms of SARS-CoV-2 infection. The virus may take advantage of this pathway as an alternative entry route.
o.
Low-density lipoprotein receptor-related protein 6 is a cell surface co-receptor essential for Wnt signaling. LRP6 acts in tandem with FZD for signal transduction and has been discussed as a potential co-receptor for SARS-CoV-2 entry.
p.
The ezrin protein links the cell membrane to the cytoskeleton (the cell's internal support structure) and plays a role in cell shape, movement, adhesion, and signaling. Drugs that occupy the same spot on ezrin where the viral spike protein would bind may hindering viral attachment, and drug binding could further stabilize ezrin, strengthening its potential natural capacity to impede viral fusion and entry.
q.
The Adipocyte Differentiation-Related Protein (ADRP, also known as Perilipin 2 or PLIN2) is a lipid droplet protein regulating the storage and breakdown of fats in cells. SARS-CoV-2 may hijack the lipid handling machinery of host cells and ADRP may play a role in this process. Disrupting ADRP's interaction with the virus may hinder the virus's ability to use lipids for replication and assembly.
r.
Neuropilin-1 (NRP1) is a cell surface receptor with roles in blood vessel development, nerve cell guidance, and immune responses. NRP1 may function as a co-receptor for SARS-CoV-2, facilitating viral entry into cells. Blocking NRP1 may disrupt an alternative route of viral entry.
s.
EP300 (E1A Binding Protein P300) is a transcriptional coactivator involved in several cellular processes, including growth, differentiation, and apoptosis, through its acetyltransferase activity that modifies histones and non-histone proteins. EP300 facilitates viral entry into cells and upregulates inflammatory cytokine production.
t.
Prostaglandin G/H synthase 2 (PTGS2, also known as COX-2) is an enzyme crucial for the production of inflammatory molecules called prostaglandins. PTGS2 plays a role in the inflammatory response that can become severe in COVID-19 and inhibitors (like some NSAIDs) may have benefits in dampening harmful inflammation, but note that prostaglandins have diverse physiological functions.
u.
Heat Shock Protein 90 Alpha Family Class A Member 1 (HSP90AA1) is a chaperone protein that helps other proteins fold correctly and maintains their stability. HSP90AA1 plays roles in cell signaling, survival, and immune responses. HSP90AA1 may interact with numerous viral proteins, but note that it has diverse physiological functions.
v.
Matrix metalloproteinase 9 (MMP9), also called gelatinase B, is a zinc-dependent enzyme that breaks down collagen and other components of the extracellular matrix. MMP9 levels increase in severe COVID-19. Overactive MMP9 can damage lung tissue and worsen inflammation. Inhibition of MMP9 may prevent excessive tissue damage and help regulate the inflammatory response.
w.
The interleukin-6 (IL-6) pro-inflammatory cytokine (signaling molecule) has a complex role in the immune response and may trigger and perpetuate inflammation. Elevated IL-6 levels are associated with severe COVID-19 cases and cytokine storm. Anti-IL-6 therapies may be beneficial in reducing excessive inflammation in severe COVID-19 cases.
x.
The interleukin-10 (IL-10) anti-inflammatory cytokine helps regulate and dampen immune responses, preventing excessive inflammation. IL-10 levels can also be elevated in severe COVID-19. IL-10 could either help control harmful inflammation or potentially contribute to immune suppression.
y.
Vascular Endothelial Growth Factor A (VEGFA) promotes the growth of new blood vessels (angiogenesis) and has roles in inflammation and immune responses. VEGFA may contribute to blood vessel leakiness and excessive inflammation associated with severe COVID-19.
z.
RELA is a transcription factor subunit of NF-kB and is a key regulator of inflammation, driving pro-inflammatory gene expression. SARS-CoV-2 may hijack and modulate NF-kB pathways.
aa.
The interaction between the SARS-CoV-2 spike protein and the human ACE2 receptor is a primary method of viral entry, inhibiting this interaction can prevent the virus from attaching to and entering host cells, halting infection at an early stage.
ab.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
ac.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
ad.
HEK293-ACE2+ is a human embryonic kidney cell line engineered for high ACE2 expression and SARS-CoV-2 susceptibility.
ae.
Huh-7 cells were derived from a liver tumor (hepatoma).
af.
Caco-2 cells come from a colorectal adenocarcinoma (cancer). They are valued for their ability to form a polarized cell layer with properties similar to the intestinal lining.
ag.
Vero E6 is an African green monkey kidney cell line with low/no ACE2 expression and high SARS-CoV-2 susceptibility. The cell line is easy to maintain and supports robust viral replication, however the monkey origin may not accurately represent human responses.
ah.
mTEC is a mouse tubular epithelial cell line.
ai.
RAW264.7 is a mouse macrophage cell line.
aj.
HLMEC (Human Lung Microvascular Endothelial Cells) are primary endothelial cells derived from the lung microvasculature. They are used to study endothelial function, inflammation, and viral interactions, particularly in the context of lung infections such as SARS-CoV-2. HLMEC express ACE2 and are susceptible to SARS-CoV-2 infection, making them a relevant model for studying viral entry and endothelial responses in the lung.
ak.
A mouse model expressing the human ACE2 receptor under the control of the K18 promoter.
al.
A mouse model of obesity and severe insulin resistance leading to type 2 diabetes due to a mutation in the leptin receptor gene that impairs satiety signaling.
am.
A mouse model commonly used in infectious disease and cancer research due to higher immune response and susceptibility to infection.
Abian et al., 31 Dec 2020, Spain, peer-reviewed, 8 authors.
Contact: oabifra@unizar.es, adrianvc@unizar.es.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening
International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.07.235
The global health emergency generated by coronavirus disease 2019 (COVID-19) has prompted the search for preventive and therapeutic treatments for its pathogen, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). There are many potential targets for drug discovery and development to tackle this disease. One of these targets is the main protease, Mpro or 3CLpro, which is highly conserved among coronaviruses. 3CLpro is an essential player in the viral replication cycle, processing the large viral polyproteins and rendering the individual proteins functional. We report a biophysical characterization of the structural stability and the catalytic activity of 3CLpro from SARS-CoV-2, from which a suitable experimental in vitro molecular screening procedure has been designed. By screening of a small chemical library consisting of about 150 compounds, the natural product quercetin was identified as reasonably potent inhibitor of SARS-CoV-2 3CLpro (K i ~7 μM). Quercetin could be shown to interact with 3CLpro using biophysical techniques and bind to the active site in molecular simulations. Quercetin, with well-known pharmacokinetic and ADMET properties, can be considered as a good candidate for further optimization and development, or repositioned for COVID-19 therapeutic treatment.
Declaration of competing interest The authors declare no conflict of interest.
Appendix A. Supplementary data Supplementary data to this article can be found online at https://doi. org/10.1016/j.ijbiomac.2020.07.235 .
References
Abad-Zapatero, Metz, Ligand efficiency indices as guideposts for drug discovery, Drug Discov. Today
Abian, Vega, Sancho, Velazquez-Campoy, Allosteric inhibitors of the NS3 protease from the hepatitis C virus, PLoS One
Adams, Walls, Supporting the Health Care Workforce During the COVID-19 Global Epidemic, JAMA
Andersen, Rambaut, Lipkin, Holmes, Garry, The proximal origin of SARS-CoV-2, Nat. Med
Atzrodt, Maknojia, Mccarthy, Oldfield, Po et al., A guide to COVID-19: a global pandemic caused by the novel coronavirus SARS-CoV-2, FEBS J, doi:10.1111/febs.15375
Bacha, Barrila, Velazquez-Campoy, Leavitt, Freire, Identification of novel inhibitors of the SARS coronavirus main protease 3CLpro, Biochemistry
Campos, Sharma, Alvira, Ruiz, Ibarra-Molero et al., Engineering protein assemblies with allosteric control via monomer fold-switching, Nat. Commun
Catanzaro, Fagiani, Racchi, Corsini, Govoni et al., Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2, Signal Transduct. Target. Ther
Cimmperman, Baranauskiene, Jachimoviciūte, Jachno, Torresan et al., A quantitative model of thermal stabilization and destabilization of proteins by ligands, Biophys. J
Clarke, An introduction to economic studies, health emergencies, and COVID-19, J. Evid. Based Med
Claveria-Gimeno, Vega, Abian, Velazquez-Campoy, A look at ligand binding thermodynamics in drug discovery, Expert. Opin. Drug Discov
Cremades, Velázquez-Campoy, Martínez-Júlvez, Neira, Pérez-Dorado et al., Discovery of specific flavodoxin inhibitors as potential therapeutic agents against Helicobacter pylori infection, ACS Chem. Biol
Ericsson, Hallberg, Detitta, Dekker, Nordlund, Thermofluor-based high-throughput stability optimization of proteins for structural studies, Anal. Biochem
González, Casado, Chueca, Salillas, Velázquez-Campoy et al., Small molecule inhibitors of the response regulator ArsR exhibit bactericidal activity against Helicobacter pylori, Microorganisms
González, Salillas, Velázquez-Campoy, Espinosa Angarica, Fillat et al., Identifying potential novel drugs against Helicobacter pylori by targeting the essential response regulator HsrA, Sci. Rep
Grande, Rizzuti, Occhiuzzi, Ioele, Casacchia et al., Identification by molecular docking of homoisoflavones from Leopoldia comosa as ligands of estrogen receptors, Molecules
Hanwell, Curtis, Lonie, Vandermeersch, Zurek et al., Avogadro: an advanced semantic chemical editor, visualization, and analysis platform, J. Cheminform
Helmy, Fawzy, Elaswad, Sobieh, Kenney et al., The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control, J. Clin. Med
Hidalgo, Latorre, Carrodeguas, Velázquez-Campoy, Sancho et al., Inhibition of pig phosphoenolpyruvate carboxykinase isoenzymes by 3mercaptopicolinic acid and novel inhibitors, PLoS One
Huang, Wang, Li, Ren, Zhao et al., Molecular docking quercetin to 3CLpro. Structure of (a) unliganded 3CLpro (from entry 6Y2E of the PDB
Jo, Kim, Kim, Shin, Kim, Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors, Chem. Biol. Drug Des
Kasimova, Milstein, Freire, The conformational equilibrium of human growth hormone, J Mol Biol
Khan, Zia, Ashraf, Uddin, Ul-Haq, Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach, J. Biomol. Struct. Dyn
Kuo, Chi, Hsu, Liang, Characterization of SARS main protease and inhibitor assay using a fluorogenic substrate, Biochem. Biophys. Res. Commun
Lakowicz, Jayaweera, Szmacinski, Wiczk, Resolution of multicomponent fluorescence emission using frequency-dependent phase angle and modulation spectra, Anal. Chem
Lee, Mittal, Patel, Gatuz, Truong et al., Identification of novel drug scaffolds for inhibition of SARS-CoV 3-Chymotrypsinlike protease using virtual and high-throughput screenings, Bioorg. Med. Chem
Morris, Goodsell, Halliday, Huey, Hart et al., Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function, J. Comput. Chem
Morse, Lalonde, Xu, Liu, Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV, Chembiochem. Eur. J. Chem. Biol
Muramatsu, Kim, Nishii, Terada, Shirouzu et al., Autoprocessing mechanism of severe acute respiratory syndrome coronavirus 3Clike protease (SARS-CoV 3CLpro) from its polyproteins, FEBS J
Neira, Bintz, Arruebo, Rizzuti, Bonacci et al., Identification of a drug targeting an intrinsically disordered protein involved in pancreatic adenocarcinoma, Sci. Rep
Pantoliano, Petrella, Kwasnoski, Lobanov, Myslik et al., High-density miniaturized thermal shift assays as a general strategy for drug discovery, J. Biomol. Screen
Pey, Ying, Cremades, Velazquez-Campoy, Scherer et al., Identification of pharmacological chaperones as potential therapeutic agents to treat phenylketonuria, J. Clin. Invest
Pirrone, Thakkar, Jacobson, Wigdahl, Krebs, Combinatorial approaches to the prevention and treatment of HIV-1 infection, Antimicrob. Agents Chemother
Pushpakom, Iorio, Eyers, Escott, Hopper et al., Drug repurposing: progress, challenges and recommendations, Nat. Rev. Drug Discov
Renu, Prasanna, Valsala Gopalakrishnan, Coronaviruses pathogenesis, comorbidities and multi-organ damage -a review, Life Sci
Sanchez-Ruiz, Theoretical analysis of Lumry-Eyring models in differential scanning calorimetry, Biophys. J
Santofimia-Castano, Xia, Lan, Zhou, Huang et al., Ligand-based design identifies a potent NUPR1 inhibitor exerting anticancer activity via necroptosis, J. Clin. Invest
Shi, Wei, Song, Dissection study on the severe acute respiratory syndrome 3Clike protease reveals the critical role of the extra domain in dimerization of the enzyme: defining the extra domain as a new target for design of highly specific protease inhibitors, J. Biol. Chem
Shriver, Edmondson, Defining the stability of multimeric proteins, Methods Mol. Biol
Tay, Poh, Rénia, Macary, Ng, The trinity of COVID-19: immunity, inflammation and intervention, Nat. Rev. Immunol
Trott, Olson, AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, J. Comput. Chem
Velazquez-Campoy, Sancho, Abian, Vega, Biophysical screening for identifying pharmacological chaperones and inhibitors against conformational and infectious diseases, Curr. Drug Targets
Villanueva, Romero-Tamayo, Laplaza, Martínez-Olivan, Velázquez-Campoy et al., Redox-and ligand binding-dependent conformational ensembles in the human apoptosis-inducing factor regulate its pro-life and cell death functions, Antioxid. Redox Signal
Wang, Jiang, Gao, Jin, Wang et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Ward, Holdgate, Isothermal titration calorimetry in drug discovery, Prog. Med. Chem
Wu, Wang, Zeng, Huang, Xu et al., Prediction and biochemical analysis of putative cleavage sites of the 3C-like protease of Middle East respiratory syndrome coronavirus, Virus Res
Yang, Yang, Ding, Liu, Lou et al., The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor, Proc. Natl. Acad. Sci
Zhang, Lin, Kusov, Nian, Ma et al., α-Ketoamides as broadspectrum inhibitors of coronavirus and enterovirus replication: structure-based design, synthesis, and activity assessment, J. Med. Chem
Zhang, Lin, Sun, Curth, Drosten et al., Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors, Science
Zhang, Zhong, Xue, Kang, Ren et al., Threedimensional domain swapping as a mechanism to lock the active conformation in a super-active octamer of SARS-CoV main protease, Protein Cell
Zhu, Zhang, Wang, Li, Yang et al., A novel coronavirus from patients with pneumonia in China, N. Engl. J. Med
DOI record:
{
"DOI": "10.1016/j.ijbiomac.2020.07.235",
"ISSN": [
"0141-8130"
],
"URL": "http://dx.doi.org/10.1016/j.ijbiomac.2020.07.235",
"alternative-id": [
"S0141813020339970"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Biological Macromolecules"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijbiomac.2020.07.235"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2020 Elsevier B.V. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Abian",
"given": "Olga",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ortega-Alarcon",
"given": "David",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jimenez-Alesanco",
"given": "Ana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ceballos-Laita",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vega",
"given": "Sonia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Reyburn",
"given": "Hugh T.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rizzuti",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Velazquez-Campoy",
"given": "Adrian",
"sequence": "additional"
}
],
"container-title": "International Journal of Biological Macromolecules",
"container-title-short": "International Journal of Biological Macromolecules",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2020,
8,
1
]
],
"date-time": "2020-08-01T01:31:21Z",
"timestamp": 1596245481000
},
"deposited": {
"date-parts": [
[
2024,
12,
18
]
],
"date-time": "2024-12-18T23:01:14Z",
"timestamp": 1734562874000
},
"funder": [
{
"name": "Fundación hna"
},
{
"DOI": "10.13039/501100004587",
"award": [
"CPII13/00017"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100004587",
"id-type": "DOI"
}
],
"name": "Instituto de Salud Carlos III"
},
{
"DOI": "10.13039/501100004587",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100004587",
"id-type": "DOI"
}
],
"name": "Instituto de Salud Carlos III"
},
{
"DOI": "10.13039/501100000780",
"award": [
"PI18/00343"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100000780",
"id-type": "DOI"
}
],
"name": "European Union"
},
{
"DOI": "10.13039/501100003329",
"award": [
"BFU2016-78232-P",
"SAF2017-83265-R"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100003329",
"id-type": "DOI"
}
],
"name": "Ministry of Economy and Competitiveness"
},
{
"DOI": "10.13039/100014440",
"award": [
"BES-2017-080739"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/100014440",
"id-type": "DOI"
}
],
"name": "Ministry of Science, Innovation and Universities"
},
{
"DOI": "10.13039/501100003339",
"award": [
"202020E079"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100003339",
"id-type": "DOI"
}
],
"name": "Spanish National Research Council"
},
{
"award": [
"E45_20R",
"B25_20R"
],
"name": "Diputación General de Aragón"
},
{
"name": "Centro de Investigación Biomédica en Red en Enfermedades Hepáticas y Digestivas"
}
],
"indexed": {
"date-parts": [
[
2025,
6,
25
]
],
"date-time": "2025-06-25T23:06:53Z",
"timestamp": 1750892813917,
"version": "3.37.3"
},
"is-referenced-by-count": 209,
"issued": {
"date-parts": [
[
2020,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
12,
1
]
],
"date-time": "2020-12-01T00:00:00Z",
"timestamp": 1606780800000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
12,
1
]
],
"date-time": "2020-12-01T00:00:00Z",
"timestamp": 1606780800000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
12,
1
]
],
"date-time": "2020-12-01T00:00:00Z",
"timestamp": 1606780800000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
12,
1
]
],
"date-time": "2020-12-01T00:00:00Z",
"timestamp": 1606780800000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
12,
1
]
],
"date-time": "2020-12-01T00:00:00Z",
"timestamp": 1606780800000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
12,
1
]
],
"date-time": "2020-12-01T00:00:00Z",
"timestamp": 1606780800000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
12,
1
]
],
"date-time": "2020-12-01T00:00:00Z",
"timestamp": 1606780800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0141813020339970?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0141813020339970?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "1693-1703",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
12
]
]
},
"published-print": {
"date-parts": [
[
2020,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A novel coronavirus from patients with pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"issue": "8",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0005",
"volume": "382",
"year": "2020"
},
{
"author": "W.H.O. (WHO)",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0010"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "497",
"issue": "10223",
"journal-title": "Lancet",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0015",
"volume": "395",
"year": "2020"
},
{
"author": "Adams",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0020",
"series-title": "Supporting the Health Care Workforce During the COVID-19 Global Epidemic",
"year": "2020"
},
{
"DOI": "10.1016/j.lfs.2020.117839",
"article-title": "Coronaviruses pathogenesis, comorbidities and multi-organ damage - a review",
"author": "Renu",
"doi-asserted-by": "crossref",
"journal-title": "Life Sci.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0025",
"volume": "255",
"year": "2020"
},
{
"DOI": "10.1038/s41392-020-0191-1",
"article-title": "Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2",
"author": "Catanzaro",
"doi-asserted-by": "crossref",
"first-page": "84",
"issue": "1",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0030",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1111/jebm.12395",
"article-title": "An introduction to economic studies, health emergencies, and COVID-19",
"author": "Clarke",
"doi-asserted-by": "crossref",
"first-page": "161",
"issue": "2",
"journal-title": "J. Evid. Based Med.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0035",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1111/febs.15375",
"article-title": "A guide to COVID-19: a global pandemic caused by the novel coronavirus SARS-CoV-2",
"author": "Atzrodt",
"doi-asserted-by": "crossref",
"journal-title": "FEBS J.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0040",
"year": "2020"
},
{
"DOI": "10.1128/AAC.00976-10",
"article-title": "Combinatorial approaches to the prevention and treatment of HIV-1 infection",
"author": "Pirrone",
"doi-asserted-by": "crossref",
"first-page": "1831",
"issue": "5",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0045",
"volume": "55",
"year": "2011"
},
{
"DOI": "10.1038/nrd.2018.168",
"article-title": "Drug repurposing: progress, challenges and recommendations",
"author": "Pushpakom",
"doi-asserted-by": "crossref",
"first-page": "41",
"issue": "1",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0050",
"volume": "18",
"year": "2019"
},
{
"DOI": "10.1038/s41591-020-0820-9",
"article-title": "The proximal origin of SARS-CoV-2",
"author": "Andersen",
"doi-asserted-by": "crossref",
"first-page": "450",
"issue": "4",
"journal-title": "Nat. Med.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0055",
"volume": "26",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-0311-8",
"article-title": "The trinity of COVID-19: immunity, inflammation and intervention",
"author": "Tay",
"doi-asserted-by": "crossref",
"first-page": "363",
"issue": "6",
"journal-title": "Nat. Rev. Immunol.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0060",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.3390/jcm9041225",
"article-title": "The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control",
"author": "Helmy",
"doi-asserted-by": "crossref",
"first-page": "1225",
"issue": "4",
"journal-title": "J. Clin. Med.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0065",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1073/pnas.1835675100",
"article-title": "The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor",
"author": "Yang",
"doi-asserted-by": "crossref",
"issue": "23",
"journal-title": "Proc. Natl. Acad. Sci.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0070",
"volume": "100",
"year": "2003"
},
{
"DOI": "10.1111/febs.12222",
"article-title": "Autoprocessing mechanism of severe acute respiratory syndrome coronavirus 3C-like protease (SARS-CoV 3CLpro) from its polyproteins",
"author": "Muramatsu",
"doi-asserted-by": "crossref",
"first-page": "2002",
"issue": "9",
"journal-title": "FEBS J.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0075",
"volume": "280",
"year": "2013"
},
{
"DOI": "10.1016/j.virusres.2015.05.018",
"article-title": "Prediction and biochemical analysis of putative cleavage sites of the 3C-like protease of Middle East respiratory syndrome coronavirus",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "56",
"journal-title": "Virus Res.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0080",
"volume": "208",
"year": "2015"
},
{
"DOI": "10.1002/cbic.202000047",
"article-title": "Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV",
"author": "Morse",
"doi-asserted-by": "crossref",
"first-page": "730",
"issue": "5",
"journal-title": "Chembiochem. Eur. J. Chem. Biol.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0085",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1021/acs.jmedchem.9b01828",
"article-title": "α-Ketoamides as broad-spectrum inhibitors of coronavirus and enterovirus replication: structure-based design, synthesis, and activity assessment",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "4562",
"issue": "9",
"journal-title": "J. Med. Chem.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0090",
"volume": "63",
"year": "2020"
},
{
"article-title": "Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach",
"author": "Khan",
"first-page": "1",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0095",
"year": "2020"
},
{
"DOI": "10.1016/j.bmc.2013.11.041",
"article-title": "Identification of novel drug scaffolds for inhibition of SARS-CoV 3-Chymotrypsin-like protease using virtual and high-throughput screenings",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "167",
"issue": "1",
"journal-title": "Bioorg. Med. Chem.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0100",
"volume": "22",
"year": "2014"
},
{
"DOI": "10.1016/j.bbrc.2004.04.098",
"article-title": "Characterization of SARS main protease and inhibitor assay using a fluorogenic substrate",
"author": "Kuo",
"doi-asserted-by": "crossref",
"first-page": "862",
"issue": "4",
"journal-title": "Biochem. Biophys. Res. Commun.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0105",
"volume": "318",
"year": "2004"
},
{
"DOI": "10.1126/science.abb3405",
"article-title": "Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "409",
"issue": "6489",
"journal-title": "Science",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0110",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1002/jcc.21334",
"article-title": "AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading",
"author": "Trott",
"doi-asserted-by": "crossref",
"first-page": "455",
"issue": "2",
"journal-title": "J. Comput. Chem.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0115",
"volume": "31",
"year": "2010"
},
{
"DOI": "10.1002/(SICI)1096-987X(19981115)19:14<1639::AID-JCC10>3.0.CO;2-B",
"article-title": "Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function",
"author": "Morris",
"doi-asserted-by": "crossref",
"first-page": "1639",
"issue": "14",
"journal-title": "J. Comput. Chem.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0120",
"volume": "19",
"year": "1998"
},
{
"DOI": "10.1186/1758-2946-4-17",
"article-title": "Avogadro: an advanced semantic chemical editor, visualization, and analysis platform",
"author": "Hanwell",
"doi-asserted-by": "crossref",
"first-page": "17",
"issue": "1",
"journal-title": "J. Cheminform.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0125",
"volume": "4",
"year": "2012"
},
{
"DOI": "10.3390/molecules23040894",
"article-title": "Identification by molecular docking of homoisoflavones from Leopoldia comosa as ligands of estrogen receptors",
"author": "Grande",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "Molecules",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0130",
"volume": "23",
"year": "2018"
},
{
"DOI": "10.1007/s13238-010-0044-8",
"article-title": "Three-dimensional domain swapping as a mechanism to lock the active conformation in a super-active octamer of SARS-CoV main protease",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "371",
"issue": "4",
"journal-title": "Protein Cell",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0135",
"volume": "1",
"year": "2010"
},
{
"DOI": "10.1007/978-1-59745-367-7_3",
"article-title": "Defining the stability of multimeric proteins",
"author": "Shriver",
"doi-asserted-by": "crossref",
"first-page": "57",
"journal-title": "Methods Mol. Biol.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0140",
"volume": "490",
"year": "2009"
},
{
"DOI": "10.1016/S0006-3495(92)81899-4",
"article-title": "Theoretical analysis of Lumry-Eyring models in differential scanning calorimetry",
"author": "Sanchez-Ruiz",
"doi-asserted-by": "crossref",
"first-page": "921",
"issue": "4",
"journal-title": "Biophys. J.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0145",
"volume": "61",
"year": "1992"
},
{
"DOI": "10.1021/bi0361766",
"article-title": "Identification of novel inhibitors of the SARS coronavirus main protease 3CLpro",
"author": "Bacha",
"doi-asserted-by": "crossref",
"first-page": "4906",
"issue": "17",
"journal-title": "Biochemistry",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0150",
"volume": "43",
"year": "2004"
},
{
"DOI": "10.1016/j.ab.2006.07.027",
"article-title": "Thermofluor-based high-throughput stability optimization of proteins for structural studies",
"author": "Ericsson",
"doi-asserted-by": "crossref",
"first-page": "289",
"journal-title": "Anal. Biochem.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0155",
"year": "2006"
},
{
"DOI": "10.1177/108705710100600609",
"article-title": "High-density miniaturized thermal shift assays as a general strategy for drug discovery",
"author": "Pantoliano",
"doi-asserted-by": "crossref",
"first-page": "429",
"issue": "6",
"journal-title": "J. Biomol. Screen.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0160",
"volume": "6",
"year": "2001"
},
{
"DOI": "10.1529/biophysj.108.134973",
"article-title": "A quantitative model of thermal stabilization and destabilization of proteins by ligands",
"author": "Cimmperman",
"doi-asserted-by": "crossref",
"first-page": "3222",
"issue": "7",
"journal-title": "Biophys. J.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0165",
"volume": "95",
"year": "2008"
},
{
"DOI": "10.3390/microorganisms8040503",
"article-title": "Small molecule inhibitors of the response regulator ArsR exhibit bactericidal activity against Helicobacter pylori",
"author": "González",
"doi-asserted-by": "crossref",
"issue": "4",
"journal-title": "Microorganisms",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0170",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1089/ars.2018.7658",
"article-title": "Redox- and ligand binding-dependent conformational ensembles in the human apoptosis-inducing factor regulate its pro-life and cell death functions",
"author": "Villanueva",
"doi-asserted-by": "crossref",
"first-page": "2013",
"issue": "18",
"journal-title": "Antioxid. Redox Signal.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0175",
"volume": "30",
"year": "2019"
},
{
"DOI": "10.1021/ac00217a020",
"article-title": "Resolution of multicomponent fluorescence emission using frequency-dependent phase angle and modulation spectra",
"author": "Lakowicz",
"doi-asserted-by": "crossref",
"first-page": "2005",
"issue": "18",
"journal-title": "Anal. Chem.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0180",
"volume": "62",
"year": "1990"
},
{
"DOI": "10.1016/S0079-6468(08)70097-3",
"article-title": "Isothermal titration calorimetry in drug discovery",
"author": "Ward",
"doi-asserted-by": "crossref",
"first-page": "309",
"journal-title": "Prog. Med. Chem.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0185",
"year": "2001"
},
{
"DOI": "10.1080/17460441.2017.1297418",
"article-title": "A look at ligand binding thermodynamics in drug discovery",
"author": "Claveria-Gimeno",
"doi-asserted-by": "crossref",
"first-page": "363",
"issue": "4",
"journal-title": "Expert. Opin. Drug Discov.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0190",
"volume": "12",
"year": "2017"
},
{
"DOI": "10.1038/s41598-019-47746-9",
"article-title": "Identifying potential novel drugs against Helicobacter pylori by targeting the essential response regulator HsrA",
"author": "González",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0195",
"volume": "9",
"year": "2019"
},
{
"article-title": "Ligand-based design identifies a potent NUPR1 inhibitor exerting anticancer activity via necroptosis",
"author": "Santofimia-Castano",
"first-page": "130",
"journal-title": "J. Clin. Invest.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0200",
"year": "2019"
},
{
"DOI": "10.1038/srep39732",
"article-title": "Identification of a drug targeting an intrinsically disordered protein involved in pancreatic adenocarcinoma",
"author": "Neira",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Rep.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0205",
"volume": "7",
"year": "2017"
},
{
"DOI": "10.1371/journal.pone.0159002",
"article-title": "Inhibition of pig phosphoenolpyruvate carboxykinase isoenzymes by 3-mercaptopicolinic acid and novel inhibitors",
"author": "Hidalgo",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "PLoS One",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0210",
"volume": "11",
"year": "2016"
},
{
"DOI": "10.1371/journal.pone.0069773",
"article-title": "Allosteric inhibitors of the NS3 protease from the hepatitis C virus",
"author": "Abian",
"doi-asserted-by": "crossref",
"issue": "7",
"journal-title": "PLoS One",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0215",
"volume": "8",
"year": "2013"
},
{
"DOI": "10.1172/JCI34355",
"article-title": "Identification of pharmacological chaperones as potential therapeutic agents to treat phenylketonuria",
"author": "Pey",
"doi-asserted-by": "crossref",
"first-page": "2858",
"issue": "8",
"journal-title": "J. Clin. Invest.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0220",
"volume": "118",
"year": "2008"
},
{
"DOI": "10.1021/cb900166q",
"article-title": "Discovery of specific flavodoxin inhibitors as potential therapeutic agents against Helicobacter pylori infection",
"author": "Cremades",
"doi-asserted-by": "crossref",
"first-page": "928",
"issue": "11",
"journal-title": "ACS Chem. Biol.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0225",
"volume": "4",
"year": "2009"
},
{
"DOI": "10.1074/jbc.M311744200",
"article-title": "Dissection study on the severe acute respiratory syndrome 3C-like protease reveals the critical role of the extra domain in dimerization of the enzyme: defining the extra domain as a new target for design of highly specific protease inhibitors",
"author": "Shi",
"doi-asserted-by": "crossref",
"first-page": "24765",
"issue": "23",
"journal-title": "J. Biol. Chem.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0230",
"volume": "279",
"year": "2004"
},
{
"DOI": "10.1038/s41467-019-13686-1",
"article-title": "Engineering protein assemblies with allosteric control via monomer fold-switching",
"author": "Campos",
"doi-asserted-by": "crossref",
"first-page": "5703",
"issue": "1",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0235",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1006/jmbi.1997.1613",
"article-title": "The conformational equilibrium of human growth hormone",
"author": "Kasimova",
"doi-asserted-by": "crossref",
"first-page": "409",
"journal-title": "J Mol Biol",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0240",
"volume": "1998",
"year": "1998"
},
{
"DOI": "10.2174/1389450117666160201110449",
"article-title": "Biophysical screening for identifying pharmacological chaperones and inhibitors against conformational and infectious diseases",
"author": "Velazquez-Campoy",
"doi-asserted-by": "crossref",
"first-page": "1492",
"issue": "13",
"journal-title": "Curr. Drug Targets",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0245",
"volume": "17",
"year": "2016"
},
{
"DOI": "10.1016/S1359-6446(05)03386-6",
"article-title": "Ligand efficiency indices as guideposts for drug discovery",
"author": "Abad-Zapatero",
"doi-asserted-by": "crossref",
"first-page": "464",
"journal-title": "Drug Discov. Today",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0250",
"year": "2005"
},
{
"DOI": "10.1111/cbdd.13604",
"article-title": "Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors",
"author": "Jo",
"doi-asserted-by": "crossref",
"first-page": "2023",
"issue": "6",
"journal-title": "Chem. Biol. Drug Des.",
"key": "10.1016/j.ijbiomac.2020.07.235_bb0255",
"volume": "94",
"year": "2019"
}
],
"reference-count": 51,
"references-count": 51,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0141813020339970"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "164"
}
