Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites
et al., Scientific Reports, doi:10.1038/s41598-023-31764-9, Apr 2023
Quercetin for COVID-19
36th treatment shown to reduce risk in
January 2022, now with p = 0.0018 from 9 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
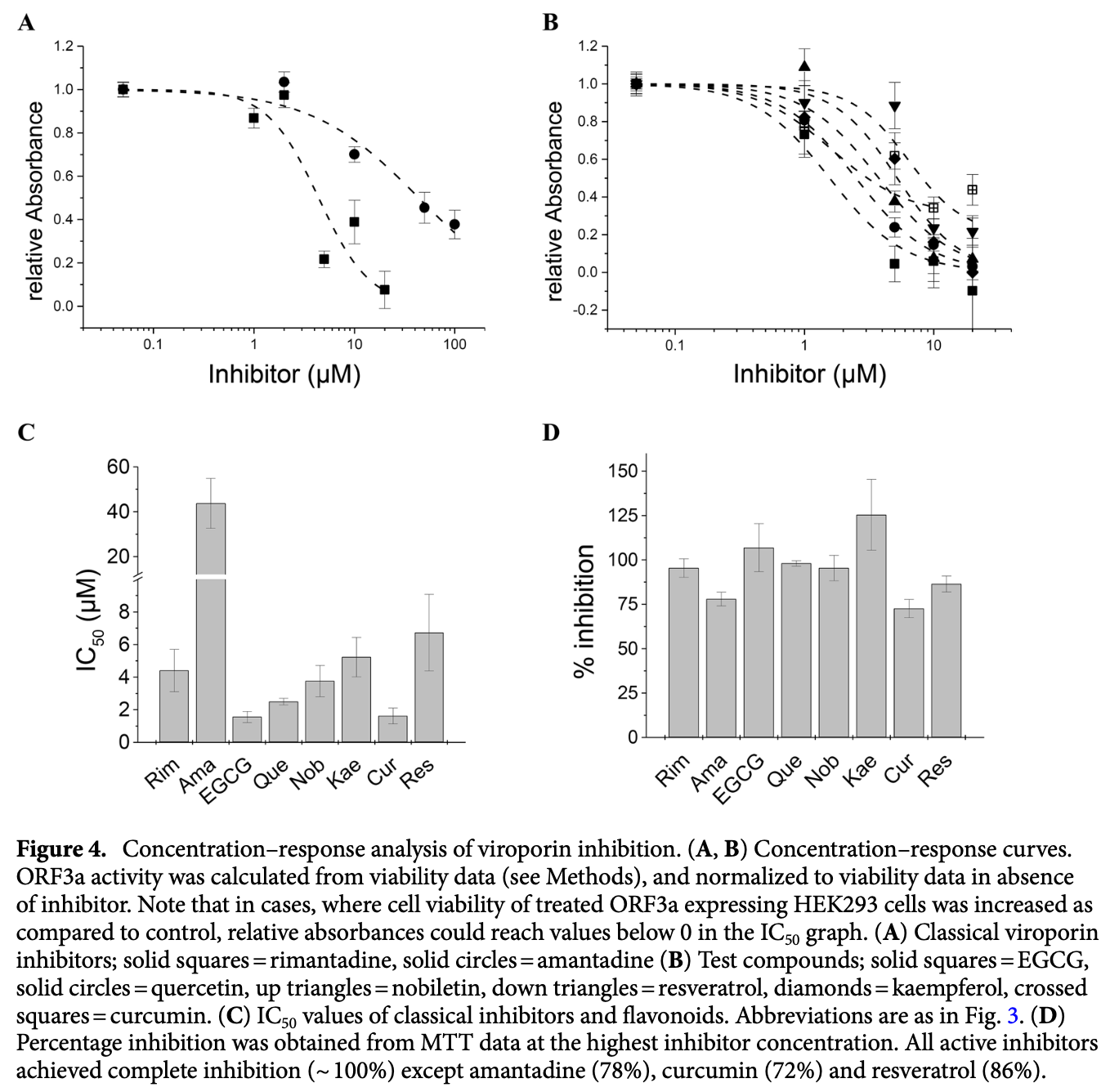
In vitro study showing that adamantane derivatives and six out of ten tested polyphenols including curcumin and quercetin inhibited the SARS-CoV-2 viroporin ORF3a, which contributes to viral pathogenicity and cytotoxicity. Authors used cell viability assays and patch-clamp electrophysiology to test rimantadine, amantadine, and ten phenolic compounds against recombinant ORF3a expressed in HEK293 cells. Rimantadine, amantadine, epigallocatechin gallate (EGCG), quercetin, nobiletin, kaempferol, curcumin and resveratrol inhibited ORF3a activity, while apigenin, genistein, naringenin and 6-gingerol were inactive. Inhibitory potency of flavonoids appeared to correlate with the pattern of OH groups on the chromone ring system.
Bioavailability. Quercetin has low bioavailability and studies typically use advanced formulations to improve bioavailability which may be required to reach therapeutic concentrations.
91 preclinical studies support the efficacy of quercetin for COVID-19:
In silico studies predict inhibition of SARS-CoV-2, or minimization of side effects, with quercetin or metabolites via binding to the spikeA,11,12,18,19,32,34,35,37,40,48,49,51,52,75 (and specifically the receptor binding domainB,8), MproC,7,8,11,12,16,18,20,22,24,26,28,30,33,34,37,40,44,46-48,52-55,72 , RNA-dependent RNA polymeraseD,8,10-12,18,42 , PLproE,12,47,55 , ACE2F,27,32,33,37,38,47,51 , TMPRSS2G,32, nucleocapsidH,12, helicaseI,12,39,44 , endoribonucleaseJ,49, NSP16/10K,15, cathepsin LL,36, Wnt-3M,32, FZDN,32, LRP6O,32, ezrinP,50, ADRPQ,48, NRP1R,51, EP300S,25, PTGS2T,33, HSP90AA1U,25,33 , matrix metalloproteinase 9V,41, IL-6W,31,45 , IL-10X,31, VEGFAY,45, and RELAZ,45 proteins, and inhibition of spike-ACE2 interactionAA,9.
In vitro studies demonstrate inhibition of the MproC,24,58,63,71 protein, and inhibition of spike-ACE2 interactionAA,59.
In vitro studies demonstrate efficacy in Calu-3AB,62, A549AC,31, HEK293-ACE2+AD,70, Huh-7AE,35, Caco-2AF,61, Vero E6AG,29,52,61 , mTECAH,64, RAW264.7AI,64, and HLMECAJ,9 cells.
Animal studies demonstrate efficacy in K18-hACE2 miceAK,67, db/db miceAL,64,74 , BALB/c miceAM,73, and rats29.
Quercetin reduced proinflammatory cytokines and protected lung and kidney tissue against LPS-induced damage in mice73, inhibits LPS-induced cytokine storm by modulating key inflammatory and antioxidant pathways in macrophages14, may block ACE2-spike interaction and NLRP3 inflammasome, limiting viral entry and inflammation5, upregulates the SIRT1/AMPK axis to inhibit oxidative injury and accelerate viral clearance76, inhibits SARS-CoV-2 ORF3a ion channel activity, which contributes to viral pathogenicity and cytotoxicity66, may alleviate COVID-19 ARDS via inhibition of EGFR and JAK2 inflammatory targets1, may destabilize the Spike protein, IL-6R, and integrins via conserved residues, blocking viral entry, hyperinflammation, and platelet aggregation77, and may reduce COVID-19 neuroinflammation and cognitive dysfunction through anti-inflammatory mechanisms and neuroprotective effects78.
Study covers quercetin and curcumin.
1.
Gupta et al., Harnessing phytoconstituents to treat COVID-19 triggered acute respiratory distress syndrome: Insights from network pharmacology, and molecular modeling, Phytochemistry Letters, doi:10.1016/j.phytol.2025.104105.
2.
Sun et al., Feasibility of the inhibitor development for SARS-CoV-2: a systematic approach for drug design, Journal of Molecular Modeling, doi:10.1007/s00894-025-06541-2.
3.
Torabfam et al., Improving quercetin solubility via structural modification enhances dual-target coronavirus entry: an integrated in-vitro and in-silico study, Scientific Reports, doi:10.1038/s41598-025-27374-2.
4.
Abdelhameed et al., Phytochemical and antiviral investigation of Cynanchum acutum L. extract and derived semi-synthetic analogs targeting SARS-CoV-2 main protease, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-025-00907-2.
5.
Manikyam et al., INP-Guided Network Pharmacology Discloses Multi-Target Therapeutic Strategy Against Cytokine and IgE Storms in the SARS-CoV-2 NB.1.8.1 Variant, Research Square, doi:10.21203/rs.3.rs-6819274/v1.
6.
Makoana et al., Integration of metabolomics and chemometrics with in-silico and in-vitro approaches to unravel SARS-Cov-2 inhibitors from South African plants, PLOS ONE, doi:10.1371/journal.pone.0320415.
7.
Bano et al., Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability, Viruses, doi:10.3390/v17030402.
8.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
9.
Moharram et al., Secondary metabolites of Alternaria alternate appraisal of their SARS-CoV-2 inhibitory and anti-inflammatory potentials, PLOS ONE, doi:10.1371/journal.pone.0313616.
10.
Metwaly et al., Integrated study of Quercetin as a potent SARS-CoV-2 RdRp inhibitor: Binding interactions, MD simulations, and In vitro assays, PLOS ONE, doi:10.1371/journal.pone.0312866.
11.
Al balawi et al., Assessing multi-target antiviral and antioxidant activities of natural compounds against SARS-CoV-2: an integrated in vitro and in silico study, Bioresources and Bioprocessing, doi:10.1186/s40643-024-00822-z.
12.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
13.
Pan et al., Decoding the mechanism of Qingjie formula in the prevention of COVID-19 based on network pharmacology and molecular docking, Heliyon, doi:10.1016/j.heliyon.2024.e39167.
14.
Xu et al., Quercetin inhibited LPS-induced cytokine storm by interacting with the AKT1-FoxO1 and Keap1-Nrf2 signaling pathway in macrophages, Scientific Reports, doi:10.1038/s41598-024-71569-y.
15.
Tamil Selvan et al., Computational Investigations to Identify Potent Natural Flavonoid Inhibitors of the Nonstructural Protein (NSP) 16/10 Complex Against Coronavirus, Cureus, doi:10.7759/cureus.68098.
16.
Sunita et al., Characterization of Phytochemical Inhibitors of the COVID-19 Primary Protease Using Molecular Modelling Approach, Asian Journal of Microbiology and Biotechnology, doi:10.56557/ajmab/2024/v9i28800.
17.
Wu et al., Biomarkers Prediction and Immune Landscape in Covid-19 and “Brain Fog”, Elsevier BV, doi:10.2139/ssrn.4897774.
18.
Raman et al., Phytoconstituents of Citrus limon (Lemon) as Potential Inhibitors Against Multi Targets of SARS‐CoV‐2 by Use of Molecular Modelling and In Vitro Determination Approaches, ChemistryOpen, doi:10.1002/open.202300198.
19.
Asad et al., Exploring the antiviral activity of Adhatoda beddomei bioactive compounds in interaction with coronavirus spike protein, Archives of Medical Reports, 1:1, archmedrep.com/index.php/amr/article/view/3.
20.
Irfan et al., Phytoconstituents of Artemisia Annua as potential inhibitors of SARS CoV2 main protease: an in silico study, BMC Infectious Diseases, doi:10.1186/s12879-024-09387-w.
21.
Yuan et al., Network pharmacology and molecular docking reveal the mechanisms of action of Panax notoginseng against post-COVID-19 thromboembolism, Review of Clinical Pharmacology and Pharmacokinetics - International Edition, doi:10.61873/DTFA3974.
22.
Nalban et al., Targeting COVID-19 (SARS-CoV-2) main protease through phytochemicals of Albizia lebbeck: molecular docking, molecular dynamics simulation, MM–PBSA free energy calculations, and DFT analysis, Journal of Proteins and Proteomics, doi:10.1007/s42485-024-00136-w.
23.
Zhou et al., Bioinformatics and system biology approaches to determine the connection of SARS-CoV-2 infection and intrahepatic cholangiocarcinoma, PLOS ONE, doi:10.1371/journal.pone.0300441.
24.
Waqas et al., Discovery of Novel Natural Inhibitors Against SARS-CoV-2 Main Protease: A Rational Approach to Antiviral Therapeutics, Current Medicinal Chemistry, doi:10.2174/0109298673292839240329081008.
25.
Hasanah et al., Decoding the therapeutic potential of empon-empon: a bioinformatics expedition unraveling mechanisms against COVID-19 and atherosclerosis, International Journal of Applied Pharmaceutics, doi:10.22159/ijap.2024v16i2.50128.
26.
Shaik et al., Computational identification of selected bioactive compounds from Cedrus deodara as inhibitors against SARS-CoV-2 main protease: a pharmacoinformatics study, Indian Drugs, doi:10.53879/id.61.02.13859.
27.
Wang et al., Investigating the Mechanism of Qu Du Qiang Fei 1 Hao Fang Formula against Coronavirus Disease 2019 Based on Network Pharmacology Method, World Journal of Traditional Chinese Medicine, doi:10.4103/2311-8571.395061.
28.
Singh et al., Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 M Pro protein - An in-silico and cell-based approach, Research Square, doi:10.21203/rs.3.rs-3888947/v1.
29.
El-Megharbel et al., Chemical and spectroscopic characterization of (Artemisinin/Quercetin/ Zinc) novel mixed ligand complex with assessment of its potent high antiviral activity against SARS-CoV-2 and antioxidant capacity against toxicity induced by acrylamide in male rats, PeerJ, doi:10.7717/peerj.15638.
30.
Akinwumi et al., Evaluation of therapeutic potentials of some bioactive compounds in selected African plants targeting main protease (Mpro) in SARS-CoV-2: a molecular docking study, Egyptian Journal of Medical Human Genetics, doi:10.1186/s43042-023-00456-4.
31.
Yang et al., Active ingredient and mechanistic analysis of traditional Chinese medicine formulas for the prevention and treatment of COVID-19: Insights from bioinformatics and in vitro experiments, Medicine, doi:10.1097/MD.0000000000036238.
32.
Chandran et al., Molecular docking analysis of quercetin with known CoVid-19 targets, Bioinformation, doi:10.6026/973206300191081.
33.
Qin et al., Exploring the bioactive compounds of Feiduqing formula for the prevention and management of COVID-19 through network pharmacology and molecular docking, Medical Data Mining, doi:10.53388/MDM202407003.
34.
Moschovou et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, International Journal of Molecular Sciences, doi:10.3390/ijms242115894.
35.
Pan (B) et al., Quercetin: A promising drug candidate against the potential SARS-CoV-2-Spike mutants with high viral infectivity, Computational and Structural Biotechnology Journal, doi:10.1016/j.csbj.2023.10.029.
36.
Ahmed et al., Evaluation of the Effect of Zinc, Quercetin, Bromelain and Vitamin C on COVID-19 Patients, International Journal of Diabetes Management, doi:10.61797/ijdm.v2i2.259.
37.
Thapa et al., In-silico Approach for Predicting the Inhibitory Effect of Home Remedies on Severe Acute Respiratory Syndrome Coronavirus-2, Makara Journal of Science, doi:10.7454/mss.v27i3.1609.
38.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
39.
Singh (B) et al., Flavonoids as Potent Inhibitor of SARS-CoV-2 Nsp13 Helicase: Grid Based Docking Approach, Middle East Research Journal of Pharmaceutical Sciences, doi:10.36348/merjps.2023.v03i04.001.
40.
Mandal et al., In silico anti-viral assessment of phytoconstituents in a traditional (Siddha Medicine) polyherbal formulation – Targeting Mpro and pan-coronavirus post-fusion Spike protein, Journal of Traditional and Complementary Medicine, doi:10.1016/j.jtcme.2023.07.004.
41.
Sai Ramesh et al., Computational analysis of the phytocompounds of Mimusops elengi against spike protein of SARS CoV2 – An Insilico model, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2023.125553.
42.
Corbo et al., Inhibitory potential of phytochemicals on five SARS-CoV-2 proteins: in silico evaluation of endemic plants of Bosnia and Herzegovina, Biotechnology & Biotechnological Equipment, doi:10.1080/13102818.2023.2222196.
43.
Azmi et al., Utilization of quercetin flavonoid compounds in onion (Allium cepa L.) as an inhibitor of SARS-CoV-2 spike protein against ACE2 receptors, 11th International Seminar on New Paradigm and Innovation on Natural Sciences and its Application, doi:10.1063/5.0140285.
44.
Alanzi et al., Structure-based virtual identification of natural inhibitors of SARS-CoV-2 and its Delta and Omicron variant proteins, Future Virology, doi:10.2217/fvl-2022-0184.
45.
Yang (B) et al., In silico evidence implicating novel mechanisms of Prunella vulgaris L. as a potential botanical drug against COVID-19-associated acute kidney injury, Frontiers in Pharmacology, doi:10.3389/fphar.2023.1188086.
46.
Wang (B) et al., Computational Analysis of Lianhua Qingwen as an Adjuvant Treatment in Patients with COVID-19, Society of Toxicology Conference, 2023, www.researchgate.net/publication/370491709_Y_Wang_A_E_Tan_O_Chew_A_Hsueh_and_D_E_Johnson_2023_Computational_Analysis_of_Lianhua_Qingwen_as_an_Adjuvant_Treatment_in_Patients_with_COVID-19_Toxicologist_1921_507.
47.
Ibeh et al., Computational studies of potential antiviral compounds from some selected Nigerian medicinal plants against SARS-CoV-2 proteins, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2023.101230.
48.
Nguyen et al., The Potential of Ameliorating COVID-19 and Sequelae From Andrographis paniculata via Bioinformatics, Bioinformatics and Biology Insights, doi:10.1177/11779322221149622.
49.
Alavi et al., Interaction of Epigallocatechin Gallate and Quercetin with Spike Glycoprotein (S-Glycoprotein) of SARS-CoV-2: In Silico Study, Biomedicines, doi:10.3390/biomedicines10123074.
50.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
51.
Şimşek et al., In silico identification of SARS-CoV-2 cell entry inhibitors from selected natural antivirals, Journal of Molecular Graphics and Modelling, doi:10.1016/j.jmgm.2021.108038.
52.
Kandeil et al., Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2, Pathogens, doi:10.3390/pathogens10060758.
53.
Rehman et al., Natural Compounds as Inhibitors of SARS-CoV-2 Main Protease (3CLpro): A Molecular Docking and Simulation Approach to Combat COVID-19, Current Pharmaceutical Design, doi:10.2174/1381612826999201116195851.
54.
Sekiou et al., In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2, ChemRxiv, doi:10.26434/chemrxiv.12181404.v1.
55.
Zhang et al., In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus, Journal of Integrative Medicine, doi:10.1016/j.joim.2020.02.005.
56.
Sisti et al., Evaluation of respiratory virus transmissibility and resilience from fomites: the case of 11 SARS-CoV-2 clinical isolates, Applied and Environmental Microbiology, doi:10.1128/aem.00774-25.
57.
Spinelli et al., Amphibian‐Derived Peptides as Natural Inhibitors of SARS‐CoV‐2 Main Protease (Mpro): A Combined In Vitro and In Silico Approach, Chemistry & Biodiversity, doi:10.1002/cbdv.202403202.
58.
Aguilera-Rodriguez et al., Inhibition of SARS-CoV-2 3CLpro by chemically modified tyrosinase from Agaricus bisporus, RSC Medicinal Chemistry, doi:10.1039/D4MD00289J.
59.
Emam et al., Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors, AMB Express, doi:10.1186/s13568-024-01739-8.
60.
Fang et al., Development of nanoparticles incorporated with quercetin and ACE2-membrane as a novel therapy for COVID-19, Journal of Nanobiotechnology, doi:10.1186/s12951-024-02435-2.
61.
Roy et al., Quercetin inhibits SARS-CoV-2 infection and prevents syncytium formation by cells co-expressing the viral spike protein and human ACE2, Virology Journal, doi:10.1186/s12985-024-02299-w.
62.
DiGuilio et al., Quercetin improves and protects Calu-3 airway epithelial barrier function, Frontiers in Cell and Developmental Biology, doi:10.3389/fcell.2023.1271201.
63.
Zhang (B) et al., Discovery of the covalent SARS‐CoV‐2 Mpro inhibitors from antiviral herbs via integrating target‐based high‐throughput screening and chemoproteomic approaches, Journal of Medical Virology, doi:10.1002/jmv.29208.
64.
Wu (B) et al., SARS-CoV-2 N protein induced acute kidney injury in diabetic db/db mice is associated with a Mincle-dependent M1 macrophage activation, Frontiers in Immunology, doi:10.3389/fimmu.2023.1264447.
65.
Xu (B) et al., Bioactive compounds from Huashi Baidu decoction possess both antiviral and anti-inflammatory effects against COVID-19, Proceedings of the National Academy of Sciences, doi:10.1073/pnas.2301775120.
66.
Fam et al., Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites, Scientific Reports, doi:10.1038/s41598-023-31764-9.
67.
Aguado et al., Senolytic therapy alleviates physiological human brain aging and COVID-19 neuropathology, bioRxiv, doi:10.1101/2023.01.17.524329.
68.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
69.
Munafò et al., Quercetin and Luteolin Are Single-digit Micromolar Inhibitors of the SARS-CoV-2 RNA-dependent RNA Polymerase, Research Square, doi:10.21203/rs.3.rs-1149846/v1.
70.
Singh (C) et al., The spike protein of SARS-CoV-2 virus induces heme oxygenase-1: Pathophysiologic implications, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, doi:10.1016/j.bbadis.2021.166322.
71.
Bahun et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594.
72.
Abian et al., Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.07.235.
73.
Shaker et al., Anti-cytokine Storm Activity of Fraxin, Quercetin, and their Combination on Lipopolysaccharide-Induced Cytokine Storm in Mice: Implications in COVID-19, Iranian Journal of Medical Sciences, doi:10.30476/ijms.2023.98947.3102.
74.
Wu (C) et al., Treatment with Quercetin inhibits SARS-CoV-2 N protein-induced acute kidney injury by blocking Smad3-dependent G1 cell cycle arrest, Molecular Therapy, doi:10.1016/j.ymthe.2022.12.002.
75.
Azmi (B) et al., The role of vitamin D receptor and IL‐6 in COVID‐19, Molecular Genetics & Genomic Medicine, doi:10.1002/mgg3.2172.
76.
Shokri-Afra et al., Targeting SIRT1: A Potential Strategy for Combating Severe COVID‐19, BioMed Research International, doi:10.1155/bmri/9507417.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The receptor binding domain is a specific region of the spike protein that binds ACE2 and is a major target of neutralizing antibodies. Focusing on the precise binding site allows highly specific disruption of viral attachment with reduced potential for off-target effects.
c.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
d.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
e.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
f.
The angiotensin converting enzyme 2 (ACE2) protein is a host cell transmembrane protein that serves as the cellular receptor for the SARS-CoV-2 spike protein. ACE2 is expressed on many cell types, including epithelial cells in the lungs, and allows the virus to enter and infect host cells. Inhibition may affect ACE2's physiological function in blood pressure control.
g.
Transmembrane protease serine 2 (TMPRSS2) is a host cell protease that primes the spike protein, facilitating cellular entry. TMPRSS2 activity helps enable cleavage of the spike protein required for membrane fusion and virus entry. Inhibition may especially protect respiratory epithelial cells, buy may have physiological effects.
h.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
i.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
j.
The endoribonuclease, also known as NendoU or nsp15, cleaves specific sequences in viral RNA which may help the virus evade detection by the host immune system. Inhibition may hinder the virus's ability to mask itself from the immune system, facilitating a stronger immune response.
k.
The NSP16/10 complex consists of non-structural proteins 16 and 10, forming a 2'-O-methyltransferase that modifies the viral RNA cap structure. This modification helps the virus evade host immune detection by mimicking host mRNA, making NSP16/10 a promising antiviral target.
l.
Cathepsin L is a host lysosomal cysteine protease that can prime the spike protein through an alternative pathway when TMPRSS2 is unavailable. Dual targeting of cathepsin L and TMPRSS2 may maximize disruption of alternative pathways for virus entry.
m.
Wingless-related integration site (Wnt) ligand 3 is a host signaling molecule that activates the Wnt signaling pathway, which is important in development, cell growth, and tissue repair. Some studies suggest that SARS-CoV-2 infection may interfere with the Wnt signaling pathway, and that Wnt3a is involved in SARS-CoV-2 entry.
n.
The frizzled (FZD) receptor is a host transmembrane receptor that binds Wnt ligands, initiating the Wnt signaling cascade. FZD serves as a co-receptor, along with ACE2, in some proposed mechanisms of SARS-CoV-2 infection. The virus may take advantage of this pathway as an alternative entry route.
o.
Low-density lipoprotein receptor-related protein 6 is a cell surface co-receptor essential for Wnt signaling. LRP6 acts in tandem with FZD for signal transduction and has been discussed as a potential co-receptor for SARS-CoV-2 entry.
p.
The ezrin protein links the cell membrane to the cytoskeleton (the cell's internal support structure) and plays a role in cell shape, movement, adhesion, and signaling. Drugs that occupy the same spot on ezrin where the viral spike protein would bind may hindering viral attachment, and drug binding could further stabilize ezrin, strengthening its potential natural capacity to impede viral fusion and entry.
q.
The Adipocyte Differentiation-Related Protein (ADRP, also known as Perilipin 2 or PLIN2) is a lipid droplet protein regulating the storage and breakdown of fats in cells. SARS-CoV-2 may hijack the lipid handling machinery of host cells and ADRP may play a role in this process. Disrupting ADRP's interaction with the virus may hinder the virus's ability to use lipids for replication and assembly.
r.
Neuropilin-1 (NRP1) is a cell surface receptor with roles in blood vessel development, nerve cell guidance, and immune responses. NRP1 may function as a co-receptor for SARS-CoV-2, facilitating viral entry into cells. Blocking NRP1 may disrupt an alternative route of viral entry.
s.
EP300 (E1A Binding Protein P300) is a transcriptional coactivator involved in several cellular processes, including growth, differentiation, and apoptosis, through its acetyltransferase activity that modifies histones and non-histone proteins. EP300 facilitates viral entry into cells and upregulates inflammatory cytokine production.
t.
Prostaglandin G/H synthase 2 (PTGS2, also known as COX-2) is an enzyme crucial for the production of inflammatory molecules called prostaglandins. PTGS2 plays a role in the inflammatory response that can become severe in COVID-19 and inhibitors (like some NSAIDs) may have benefits in dampening harmful inflammation, but note that prostaglandins have diverse physiological functions.
u.
Heat Shock Protein 90 Alpha Family Class A Member 1 (HSP90AA1) is a chaperone protein that helps other proteins fold correctly and maintains their stability. HSP90AA1 plays roles in cell signaling, survival, and immune responses. HSP90AA1 may interact with numerous viral proteins, but note that it has diverse physiological functions.
v.
Matrix metalloproteinase 9 (MMP9), also called gelatinase B, is a zinc-dependent enzyme that breaks down collagen and other components of the extracellular matrix. MMP9 levels increase in severe COVID-19. Overactive MMP9 can damage lung tissue and worsen inflammation. Inhibition of MMP9 may prevent excessive tissue damage and help regulate the inflammatory response.
w.
The interleukin-6 (IL-6) pro-inflammatory cytokine (signaling molecule) has a complex role in the immune response and may trigger and perpetuate inflammation. Elevated IL-6 levels are associated with severe COVID-19 cases and cytokine storm. Anti-IL-6 therapies may be beneficial in reducing excessive inflammation in severe COVID-19 cases.
x.
The interleukin-10 (IL-10) anti-inflammatory cytokine helps regulate and dampen immune responses, preventing excessive inflammation. IL-10 levels can also be elevated in severe COVID-19. IL-10 could either help control harmful inflammation or potentially contribute to immune suppression.
y.
Vascular Endothelial Growth Factor A (VEGFA) promotes the growth of new blood vessels (angiogenesis) and has roles in inflammation and immune responses. VEGFA may contribute to blood vessel leakiness and excessive inflammation associated with severe COVID-19.
z.
RELA is a transcription factor subunit of NF-kB and is a key regulator of inflammation, driving pro-inflammatory gene expression. SARS-CoV-2 may hijack and modulate NF-kB pathways.
aa.
The interaction between the SARS-CoV-2 spike protein and the human ACE2 receptor is a primary method of viral entry, inhibiting this interaction can prevent the virus from attaching to and entering host cells, halting infection at an early stage.
ab.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
ac.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
ad.
HEK293-ACE2+ is a human embryonic kidney cell line engineered for high ACE2 expression and SARS-CoV-2 susceptibility.
ae.
Huh-7 cells were derived from a liver tumor (hepatoma).
af.
Caco-2 cells come from a colorectal adenocarcinoma (cancer). They are valued for their ability to form a polarized cell layer with properties similar to the intestinal lining.
ag.
Vero E6 is an African green monkey kidney cell line with low/no ACE2 expression and high SARS-CoV-2 susceptibility. The cell line is easy to maintain and supports robust viral replication, however the monkey origin may not accurately represent human responses.
ah.
mTEC is a mouse tubular epithelial cell line.
ai.
RAW264.7 is a mouse macrophage cell line.
aj.
HLMEC (Human Lung Microvascular Endothelial Cells) are primary endothelial cells derived from the lung microvasculature. They are used to study endothelial function, inflammation, and viral interactions, particularly in the context of lung infections such as SARS-CoV-2. HLMEC express ACE2 and are susceptible to SARS-CoV-2 infection, making them a relevant model for studying viral entry and endothelial responses in the lung.
ak.
A mouse model expressing the human ACE2 receptor under the control of the K18 promoter.
al.
A mouse model of obesity and severe insulin resistance leading to type 2 diabetes due to a mutation in the leptin receptor gene that impairs satiety signaling.
am.
A mouse model commonly used in infectious disease and cancer research due to higher immune response and susceptibility to infection.
Fam et al., 1 Apr 2023, peer-reviewed, 5 authors.
Contact: ulrike.breitinger@guc.edu.eg.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites
Scientific Reports, doi:10.1038/s41598-023-31764-9
SARS-CoV-2 has been responsible for the major worldwide pandemic of COVID-19. Despite the enormous success of vaccination campaigns, virus infections are still prevalent and effective antiviral therapies are urgently needed. Viroporins are essential for virus replication and release, and are thus promising therapeutic targets. Here, we studied the expression and function of recombinant ORF3a viroporin of SARS-CoV-2 using a combination of cell viability assays and patch-clamp electrophysiology. ORF3a was expressed in HEK293 cells and transport to the plasma membrane verified by a dot blot assay. Incorporation of a membrane-directing signal peptide increased plasma membrane expression. Cell viability tests were carried out to measure cell damage associated with ORF3a activity, and voltage-clamp recordings verified its channel activity. The classical viroporin inhibitors amantadine and rimantadine inhibited ORF3a channels. A series of ten flavonoids and polyphenolics were studied. Kaempferol, quercetin, epigallocatechin gallate, nobiletin, resveratrol and curcumin were ORF3a inhibitors, with IC 50 values ranging between 1 and 6 µM, while 6-gingerol, apigenin, naringenin and genistein were inactive. For flavonoids, inhibitory activity could be related to the pattern of OH groups on the chromone ring system. Thus, the ORF3a viroporin of SARS-CoV-2 may indeed be a promising target for antiviral drugs. Coronaviruses (CoVs) belong to the order Nidovirales, family Coronaviridae, and subfamily Coronavirinae 1 . They are subdivided into four different genera named α-, β-, γ-, and δ-CoVs 2 . Coronaviruses have been known to infect humans 2-4 , usually causing mild respiratory infections such as a common cold. However, in the past 20 years, two major outbreaks occurred due to crossover of animal β-coronavirus to humans 5 . In 2002-03 humans were infected by bat coronavirus resulting in severe acute respiratory syndrome coronavirus (SARS-CoV) and in 2019, a novel coronavirus of bat origin that had spread to humans, had been discovered in Wuhan, China 6 . This new virus, named SARS-CoV-2, is a member of the β-coronavirus family and is responsible for the ongoing pandemic of COVID-19 1, 7, 8 . SARS-CoVs are enveloped, positive sense single-stranded RNA viruses, with a genome of approximately 30 kb arranged into 14 open reading frames (ORF) encoding 31 proteins [8] [9] [10] [11] . Spike (S), envelope (E), membrane (M) and nucleoprotein (N) are the four structural proteins forming the virus capsid. The S protein binds to the host receptor through the receptor-binding domain in the S1 subunit, while S2 subunit is responsible for membrane fusion 8 . The E protein belongs to the class of viroporins, integral membrane proteins functioning as ion channels and promoting virus release. It was found to be expressed in the ER and the Golgi apparatus forming an ion channel allowing the efflux of cations Na + , K + and Ca 2+ , and is required for pathogenesis and..
Author contributions M.S.F.: design of work, data acquisition and analysis, interpretation of data, revising the manuscript. C.A.S.: data acquisition and analysis, interpretation of data, revising the manuscript. N.O.T.: data acquisition and analysis, interpretation of data, revising the manuscript. H.G.B.: conception, design of work, data acquisition and analysis, interpretation of data, drafting and revising the manuscript. U.B.: conception, design of work, data acquisition and analysis, interpretation of data, drafting, writing and revising the manuscript. All authors have read and approved the submitted manuscript.
Competing interests The authors declare no competing interests.
References
Abba, Hassim, Hamzah, Noordin, Antiviral activity of resveratrol against human and animal viruses, Adv. Virol
Aboubakr, In vitro antiviral activity of clove and ginger aqueous extracts against feline calicivirus, a surrogate for human norovirus, J. Food Prot, doi:10.4315/0362-028X.JFP-15-593
Agrawal, Agrawal, Blunden, Quercetin: antiviral significance and possible COVID-19 integrative considerations, Nat. Prod. Comm, doi:10.1177/1934578X20976293
Arshad, SARS-CoV-2 accessory proteins ORF7a and ORF3a use distinct mechanisms to downregulate MHC-I surface expression, bioRxiv
Azad, Khan, Variations in ORF3a protein of SARS-CoV-2 alter its structure and function, Biochem. Biophys. Rep, doi:10.1016/j.bbrep.2021.100933
Bhowmik, Identification of potential inhibitors against SARS-CoV-2 by targeting proteins responsible for envelope formation and virion assembly using docking based virtual screening, and pharmacokinetics approaches, Infect. Genet. Evol, doi:10.1016/j.meegid.2020.104451
Bianchi, Borsetti, Ciccozzi, Pascarella, SARS-CoV-2 ORF3a: mutability and function, Int. J. Biol. Macromol, doi:10.1016/j.ijbiomac.2020.12.142
Breitinger, Ali, Sticht, Breitinger, Inhibition of SARS CoV envelope protein by flavonoids and classical viroporin inhibitors, Front. Microbiol, doi:10.3389/fmicb.2021.692423
Breitinger, Cell viability assay as a tool to study activity and inhibition of hepatitis C p7 channels, J. Gen. Virol, doi:10.1099/jgv.0.001571
Breitinger, Farag, Ali, Breitinger, Patch-clamp study of hepatitis C p7 channels reveals genotype-specific sensitivity to inhibitors, Biophys. J
Breitinger, Farag, Sticht, Breitinger, Viroporins, Structure, function, and their role in the life cycle of SARS-CoV-2, Int. J. Biochem. Cell Biol, doi:10.1016/j.biocel.2022.106185
Campagna, Rivas, Antiviral activity of resveratrol, Biochem. Soc. Trans, doi:10.1042/BST0380050
Castano-Rodriguez, Role of severe acute respiratory syndrome coronavirus viroporins E, 3a, and 8a in replication and pathogenesis, doi:10.1128/mBio.02325-17
Chan, The ion channel activity of the SARS-coronavirus 3a protein is linked to its pro-apoptotic function, Int. J. Biochem. Cell Biol, doi:10.1016/j.biocel.2009.04.019
Chang, Wang, Yeh, Shieh, Chiang, Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines, J. Ethnopharmacol, doi:10.1016/j.jep.2012.10.043
Chen, Insights into the anti-inflammatory and antiviral mechanisms of resveratrol, Mediat. Inflamm, doi:10.1155/2022/7138756
Chen, Lo, Ma, Li, Expression and membrane integration of SARS-CoV E protein and its interaction with M protein, Virus Genes, doi:10.1007/s11262-009-0341-6
Chen, Moriyama, Chang, Ichinohe, Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome, Front. Microbiol, doi:10.3389/fmicb.2019.00050
Cione, Quercetin, epigallocatechin gallate, curcumin, and resveratrol: from dietary sources to human MicroRNA modulation, Molecules, doi:10.3390/molecules25010063
De Wit, Van Doremalen, Falzarano, Munster, SARS and MERS: recent insights into emerging coronaviruses, Nat. Rev. Microbiol, doi:10.1038/nrmicro.2016.81
Dey, The effect of amantadine on an ion channel protein from Chikungunya virus, PLoS Negl. Trop. Dis, doi:10.1371/journal.pntd.0007548
Drago, Nicola, Ossola, De Vecchi, In vitro antiviral activity of resveratrol against respiratory viruses, J. Chemother, doi:10.1179/joc.2008.20.3.393
Duff, Ashley, The transmembrane domain of influenza A M2 protein forms amantadine-sensitive proton channels in planar lipid bilayers, Virology, doi:10.1016/0042-6822(92)91239-q
Farag, Breitinger, El-Azizi, Breitinger, The p7 viroporin of the hepatitis C virus contributes to liver inflammation by stimulating production of Interleukin-1β, Biochim. Biophys. Acta Mol. Basis Dis
Fleming, Managing influenza: amantadine, rimantadine and beyond, Int. J. Clin. Pract
Freundt, The open reading frame 3a protein of severe acute respiratory syndrome-associated coronavirus promotes membrane rearrangement and cell death, J. Virol, doi:10.1128/JVI.01662-09
Gligorijevic, Molecular mechanisms of possible action of phenolic compounds in COVID-19 protection and prevention, Int. J. Mol. Sci
Gonzalez, Carrasco, Viroporins, None, FEBS Lett
Gorbalenya, The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2, Nat. Microbiol, doi:10.1038/s41564-020-0695-z
Gordon, A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature, doi:10.1038/s41586-020-2286-9
Griffin, Genotype-dependent sensitivity of hepatitis C virus to inhibitors of the p7 ion channel, Hepatology
Griffin, The p7 protein of hepatitis C virus forms an ion channel that is blocked by the antiviral drug Amantadine, FEBS Lett
Gupta, D155Y substitution of SARS-CoV-2 ORF3a weakens binding with Caveolin-1, Comput. Struct. Biotechnol. J, doi:10.1016/j.csbj.2022.01.017
Hassan, Attrish, Ghosh, Choudhury, Roy, Pathogenic perspective of missense mutations of ORF3a protein of SARS-CoV-2, Virus Res, doi:10.1016/j.virusres.2021.198441
Hayati, 6]-Gingerol inhibits chikungunya virus infection by suppressing viral replication, Biomed. Res. Int, doi:10.1155/2021/6623400
Intharathep, How amantadine and rimantadine inhibit proton transport in the M2 protein channel, J. Mol. Graph Model, doi:10.1016/j.jmgm.2008.06.002
Issa, Merhi, Panossian, Salloum, Tokajian, SARS-CoV-2 and ORF3a: nonsynonymous mutations, functional domains, and viral pathogenesis, doi:10.1128/mSystems.00266-20
Jennings, Parks, Curcumin as an antiviral agent, Viruses
Jimenez-Guardeno, The PDZ-binding motif of severe acute respiratory syndrome coronavirus envelope protein is a determinant of viral pathogenesis, PLoS Pathog, doi:10.1371/journal.ppat.1004320
Jing, Functional studies indicate amantadine binds to the pore of the influenza A virus M2 proton-selective ion channel, Proc. Natl. Acad. Sci. U. S. A, doi:10.1073/pnas.0804958105
Kanjanasirirat, High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents, Sci. Rep
Kanzawa, Augmentation of chemokine production by severe acute respiratory syndrome coronavirus 3a/X1 and 7a/X4 proteins through NF-kappaB activation, FEBS Lett, doi:10.1016/j.febslet.2006.11.046
Kaushik, Jangra, Kundu, Yadav, Kaushik, Anti-viral activity of Zingiber officinale (Ginger) ingredients against the Chikungunya virus, Virusdisease, doi:10.1007/s13337-020-00584-0
Kern, Cryo-EM structure of the SARS-CoV-2 3a ion channel in lipid nanodiscs, bioRxiv
Kien, Ma, Gaisenband, Nal, Microbial Pathogenesis: Infection and Immunity
Kongpichitchoke, Hsu, Huang, Number of hydroxyl groups on the B-ring of flavonoids affects their antioxidant activity and interaction with phorbol ester binding site of PKCδ C1B domain: in vitro and in silico studies, J. Agric. Food Chem, doi:10.1021/acs.jafc.5b00312
Kumar, Pandey, Chemistry and biological activities of flavonoids: an overview, Sci.WorldJ, doi:10.1155/2013/162750
Law, The 3a protein of severe acute respiratory syndrome-associated coronavirus induces apoptosis in Vero E6 cells, J. Gen. Virol, doi:10.1099/vir.0.80813-0
Lebedeva, Theoretical and experimental study of interaction of macroheterocyclic compounds with ORF3a of SARS-CoV-2, Sci. Rep, doi:10.1038/s41598-021-99072-8
Liao, Tam, Liu, Viroporin activity of SARS-CoV E protein, Adv. Exp. Med. Biol, doi:10.1007/978-0-387-33012-9_34
Lim, Ng, Tam, Liu, Human coronaviruses: a review of virus-host interactions, Diseases
Liu, A comparative overview of COVID-19, MERS and SARS: review article, Int. J. Surg, doi:10.1016/j.ijsu.2020.07.032
Mahrosh, Mustafa, An in silico approach to target RNA-dependent RNA polymerase of COVID-19 with naturally occurring phytochemicals, Environ. Dev. Sustain, doi:10.1007/s10668-021-01373-5
Majumdar, Niyogi, ORF3a mutation associated with higher mortality rate in SARS-CoV-2 infection, Epidemiol. Infect, doi:10.1017/S0950268820002599
Marra, The Genome sequence of the SARS-associated coronavirus, Science, doi:10.1126/science.1085953
Miao, ORF3a of the COVID-19 virus SARS-CoV-2 blocks HOPS complex-mediated assembly of the SNARE complex required for autolysosome formation, Dev. Cell, doi:10.1016/j.devcel.2020.12.010
Michel, Mayer, Poch, Thompson, Characterization of accessory genes in coronavirus genomes, Virol. J, doi:10.1186/s12985-020-01402-1
Moghadamtousi, A review on antibacterial, antiviral, and antifungal activity of curcumin, Biomed. Res. Int, doi:10.1155/2014/186864
Naqvi, Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach, Biochim. Biophys. Acta Mol. Basis Dis, doi:10.1016/j.bbadis.2020.165878
Nieto-Torres, Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome, Virology, doi:10.1016/j.virol.2015.08.010
Nieva, Madan, Carrasco, Viroporins: structure and biological functions, Nat. Rev. Microbiol, doi:10.1038/nrmicro2820
Oso, Adeoye, Olaoye, Pharmacoinformatics and hypothetical studies on allicin, curcumin, and gingerol as potential candidates against COVID-19-associated proteases, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2020.1813630
Padhan, Minakshi, Towheed, Jameel, Severe acute respiratory syndrome coronavirus 3a protein activates the mitochondrial death pathway through p38 MAP kinase activation, J. Gen. Virol, doi:10.1099/vir.0.83665-0
Padhan, Severe acute respiratory syndrome coronavirus ORF3a protein interacts with caveolin, J. Gen. Virol, doi:10.1099/vir.0.82856-0
Panche, Diwan, Chandra, Flavonoids: an overview, J. Nutr. Sci
Pecheur, Curcumin against hepatitis C virus infection: Spicing up antiviral therapies with 'nutraceuticals'?, Gut, doi:10.1136/gutjnl-2013-305646
Qu, ORF3a-mediated incomplete autophagy facilitates severe acute respiratory syndrome coronavirus-2 replication, Front. Cell Dev. Biol, doi:10.3389/fcell.2021.716208
Rattis, Ramos, Celes, Curcumin as a potential treatment for COVID-19, Front. Pharmacol, doi:10.3389/fphar.2021.675287
Redondo, Zaldívar-López, Garrido, Montoya, SARS-CoV-2 accessory proteins in viral pathogenesis: knowns and unknowns, Front. Immunol
Regla-Nava, Severe acute respiratory syndrome coronaviruses with mutations in the E protein are attenuated and promising vaccine candidates, J. Virol, doi:10.1128/JVI.03566-14
Ren, Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study, Chin. Med. J. (Engl.), doi:10.1097/CM9.0000000000000722
Ren, The ORF3a protein of SARS-CoV-2 induces apoptosis in cells, Cell Mol. Immunol, doi:10.1038/s41423-020-0485-9
Ruch, Machamer, The coronavirus E protein: assembly and beyond, Viruses, doi:10.3390/v4030363
Salom, Hill, Lear, Degrado, pH-dependent tetramerization and amantadine binding of the transmembrane helix of M2 from the influenza A virus, Biochemistry, doi:10.1021/bi001799u
Schwarz, Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus, Planta Med, doi:10.1055/s-0033-1360277
Schwarz, Wang, Yu, Sun, Schwarz, Emodin inhibits current through SARS-associated coronavirus 3a protein, Antiviral Res, doi:10.1016/j.antiviral.2011.02.008
Scott, Griffin, Viroporins: structure, function and potential as antiviral targets, J. Gen. Virol, doi:10.1099/vir.0.000201
Singhal, A review of coronavirus disease-2019 (COVID-19), Indian J. Pediatr, doi:10.1007/s12098-020-03263-6
Siu, Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC, FASEB J, doi:10.1096/fj.201802418R
Su, Yu, Zhou, SARS-CoV-2 ORF3a induces incomplete autophagy via the unfolded protein response, Viruses
Tahmasebi, Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients, J. Cell Physiol
Teoh, The SARS coronavirus E protein interacts with PALS1 and alters tight junction formation and epithelial morphogenesis, Mol. Biol. Cell, doi:10.1091/mbc.E10-04-0338
Thimmulappa, Antiviral and immunomodulatory activity of curcumin: a case for prophylactic therapy for COVID-19, Heliyon, doi:10.1016/j.heliyon.2021.e06350
Torres, Conductance and amantadine binding of a pore formed by a lysine-flanked transmembrane domain of SARS coronavirus envelope protein, Protein Sci, doi:10.1110/ps.062730007
Tungmunnithum, Thongboonyou, Pholboon, Yangsabai, Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview, Medicines (Basel)
Vakulenko, Deviatkin, Drexler, Lukashev, Modular evolution of coronavirus genomes, Viruses
Verdia-Baguena, Coronavirus E protein forms ion channels with functionally and structurally-involved membrane lipids, Virology, doi:10.1016/j.virol.2012.07.005
Wang, Grunewald, Perlman, coronaviruses: an updated overview of their replication and pathogenesis, doi:10.1007/978-1-0716-0900-2_1
Wilson, Gage, Ewart, Hexamethylene amiloride blocks E protein ion channels and inhibits coronavirus replication, Virology, doi:10.1016/j.virol.2006.05.028
Xu, SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway, Virology, doi:10.1016/j.virol.2022.01.003
Yue, SARS-coronavirus open reading frame-3a drives multimodal necrotic cell death, Cell Death Dis, doi:10.1038/s41419-018-0917-y
Zhang, The SARS-CoV-2 protein ORF3a inhibits fusion of autophagosomes with lysosomes, Cell Discov, doi:10.1038/s41421-021-00268-z
Zhang, Understanding the role of SARS-CoV-2 ORF3a in viral pathogenesis and COVID-19, Front Microbiol, doi:10.3389/fmicb.2022.854567
Zhou, Efficacy of ion-channel inhibitors amantadine, memantine and rimantadine for the treatment of SARS-CoV-2 in vitro, Viruses, doi:10.3390/v13102082
DOI record:
{
"DOI": "10.1038/s41598-023-31764-9",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-023-31764-9",
"abstract": "<jats:title>Abstract</jats:title><jats:p>SARS-CoV-2 has been responsible for the major worldwide pandemic of COVID-19. Despite the enormous success of vaccination campaigns, virus infections are still prevalent and effective antiviral therapies are urgently needed. Viroporins are essential for virus replication and release, and are thus promising therapeutic targets. Here, we studied the expression and function of recombinant ORF3a viroporin of SARS-CoV-2 using a combination of cell viability assays and patch-clamp electrophysiology. ORF3a was expressed in HEK293 cells and transport to the plasma membrane verified by a dot blot assay. Incorporation of a membrane-directing signal peptide increased plasma membrane expression. Cell viability tests were carried out to measure cell damage associated with ORF3a activity, and voltage-clamp recordings verified its channel activity. The classical viroporin inhibitors amantadine and rimantadine inhibited ORF3a channels. A series of ten flavonoids and polyphenolics were studied. Kaempferol, quercetin, epigallocatechin gallate, nobiletin, resveratrol and curcumin were ORF3a inhibitors, with IC<jats:sub>50</jats:sub> values ranging between 1 and 6 µM, while 6-gingerol, apigenin, naringenin and genistein were inactive. For flavonoids, inhibitory activity could be related to the pattern of OH groups on the chromone ring system. Thus, the ORF3a viroporin of SARS-CoV-2 may indeed be a promising target for antiviral drugs.</jats:p>",
"alternative-id": [
"31764"
],
"article-number": "5328",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "28 September 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "16 March 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "1 April 2023"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Fam",
"given": "Marina Sherif",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sedky",
"given": "Christine Adel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Turky",
"given": "Nancy Osama",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Breitinger",
"given": "Hans-Georg",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Breitinger",
"given": "Ulrike",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
4,
3
]
],
"date-time": "2023-04-03T05:50:55Z",
"timestamp": 1680501055000
},
"deposited": {
"date-parts": [
[
2023,
4,
3
]
],
"date-time": "2023-04-03T05:58:06Z",
"timestamp": 1680501486000
},
"funder": [
{
"DOI": "10.13039/501100003009",
"award": [
"45420"
],
"doi-asserted-by": "publisher",
"name": "Science and Technology Development Fund"
},
{
"DOI": "10.13039/501100007637",
"doi-asserted-by": "crossref",
"name": "German University in Cairo"
}
],
"indexed": {
"date-parts": [
[
2023,
4,
4
]
],
"date-time": "2023-04-04T05:31:46Z",
"timestamp": 1680586306786
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
4,
1
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
4,
1
]
],
"date-time": "2023-04-01T00:00:00Z",
"timestamp": 1680307200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
4,
1
]
],
"date-time": "2023-04-01T00:00:00Z",
"timestamp": 1680307200000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-023-31764-9.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-023-31764-9",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-023-31764-9.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2023,
4,
1
]
]
},
"published-online": {
"date-parts": [
[
2023,
4,
1
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1038/s41564-020-0695-z",
"author": "AE Gorbalenya",
"doi-asserted-by": "publisher",
"first-page": "536",
"issue": "4",
"journal-title": "Nat. Microbiol.",
"key": "31764_CR1",
"unstructured": "Gorbalenya, A. E. et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 5(4), 536–544. https://doi.org/10.1038/s41564-020-0695-z (2020).",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1007/978-1-0716-0900-2_1",
"author": "Y Wang",
"doi-asserted-by": "publisher",
"first-page": "1",
"key": "31764_CR2",
"unstructured": "Wang, Y., Grunewald, M. & Perlman, S. coronaviruses: an updated overview of their replication and pathogenesis. In Coronaviruses: Methods and Protocols (eds Maier, H. J. & Bickerton, E.) 1–29 (Springer US, 2020). https://doi.org/10.1007/978-1-0716-0900-2_1.",
"volume-title": "Coronaviruses: Methods and Protocols",
"year": "2020"
},
{
"DOI": "10.1038/nrmicro.2016.81",
"author": "E de Wit",
"doi-asserted-by": "publisher",
"first-page": "523",
"journal-title": "Nat. Rev. Microbiol.",
"key": "31764_CR3",
"unstructured": "de Wit, E., van Doremalen, N., Falzarano, D. & Munster, V. J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 14, 523–534. https://doi.org/10.1038/nrmicro.2016.81 (2016).",
"volume": "14",
"year": "2016"
},
{
"DOI": "10.3390/diseases4030026",
"author": "YX Lim",
"doi-asserted-by": "publisher",
"first-page": "26",
"journal-title": "Diseases",
"key": "31764_CR4",
"unstructured": "Lim, Y. X., Ng, Y. L., Tam, J. P. & Liu, D. X. Human coronaviruses: a review of virus-host interactions. Diseases 4, 26 (2016).",
"volume": "4",
"year": "2016"
},
{
"DOI": "10.1007/s12098-020-03263-6",
"author": "T Singhal",
"doi-asserted-by": "publisher",
"first-page": "281",
"journal-title": "Indian J. Pediatr.",
"key": "31764_CR5",
"unstructured": "Singhal, T. A review of coronavirus disease-2019 (COVID-19). Indian J. Pediatr. 87, 281–286. https://doi.org/10.1007/s12098-020-03263-6 (2020).",
"volume": "87",
"year": "2020"
},
{
"DOI": "10.1097/CM9.0000000000000722",
"author": "LL Ren",
"doi-asserted-by": "publisher",
"first-page": "1015",
"journal-title": "Chin. Med. J. (Engl.)",
"key": "31764_CR6",
"unstructured": "Ren, L. L. et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin. Med. J. (Engl.) 133, 1015–1024. https://doi.org/10.1097/CM9.0000000000000722 (2020).",
"volume": "133",
"year": "2020"
},
{
"DOI": "10.1016/j.ijsu.2020.07.032",
"author": "J Liu",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Int. J. Surg.",
"key": "31764_CR7",
"unstructured": "Liu, J. et al. A comparative overview of COVID-19, MERS and SARS: review article. Int. J. Surg. 81, 1–8. https://doi.org/10.1016/j.ijsu.2020.07.032 (2020).",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1016/j.bbadis.2020.165878",
"author": "AAT Naqvi",
"doi-asserted-by": "publisher",
"first-page": "165878",
"journal-title": "Biochim. Biophys. Acta Mol. Basis Dis.",
"key": "31764_CR8",
"unstructured": "Naqvi, A. A. T. et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165878. https://doi.org/10.1016/j.bbadis.2020.165878 (2020).",
"volume": "1866",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2021.708264",
"author": "N Redondo",
"doi-asserted-by": "publisher",
"first-page": "708264",
"journal-title": "Front. Immunol.",
"key": "31764_CR9",
"unstructured": "Redondo, N., Zaldívar-López, S., Garrido, J. J. & Montoya, M. SARS-CoV-2 accessory proteins in viral pathogenesis: knowns and unknowns. Front. Immunol. 12, 708264 (2021).",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1126/science.1085953",
"author": "MA Marra",
"doi-asserted-by": "publisher",
"first-page": "1399",
"journal-title": "Science",
"key": "31764_CR10",
"unstructured": "Marra, M. A. et al. The Genome sequence of the SARS-associated coronavirus. Science 300, 1399–1404. https://doi.org/10.1126/science.1085953 (2003).",
"volume": "300",
"year": "2003"
},
{
"DOI": "10.3390/v13071270",
"author": "Y Vakulenko",
"doi-asserted-by": "publisher",
"first-page": "1270",
"journal-title": "Viruses",
"key": "31764_CR11",
"unstructured": "Vakulenko, Y., Deviatkin, A., Drexler, J. F. & Lukashev, A. Modular evolution of coronavirus genomes. Viruses 13, 1270 (2021).",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.biocel.2022.106185",
"author": "U Breitinger",
"doi-asserted-by": "publisher",
"first-page": "106185",
"journal-title": "Int. J. Biochem. Cell Biol.",
"key": "31764_CR12",
"unstructured": "Breitinger, U., Farag, N. S., Sticht, H. & Breitinger, H. G. Viroporins: Structure, function, and their role in the life cycle of SARS-CoV-2. Int. J. Biochem. Cell Biol. 145, 106185. https://doi.org/10.1016/j.biocel.2022.106185 (2022).",
"volume": "145",
"year": "2022"
},
{
"DOI": "10.1016/S0014-5793(03)00780-4",
"author": "ME Gonzalez",
"doi-asserted-by": "publisher",
"first-page": "28",
"journal-title": "FEBS Lett.",
"key": "31764_CR13",
"unstructured": "Gonzalez, M. E. & Carrasco, L. Viroporins. FEBS Lett. 552, 28–34 (2003).",
"volume": "552",
"year": "2003"
},
{
"DOI": "10.1038/nrmicro2820",
"author": "JL Nieva",
"doi-asserted-by": "publisher",
"first-page": "563",
"journal-title": "Nat. Rev. Microbiol.",
"key": "31764_CR14",
"unstructured": "Nieva, J. L., Madan, V. & Carrasco, L. Viroporins: structure and biological functions. Nat. Rev. Microbiol. 10, 563–574. https://doi.org/10.1038/nrmicro2820 (2012).",
"volume": "10",
"year": "2012"
},
{
"DOI": "10.1099/vir.0.000201",
"author": "C Scott",
"doi-asserted-by": "publisher",
"first-page": "2000",
"journal-title": "J. Gen. Virol.",
"key": "31764_CR15",
"unstructured": "Scott, C. & Griffin, S. Viroporins: structure, function and potential as antiviral targets. J. Gen. Virol. 96, 2000–2027. https://doi.org/10.1099/vir.0.000201 (2015).",
"volume": "96",
"year": "2015"
},
{
"DOI": "10.1007/s11262-009-0341-6",
"author": "SC Chen",
"doi-asserted-by": "publisher",
"first-page": "365",
"journal-title": "Virus Genes",
"key": "31764_CR16",
"unstructured": "Chen, S. C., Lo, S. Y., Ma, H. C. & Li, H. C. Expression and membrane integration of SARS-CoV E protein and its interaction with M protein. Virus Genes 38, 365–371. https://doi.org/10.1007/s11262-009-0341-6 (2009).",
"volume": "38",
"year": "2009"
},
{
"DOI": "10.1371/journal.ppat.1004320",
"author": "JM Jimenez-Guardeno",
"doi-asserted-by": "publisher",
"first-page": "e1004320",
"journal-title": "PLoS Pathog.",
"key": "31764_CR17",
"unstructured": "Jimenez-Guardeno, J. M. et al. The PDZ-binding motif of severe acute respiratory syndrome coronavirus envelope protein is a determinant of viral pathogenesis. PLoS Pathog. 10, e1004320. https://doi.org/10.1371/journal.ppat.1004320 (2014).",
"volume": "10",
"year": "2014"
},
{
"DOI": "10.1007/978-0-387-33012-9_34",
"author": "Y Liao",
"doi-asserted-by": "publisher",
"first-page": "199",
"journal-title": "Adv. Exp. Med. Biol.",
"key": "31764_CR18",
"unstructured": "Liao, Y., Tam, J. P. & Liu, D. X. Viroporin activity of SARS-CoV E protein. Adv. Exp. Med. Biol. 581, 199–202. https://doi.org/10.1007/978-0-387-33012-9_34 (2006).",
"volume": "581",
"year": "2006"
},
{
"DOI": "10.1128/JVI.03566-14",
"author": "JA Regla-Nava",
"doi-asserted-by": "publisher",
"first-page": "3870",
"journal-title": "J. Virol.",
"key": "31764_CR19",
"unstructured": "Regla-Nava, J. A. et al. Severe acute respiratory syndrome coronaviruses with mutations in the E protein are attenuated and promising vaccine candidates. J. Virol. 89, 3870–3887. https://doi.org/10.1128/JVI.03566-14 (2015).",
"volume": "89",
"year": "2015"
},
{
"DOI": "10.3390/v4030363",
"author": "TR Ruch",
"doi-asserted-by": "publisher",
"first-page": "363",
"journal-title": "Viruses",
"key": "31764_CR20",
"unstructured": "Ruch, T. R. & Machamer, C. E. The coronavirus E protein: assembly and beyond. Viruses 4, 363–382. https://doi.org/10.3390/v4030363 (2012).",
"volume": "4",
"year": "2012"
},
{
"DOI": "10.1091/mbc.E10-04-0338",
"author": "KT Teoh",
"doi-asserted-by": "publisher",
"first-page": "3838",
"journal-title": "Mol. Biol. Cell",
"key": "31764_CR21",
"unstructured": "Teoh, K. T. et al. The SARS coronavirus E protein interacts with PALS1 and alters tight junction formation and epithelial morphogenesis. Mol. Biol. Cell 21, 3838–3852. https://doi.org/10.1091/mbc.E10-04-0338 (2010).",
"volume": "21",
"year": "2010"
},
{
"DOI": "10.1016/j.virol.2012.07.005",
"author": "C Verdia-Baguena",
"doi-asserted-by": "publisher",
"first-page": "485",
"journal-title": "Virology",
"key": "31764_CR22",
"unstructured": "Verdia-Baguena, C. et al. Coronavirus E protein forms ion channels with functionally and structurally-involved membrane lipids. Virology 432, 485–494. https://doi.org/10.1016/j.virol.2012.07.005 (2012).",
"volume": "432",
"year": "2012"
},
{
"DOI": "10.1016/j.virol.2006.05.028",
"author": "L Wilson",
"doi-asserted-by": "publisher",
"first-page": "294",
"journal-title": "Virology",
"key": "31764_CR23",
"unstructured": "Wilson, L., Gage, P. & Ewart, G. Hexamethylene amiloride blocks E protein ion channels and inhibits coronavirus replication. Virology 353, 294–306. https://doi.org/10.1016/j.virol.2006.05.028 (2006).",
"volume": "353",
"year": "2006"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"author": "DE Gordon",
"doi-asserted-by": "publisher",
"first-page": "459",
"journal-title": "Nature",
"key": "31764_CR24",
"unstructured": "Gordon, D. E. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583, 459–468. https://doi.org/10.1038/s41586-020-2286-9 (2020).",
"volume": "583",
"year": "2020"
},
{
"DOI": "10.1186/s12985-020-01402-1",
"author": "CJ Michel",
"doi-asserted-by": "publisher",
"first-page": "131",
"journal-title": "Virol. J.",
"key": "31764_CR25",
"unstructured": "Michel, C. J., Mayer, C., Poch, O. & Thompson, J. D. Characterization of accessory genes in coronavirus genomes. Virol. J. 17, 131. https://doi.org/10.1186/s12985-020-01402-1 (2020).",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.3389/fmicb.2022.854567",
"author": "J Zhang",
"doi-asserted-by": "publisher",
"first-page": "854567",
"journal-title": "Front Microbiol",
"key": "31764_CR26",
"unstructured": "Zhang, J. et al. Understanding the role of SARS-CoV-2 ORF3a in viral pathogenesis and COVID-19. Front Microbiol 13, 854567. https://doi.org/10.3389/fmicb.2022.854567 (2022).",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1128/mBio.02325-17",
"author": "C Castano-Rodriguez",
"doi-asserted-by": "publisher",
"journal-title": "mBio",
"key": "31764_CR27",
"unstructured": "Castano-Rodriguez, C. et al. Role of severe acute respiratory syndrome coronavirus viroporins E, 3a, and 8a in replication and pathogenesis. mBio https://doi.org/10.1128/mBio.02325-17 (2018).",
"year": "2018"
},
{
"author": "DM Kern",
"first-page": "439",
"journal-title": "bioRxiv",
"key": "31764_CR28",
"unstructured": "Kern, D. M. et al. Cryo-EM structure of the SARS-CoV-2 3a ion channel in lipid nanodiscs. bioRxiv 9, 439 (2021).",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1016/j.biocel.2009.04.019",
"author": "CM Chan",
"doi-asserted-by": "publisher",
"first-page": "2232",
"journal-title": "Int. J. Biochem. Cell Biol.",
"key": "31764_CR29",
"unstructured": "Chan, C. M. et al. The ion channel activity of the SARS-coronavirus 3a protein is linked to its pro-apoptotic function. Int. J. Biochem. Cell Biol. 41, 2232–2239. https://doi.org/10.1016/j.biocel.2009.04.019 (2009).",
"volume": "41",
"year": "2009"
},
{
"DOI": "10.3389/fmicb.2019.00050",
"author": "IY Chen",
"doi-asserted-by": "publisher",
"first-page": "50",
"journal-title": "Front. Microbiol.",
"key": "31764_CR30",
"unstructured": "Chen, I. Y., Moriyama, M., Chang, M. F. & Ichinohe, T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front. Microbiol. 10, 50. https://doi.org/10.3389/fmicb.2019.00050 (2019).",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1099/vir.0.80813-0",
"author": "PTW Law",
"doi-asserted-by": "publisher",
"first-page": "1921",
"journal-title": "J. Gen. Virol.",
"key": "31764_CR31",
"unstructured": "Law, P. T. W. et al. The 3a protein of severe acute respiratory syndrome-associated coronavirus induces apoptosis in Vero E6 cells. J. Gen. Virol. 86, 1921–1930. https://doi.org/10.1099/vir.0.80813-0 (2005).",
"volume": "86",
"year": "2005"
},
{
"DOI": "10.1016/j.febslet.2006.11.046",
"author": "N Kanzawa",
"doi-asserted-by": "publisher",
"first-page": "6807",
"journal-title": "FEBS Lett.",
"key": "31764_CR32",
"unstructured": "Kanzawa, N. et al. Augmentation of chemokine production by severe acute respiratory syndrome coronavirus 3a/X1 and 7a/X4 proteins through NF-kappaB activation. FEBS Lett. 580, 6807–6812. https://doi.org/10.1016/j.febslet.2006.11.046 (2006).",
"volume": "580",
"year": "2006"
},
{
"DOI": "10.1099/vir.0.83665-0",
"author": "K Padhan",
"doi-asserted-by": "publisher",
"first-page": "1960",
"journal-title": "J. Gen. Virol.",
"key": "31764_CR33",
"unstructured": "Padhan, K., Minakshi, R., Towheed, M. A. B. & Jameel, S. Severe acute respiratory syndrome coronavirus 3a protein activates the mitochondrial death pathway through p38 MAP kinase activation. J. Gen. Virol. 89, 1960–1969. https://doi.org/10.1099/vir.0.83665-0 (2008).",
"volume": "89",
"year": "2008"
},
{
"DOI": "10.1128/JVI.01662-09",
"author": "EC Freundt",
"doi-asserted-by": "publisher",
"first-page": "1097",
"journal-title": "J. Virol.",
"key": "31764_CR34",
"unstructured": "Freundt, E. C. et al. The open reading frame 3a protein of severe acute respiratory syndrome-associated coronavirus promotes membrane rearrangement and cell death. J. Virol. 84, 1097–1109. https://doi.org/10.1128/JVI.01662-09 (2010).",
"volume": "84",
"year": "2010"
},
{
"key": "31764_CR35",
"unstructured": "Kien, F., Ma, H., Gaisenband, S. D. & Nal, B. in Microbial Pathogenesis: Infection and Immunity (ed Uday Kishore and Annapurna Nayak) Ch. 3, 38–62 (Landes Bioscience and Springer Science+Business Media, 2013)."
},
{
"DOI": "10.1055/s-0033-1360277",
"author": "S Schwarz",
"doi-asserted-by": "publisher",
"first-page": "177",
"journal-title": "Planta Med.",
"key": "31764_CR36",
"unstructured": "Schwarz, S. et al. Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 80, 177–182. https://doi.org/10.1055/s-0033-1360277 (2014).",
"volume": "80",
"year": "2014"
},
{
"DOI": "10.1016/j.antiviral.2011.02.008",
"author": "S Schwarz",
"doi-asserted-by": "publisher",
"first-page": "64",
"journal-title": "Antiviral Res.",
"key": "31764_CR37",
"unstructured": "Schwarz, S., Wang, K., Yu, W., Sun, B. & Schwarz, W. Emodin inhibits current through SARS-associated coronavirus 3a protein. Antiviral Res. 90, 64–69. https://doi.org/10.1016/j.antiviral.2011.02.008 (2011).",
"volume": "90",
"year": "2011"
},
{
"DOI": "10.1016/j.devcel.2020.12.010",
"author": "G Miao",
"doi-asserted-by": "publisher",
"first-page": "427",
"journal-title": "Dev. Cell",
"key": "31764_CR38",
"unstructured": "Miao, G. et al. ORF3a of the COVID-19 virus SARS-CoV-2 blocks HOPS complex-mediated assembly of the SNARE complex required for autolysosome formation. Dev. Cell 56, 427–442. https://doi.org/10.1016/j.devcel.2020.12.010 (2021).",
"volume": "56",
"year": "2021"
},
{
"DOI": "10.3389/fcell.2021.716208",
"author": "Y Qu",
"doi-asserted-by": "publisher",
"first-page": "716208",
"journal-title": "Front. Cell Dev. Biol.",
"key": "31764_CR39",
"unstructured": "Qu, Y. et al. ORF3a-mediated incomplete autophagy facilitates severe acute respiratory syndrome coronavirus-2 replication. Front. Cell Dev. Biol. 9, 716208. https://doi.org/10.3389/fcell.2021.716208 (2021).",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1038/s41421-021-00268-z",
"author": "Y Zhang",
"doi-asserted-by": "publisher",
"first-page": "31",
"journal-title": "Cell Discov.",
"key": "31764_CR40",
"unstructured": "Zhang, Y. et al. The SARS-CoV-2 protein ORF3a inhibits fusion of autophagosomes with lysosomes. Cell Discov. 7, 31. https://doi.org/10.1038/s41421-021-00268-z (2021).",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.3390/v13122467",
"author": "WQ Su",
"doi-asserted-by": "publisher",
"first-page": "2467",
"journal-title": "Viruses",
"key": "31764_CR41",
"unstructured": "Su, W. Q., Yu, X. J. & Zhou, C. M. SARS-CoV-2 ORF3a induces incomplete autophagy via the unfolded protein response. Viruses 13, 2467 (2021).",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1016/j.virol.2022.01.003",
"author": "H Xu",
"doi-asserted-by": "publisher",
"first-page": "13",
"journal-title": "Virology",
"key": "31764_CR42",
"unstructured": "Xu, H. et al. SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway. Virology 568, 13–22. https://doi.org/10.1016/j.virol.2022.01.003 (2022).",
"volume": "568",
"year": "2022"
},
{
"DOI": "10.1038/s41423-020-0485-9",
"author": "Y Ren",
"doi-asserted-by": "publisher",
"first-page": "881",
"journal-title": "Cell Mol. Immunol.",
"key": "31764_CR43",
"unstructured": "Ren, Y. et al. The ORF3a protein of SARS-CoV-2 induces apoptosis in cells. Cell Mol. Immunol. 17, 881–883. https://doi.org/10.1038/s41423-020-0485-9 (2020).",
"volume": "17",
"year": "2020"
},
{
"author": "N Arshad",
"first-page": "8",
"journal-title": "bioRxiv",
"key": "31764_CR44",
"unstructured": "Arshad, N. et al. SARS-CoV-2 accessory proteins ORF7a and ORF3a use distinct mechanisms to downregulate MHC-I surface expression. bioRxiv 204, 8 (2022).",
"volume": "204",
"year": "2022"
},
{
"DOI": "10.1016/j.csbj.2022.01.017",
"author": "S Gupta",
"doi-asserted-by": "publisher",
"first-page": "766",
"journal-title": "Comput. Struct. Biotechnol. J.",
"key": "31764_CR45",
"unstructured": "Gupta, S. et al. D155Y substitution of SARS-CoV-2 ORF3a weakens binding with Caveolin-1. Comput. Struct. Biotechnol. J. 20, 766–778. https://doi.org/10.1016/j.csbj.2022.01.017 (2022).",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1099/vir.0.82856-0",
"author": "K Padhan",
"doi-asserted-by": "publisher",
"first-page": "3067",
"journal-title": "J. Gen. Virol.",
"key": "31764_CR46",
"unstructured": "Padhan, K. et al. Severe acute respiratory syndrome coronavirus ORF3a protein interacts with caveolin. J. Gen. Virol. 88, 3067–3077. https://doi.org/10.1099/vir.0.82856-0 (2007).",
"volume": "88",
"year": "2007"
},
{
"DOI": "10.1038/s41419-018-0917-y",
"author": "Y Yue",
"doi-asserted-by": "publisher",
"first-page": "904",
"journal-title": "Cell Death Dis.",
"key": "31764_CR47",
"unstructured": "Yue, Y. et al. SARS-coronavirus open reading frame-3a drives multimodal necrotic cell death. Cell Death Dis. 9, 904. https://doi.org/10.1038/s41419-018-0917-y (2018).",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1016/j.bbrep.2021.100933",
"author": "GK Azad",
"doi-asserted-by": "publisher",
"first-page": "100933",
"journal-title": "Biochem. Biophys. Rep.",
"key": "31764_CR48",
"unstructured": "Azad, G. K. & Khan, P. K. Variations in ORF3a protein of SARS-CoV-2 alter its structure and function. Biochem. Biophys. Rep. 26, 100933. https://doi.org/10.1016/j.bbrep.2021.100933 (2021).",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1016/j.ijbiomac.2020.12.142",
"author": "M Bianchi",
"doi-asserted-by": "publisher",
"first-page": "820",
"journal-title": "Int. J. Biol. Macromol.",
"key": "31764_CR49",
"unstructured": "Bianchi, M., Borsetti, A., Ciccozzi, M. & Pascarella, S. SARS-CoV-2 ORF3a: mutability and function. Int. J. Biol. Macromol. 170, 820–826. https://doi.org/10.1016/j.ijbiomac.2020.12.142 (2021).",
"volume": "170",
"year": "2021"
},
{
"DOI": "10.1016/j.virusres.2021.198441",
"author": "SS Hassan",
"doi-asserted-by": "publisher",
"first-page": "198441",
"journal-title": "Virus Res.",
"key": "31764_CR50",
"unstructured": "Hassan, S. S., Attrish, D., Ghosh, S., Choudhury, P. P. & Roy, B. Pathogenic perspective of missense mutations of ORF3a protein of SARS-CoV-2. Virus Res. 300, 198441. https://doi.org/10.1016/j.virusres.2021.198441 (2021).",
"volume": "300",
"year": "2021"
},
{
"DOI": "10.1128/mSystems.00266-20",
"author": "E Issa",
"doi-asserted-by": "publisher",
"journal-title": "Msystems",
"key": "31764_CR51",
"unstructured": "Issa, E., Merhi, G., Panossian, B., Salloum, T. & Tokajian, S. SARS-CoV-2 and ORF3a: nonsynonymous mutations, functional domains, and viral pathogenesis. Msystems https://doi.org/10.1128/mSystems.00266-20 (2020).",
"year": "2020"
},
{
"DOI": "10.1017/S0950268820002599",
"author": "P Majumdar",
"doi-asserted-by": "publisher",
"first-page": "e262",
"journal-title": "Epidemiol. Infect.",
"key": "31764_CR52",
"unstructured": "Majumdar, P. & Niyogi, S. ORF3a mutation associated with higher mortality rate in SARS-CoV-2 infection. Epidemiol. Infect. 148, e262. https://doi.org/10.1017/S0950268820002599 (2020).",
"volume": "148",
"year": "2020"
},
{
"DOI": "10.3390/v13102082",
"author": "Y Zhou",
"doi-asserted-by": "publisher",
"first-page": "2082",
"journal-title": "Viruses",
"key": "31764_CR53",
"unstructured": "Zhou, Y. et al. Efficacy of ion-channel inhibitors amantadine, memantine and rimantadine for the treatment of SARS-CoV-2 in vitro. Viruses 13, 2082. https://doi.org/10.3390/v13102082 (2021).",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-99072-8",
"author": "NS Lebedeva",
"doi-asserted-by": "publisher",
"first-page": "19481",
"journal-title": "Sci. Rep.",
"key": "31764_CR54",
"unstructured": "Lebedeva, N. S. et al. Theoretical and experimental study of interaction of macroheterocyclic compounds with ORF3a of SARS-CoV-2. Sci. Rep. 11, 19481. https://doi.org/10.1038/s41598-021-99072-8 (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1371/journal.pntd.0007548",
"author": "D Dey",
"doi-asserted-by": "publisher",
"first-page": "e0007548",
"journal-title": "PLoS Negl. Trop. Dis.",
"key": "31764_CR55",
"unstructured": "Dey, D. et al. The effect of amantadine on an ion channel protein from Chikungunya virus. PLoS Negl. Trop. Dis. 13, e0007548. https://doi.org/10.1371/journal.pntd.0007548 (2019).",
"volume": "13",
"year": "2019"
},
{
"DOI": "10.1016/0042-6822(92)91239-q",
"author": "KC Duff",
"doi-asserted-by": "publisher",
"first-page": "485",
"journal-title": "Virology",
"key": "31764_CR56",
"unstructured": "Duff, K. C. & Ashley, R. H. The transmembrane domain of influenza A M2 protein forms amantadine-sensitive proton channels in planar lipid bilayers. Virology 190, 485–489. https://doi.org/10.1016/0042-6822(92)91239-q (1992).",
"volume": "190",
"year": "1992"
},
{
"author": "DM Fleming",
"first-page": "189",
"journal-title": "Int. J. Clin. Pract.",
"key": "31764_CR57",
"unstructured": "Fleming, D. M. Managing influenza: amantadine, rimantadine and beyond. Int. J. Clin. Pract. 55, 189–195 (2001).",
"volume": "55",
"year": "2001"
},
{
"DOI": "10.1016/S0014-5793(02)03851-6",
"author": "SD Griffin",
"doi-asserted-by": "publisher",
"first-page": "34",
"journal-title": "FEBS Lett.",
"key": "31764_CR58",
"unstructured": "Griffin, S. D. et al. The p7 protein of hepatitis C virus forms an ion channel that is blocked by the antiviral drug Amantadine. FEBS Lett. 535, 34–38 (2003).",
"volume": "535",
"year": "2003"
},
{
"DOI": "10.1016/j.jmgm.2008.06.002",
"author": "P Intharathep",
"doi-asserted-by": "publisher",
"first-page": "342",
"journal-title": "J. Mol. Graph Model.",
"key": "31764_CR59",
"unstructured": "Intharathep, P. et al. How amantadine and rimantadine inhibit proton transport in the M2 protein channel. J. Mol. Graph Model. 27, 342–348. https://doi.org/10.1016/j.jmgm.2008.06.002 (2008).",
"volume": "27",
"year": "2008"
},
{
"DOI": "10.1073/pnas.0804958105",
"author": "X Jing",
"doi-asserted-by": "publisher",
"first-page": "10967",
"journal-title": "Proc. Natl. Acad. Sci. U. S. A.",
"key": "31764_CR60",
"unstructured": "Jing, X. et al. Functional studies indicate amantadine binds to the pore of the influenza A virus M2 proton-selective ion channel. Proc. Natl. Acad. Sci. U. S. A. 105, 10967–10972. https://doi.org/10.1073/pnas.0804958105 (2008).",
"volume": "105",
"year": "2008"
},
{
"DOI": "10.1110/ps.062730007",
"author": "J Torres",
"doi-asserted-by": "publisher",
"first-page": "2065",
"journal-title": "Protein Sci.",
"key": "31764_CR61",
"unstructured": "Torres, J. et al. Conductance and amantadine binding of a pore formed by a lysine-flanked transmembrane domain of SARS coronavirus envelope protein. Protein Sci. 16, 2065–2071. https://doi.org/10.1110/ps.062730007 (2007).",
"volume": "16",
"year": "2007"
},
{
"DOI": "10.3390/molecules25010063",
"author": "E Cione",
"doi-asserted-by": "publisher",
"first-page": "63",
"journal-title": "Molecules",
"key": "31764_CR62",
"unstructured": "Cione, E. et al. Quercetin, epigallocatechin gallate, curcumin, and resveratrol: from dietary sources to human MicroRNA modulation. Molecules 25, 63. https://doi.org/10.3390/molecules25010063 (2019).",
"volume": "25",
"year": "2019"
},
{
"DOI": "10.1017/jns.2016.41",
"author": "AN Panche",
"doi-asserted-by": "publisher",
"first-page": "e47",
"journal-title": "J. Nutr. Sci.",
"key": "31764_CR63",
"unstructured": "Panche, A. N., Diwan, A. D. & Chandra, S. R. Flavonoids: an overview. J. Nutr. Sci. 5, e47 (2016).",
"volume": "5",
"year": "2016"
},
{
"DOI": "10.3390/ijms222212385",
"author": "N Gligorijevic",
"doi-asserted-by": "publisher",
"first-page": "12385",
"journal-title": "Int. J. Mol. Sci.",
"key": "31764_CR64",
"unstructured": "Gligorijevic, N. et al. Molecular mechanisms of possible action of phenolic compounds in COVID-19 protection and prevention. Int. J. Mol. Sci. 22, 12385 (2021).",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.3390/medicines5030093",
"author": "D Tungmunnithum",
"doi-asserted-by": "publisher",
"first-page": "93",
"journal-title": "Medicines (Basel)",
"key": "31764_CR65",
"unstructured": "Tungmunnithum, D., Thongboonyou, A., Pholboon, A. & Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines (Basel) 5, 93 (2018).",
"volume": "5",
"year": "2018"
},
{
"DOI": "10.1155/2015/184241",
"author": "Y Abba",
"doi-asserted-by": "publisher",
"first-page": "184241",
"journal-title": "Adv. Virol.",
"key": "31764_CR66",
"unstructured": "Abba, Y., Hassim, H., Hamzah, H. & Noordin, M. M. Antiviral activity of resveratrol against human and animal viruses. Adv. Virol. 2015, 184241 (2015).",
"volume": "2015",
"year": "2015"
},
{
"DOI": "10.1042/BST0380050",
"author": "M Campagna",
"doi-asserted-by": "publisher",
"first-page": "50",
"journal-title": "Biochem. Soc. Trans.",
"key": "31764_CR67",
"unstructured": "Campagna, M. & Rivas, C. Antiviral activity of resveratrol. Biochem. Soc. Trans. 38, 50–53. https://doi.org/10.1042/BST0380050 (2010).",
"volume": "38",
"year": "2010"
},
{
"DOI": "10.1155/2022/7138756",
"author": "X Chen",
"doi-asserted-by": "publisher",
"first-page": "7138756",
"journal-title": "Mediat. Inflamm.",
"key": "31764_CR68",
"unstructured": "Chen, X. et al. Insights into the anti-inflammatory and antiviral mechanisms of resveratrol. Mediat. Inflamm. 2022, 7138756. https://doi.org/10.1155/2022/7138756 (2022).",
"volume": "2022",
"year": "2022"
},
{
"DOI": "10.1179/joc.2008.20.3.393",
"author": "L Drago",
"doi-asserted-by": "publisher",
"first-page": "393",
"journal-title": "J. Chemother.",
"key": "31764_CR69",
"unstructured": "Drago, L., Nicola, L., Ossola, F. & De Vecchi, E. In vitro antiviral activity of resveratrol against respiratory viruses. J. Chemother. 20, 393–394. https://doi.org/10.1179/joc.2008.20.3.393 (2008).",
"volume": "20",
"year": "2008"
},
{
"DOI": "10.3390/v12111242",
"author": "MR Jennings",
"doi-asserted-by": "publisher",
"first-page": "1242",
"journal-title": "Viruses",
"key": "31764_CR70",
"unstructured": "Jennings, M. R. & Parks, R. J. Curcumin as an antiviral agent. Viruses 12, 1242 (2020).",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1155/2014/186864",
"author": "SZ Moghadamtousi",
"doi-asserted-by": "publisher",
"first-page": "186864",
"journal-title": "Biomed. Res. Int.",
"key": "31764_CR71",
"unstructured": "Moghadamtousi, S. Z. et al. A review on antibacterial, antiviral, and antifungal activity of curcumin. Biomed. Res. Int. 2014, 186864. https://doi.org/10.1155/2014/186864 (2014).",
"volume": "2014",
"year": "2014"
},
{
"DOI": "10.1136/gutjnl-2013-305646",
"author": "EI Pecheur",
"doi-asserted-by": "publisher",
"first-page": "1035",
"journal-title": "Gut",
"key": "31764_CR72",
"unstructured": "Pecheur, E. I. Curcumin against hepatitis C virus infection: Spicing up antiviral therapies with ‘nutraceuticals’?. Gut 63, 1035–1037. https://doi.org/10.1136/gutjnl-2013-305646 (2014).",
"volume": "63",
"year": "2014"
},
{
"DOI": "10.1016/j.heliyon.2021.e06350",
"author": "RK Thimmulappa",
"doi-asserted-by": "publisher",
"first-page": "e06350",
"journal-title": "Heliyon",
"key": "31764_CR73",
"unstructured": "Thimmulappa, R. K. et al. Antiviral and immunomodulatory activity of curcumin: a case for prophylactic therapy for COVID-19. Heliyon 7, e06350. https://doi.org/10.1016/j.heliyon.2021.e06350 (2021).",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1155/2021/6623400",
"author": "RF Hayati",
"doi-asserted-by": "publisher",
"first-page": "6623400",
"journal-title": "Biomed. Res. Int.",
"key": "31764_CR74",
"unstructured": "Hayati, R. F. et al. [6]-Gingerol inhibits chikungunya virus infection by suppressing viral replication. Biomed. Res. Int. 2021, 6623400. https://doi.org/10.1155/2021/6623400 (2021).",
"volume": "2021",
"year": "2021"
},
{
"DOI": "10.1080/07391102.2020.1813630",
"author": "BJ Oso",
"doi-asserted-by": "publisher",
"first-page": "389",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "31764_CR75",
"unstructured": "Oso, B. J., Adeoye, A. O. & Olaoye, I. F. Pharmacoinformatics and hypothetical studies on allicin, curcumin, and gingerol as potential candidates against COVID-19-associated proteases. J. Biomol. Struct. Dyn. 40, 389–400. https://doi.org/10.1080/07391102.2020.1813630 (2022).",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.4315/0362-028X.JFP-15-593",
"author": "HA Aboubakr",
"doi-asserted-by": "publisher",
"first-page": "1001",
"journal-title": "J. Food Prot.",
"key": "31764_CR76",
"unstructured": "Aboubakr, H. A. et al. In vitro antiviral activity of clove and ginger aqueous extracts against feline calicivirus, a surrogate for human norovirus. J. Food Prot. 79, 1001–1012. https://doi.org/10.4315/0362-028X.JFP-15-593 (2016).",
"volume": "79",
"year": "2016"
},
{
"DOI": "10.1016/j.jep.2012.10.043",
"author": "JS Chang",
"doi-asserted-by": "publisher",
"first-page": "146",
"journal-title": "J. Ethnopharmacol.",
"key": "31764_CR77",
"unstructured": "Chang, J. S., Wang, K. C., Yeh, C. F., Shieh, D. E. & Chiang, L. C. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 145, 146–151. https://doi.org/10.1016/j.jep.2012.10.043 (2013).",
"volume": "145",
"year": "2013"
},
{
"DOI": "10.1007/s13337-020-00584-0",
"author": "S Kaushik",
"doi-asserted-by": "publisher",
"first-page": "270",
"journal-title": "Virusdisease",
"key": "31764_CR78",
"unstructured": "Kaushik, S., Jangra, G., Kundu, V., Yadav, J. P. & Kaushik, S. Anti-viral activity of Zingiber officinale (Ginger) ingredients against the Chikungunya virus. Virusdisease 31, 270–276. https://doi.org/10.1007/s13337-020-00584-0 (2020).",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1099/jgv.0.001571",
"author": "U Breitinger",
"doi-asserted-by": "publisher",
"journal-title": "J. Gen. Virol.",
"key": "31764_CR79",
"unstructured": "Breitinger, U. et al. Cell viability assay as a tool to study activity and inhibition of hepatitis C p7 channels. J. Gen. Virol. https://doi.org/10.1099/jgv.0.001571 (2021).",
"year": "2021"
},
{
"DOI": "10.3389/fmicb.2021.692423",
"author": "U Breitinger",
"doi-asserted-by": "publisher",
"first-page": "692423",
"journal-title": "Front. Microbiol.",
"key": "31764_CR80",
"unstructured": "Breitinger, U., Ali, N. K. M., Sticht, H. & Breitinger, H. G. Inhibition of SARS CoV envelope protein by flavonoids and classical viroporin inhibitors. Front. Microbiol. 12, 692423. https://doi.org/10.3389/fmicb.2021.692423 (2021).",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1021/bi001799u",
"author": "D Salom",
"doi-asserted-by": "publisher",
"first-page": "14160",
"journal-title": "Biochemistry",
"key": "31764_CR81",
"unstructured": "Salom, D., Hill, B. R., Lear, J. D. & DeGrado, W. F. pH-dependent tetramerization and amantadine binding of the transmembrane helix of M2 from the influenza A virus. Biochemistry 39, 14160–14170. https://doi.org/10.1021/bi001799u (2000).",
"volume": "39",
"year": "2000"
},
{
"DOI": "10.1016/j.bpj.2016.04.018",
"author": "U Breitinger",
"doi-asserted-by": "publisher",
"first-page": "2419",
"journal-title": "Biophys. J.",
"key": "31764_CR82",
"unstructured": "Breitinger, U., Farag, N. S., Ali, N. K. & Breitinger, H. G. Patch-clamp study of hepatitis C p7 channels reveals genotype-specific sensitivity to inhibitors. Biophys. J. 110, 2419–2429 (2016).",
"volume": "110",
"year": "2016"
},
{
"DOI": "10.1016/j.virol.2015.08.010",
"author": "JL Nieto-Torres",
"doi-asserted-by": "publisher",
"first-page": "330",
"journal-title": "Virology",
"key": "31764_CR83",
"unstructured": "Nieto-Torres, J. L. et al. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology 485, 330–339. https://doi.org/10.1016/j.virol.2015.08.010 (2015).",
"volume": "485",
"year": "2015"
},
{
"DOI": "10.1096/fj.201802418R",
"author": "KL Siu",
"doi-asserted-by": "publisher",
"first-page": "8865",
"journal-title": "FASEB J.",
"key": "31764_CR84",
"unstructured": "Siu, K. L. et al. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 33, 8865–8877. https://doi.org/10.1096/fj.201802418R (2019).",
"volume": "33",
"year": "2019"
},
{
"DOI": "10.1002/hep.22555",
"author": "SD Griffin",
"doi-asserted-by": "publisher",
"first-page": "1779",
"journal-title": "Hepatology",
"key": "31764_CR85",
"unstructured": "Griffin, S. D. et al. Genotype-dependent sensitivity of hepatitis C virus to inhibitors of the p7 ion channel. Hepatology 48, 1779–1790 (2008).",
"volume": "48",
"year": "2008"
},
{
"DOI": "10.1021/acs.jafc.5b00312",
"author": "T Kongpichitchoke",
"doi-asserted-by": "publisher",
"first-page": "4580",
"issue": "18",
"journal-title": "J. Agric. Food Chem.",
"key": "31764_CR86",
"unstructured": "Kongpichitchoke, T., Hsu, J.-L. & Huang, T.-C. Number of hydroxyl groups on the B-ring of flavonoids affects their antioxidant activity and interaction with phorbol ester binding site of PKCδ C1B domain: in vitro and in silico studies. J. Agric. Food Chem. 63(18), 4580–4586. https://doi.org/10.1021/acs.jafc.5b00312 (2015).",
"volume": "63",
"year": "2015"
},
{
"DOI": "10.1155/2013/162750",
"author": "S Kumar",
"doi-asserted-by": "publisher",
"first-page": "162750",
"journal-title": "Sci.WorldJ.",
"key": "31764_CR87",
"unstructured": "Kumar, S. & Pandey, A. K. Chemistry and biological activities of flavonoids: an overview. Sci.WorldJ. 2013, 162750. https://doi.org/10.1155/2013/162750 (2013).",
"volume": "2013",
"year": "2013"
},
{
"DOI": "10.1016/j.bbadis.2016.12.006",
"author": "NS Farag",
"doi-asserted-by": "publisher",
"first-page": "712",
"journal-title": "Biochim. Biophys. Acta Mol. Basis Dis.",
"key": "31764_CR88",
"unstructured": "Farag, N. S., Breitinger, U., El-Azizi, M. & Breitinger, H. G. The p7 viroporin of the hepatitis C virus contributes to liver inflammation by stimulating production of Interleukin-1β. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 712–720 (2017).",
"volume": "1863",
"year": "2017"
},
{
"DOI": "10.3389/fphar.2021.675287",
"author": "BAC Rattis",
"doi-asserted-by": "publisher",
"first-page": "675287",
"journal-title": "Front. Pharmacol.",
"key": "31764_CR89",
"unstructured": "Rattis, B. A. C., Ramos, S. G. & Celes, M. R. N. Curcumin as a potential treatment for COVID-19. Front. Pharmacol. 12, 675287. https://doi.org/10.3389/fphar.2021.675287 (2021).",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41598-020-77003-3",
"author": "P Kanjanasirirat",
"doi-asserted-by": "publisher",
"first-page": "19963",
"journal-title": "Sci. Rep.",
"key": "31764_CR90",
"unstructured": "Kanjanasirirat, P. et al. High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents. Sci. Rep. 10, 19963 (2020).",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1177/1934578X20976293",
"author": "PK Agrawal",
"doi-asserted-by": "publisher",
"journal-title": "Nat. Prod. Comm.",
"key": "31764_CR91",
"unstructured": "Agrawal, P. K., Agrawal, C. & Blunden, G. Quercetin: antiviral significance and possible COVID-19 integrative considerations. Nat. Prod. Comm. https://doi.org/10.1177/1934578X20976293 (2020).",
"year": "2020"
},
{
"DOI": "10.1002/jcp.30233",
"author": "S Tahmasebi",
"doi-asserted-by": "publisher",
"first-page": "5325",
"journal-title": "J. Cell Physiol.",
"key": "31764_CR92",
"unstructured": "Tahmasebi, S. et al. Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients. J. Cell Physiol. 236, 5325–5338 (2020).",
"volume": "236",
"year": "2020"
},
{
"DOI": "10.1016/j.meegid.2020.104451",
"author": "D Bhowmik",
"doi-asserted-by": "publisher",
"first-page": "104451",
"journal-title": "Infect. Genet. Evol.",
"key": "31764_CR93",
"unstructured": "Bhowmik, D. et al. Identification of potential inhibitors against SARS-CoV-2 by targeting proteins responsible for envelope formation and virion assembly using docking based virtual screening, and pharmacokinetics approaches. Infect. Genet. Evol. 84, 104451. https://doi.org/10.1016/j.meegid.2020.104451 (2020).",
"volume": "84",
"year": "2020"
},
{
"DOI": "10.1007/s10668-021-01373-5",
"author": "HS Mahrosh",
"doi-asserted-by": "publisher",
"first-page": "16674",
"journal-title": "Environ. Dev. Sustain.",
"key": "31764_CR94",
"unstructured": "Mahrosh, H. S. & Mustafa, G. An in silico approach to target RNA-dependent RNA polymerase of COVID-19 with naturally occurring phytochemicals. Environ. Dev. Sustain. 23, 16674–16687. https://doi.org/10.1007/s10668-021-01373-5 (2021).",
"volume": "23",
"year": "2021"
}
],
"reference-count": 94,
"references-count": 94,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-023-31764-9"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "13"
}
