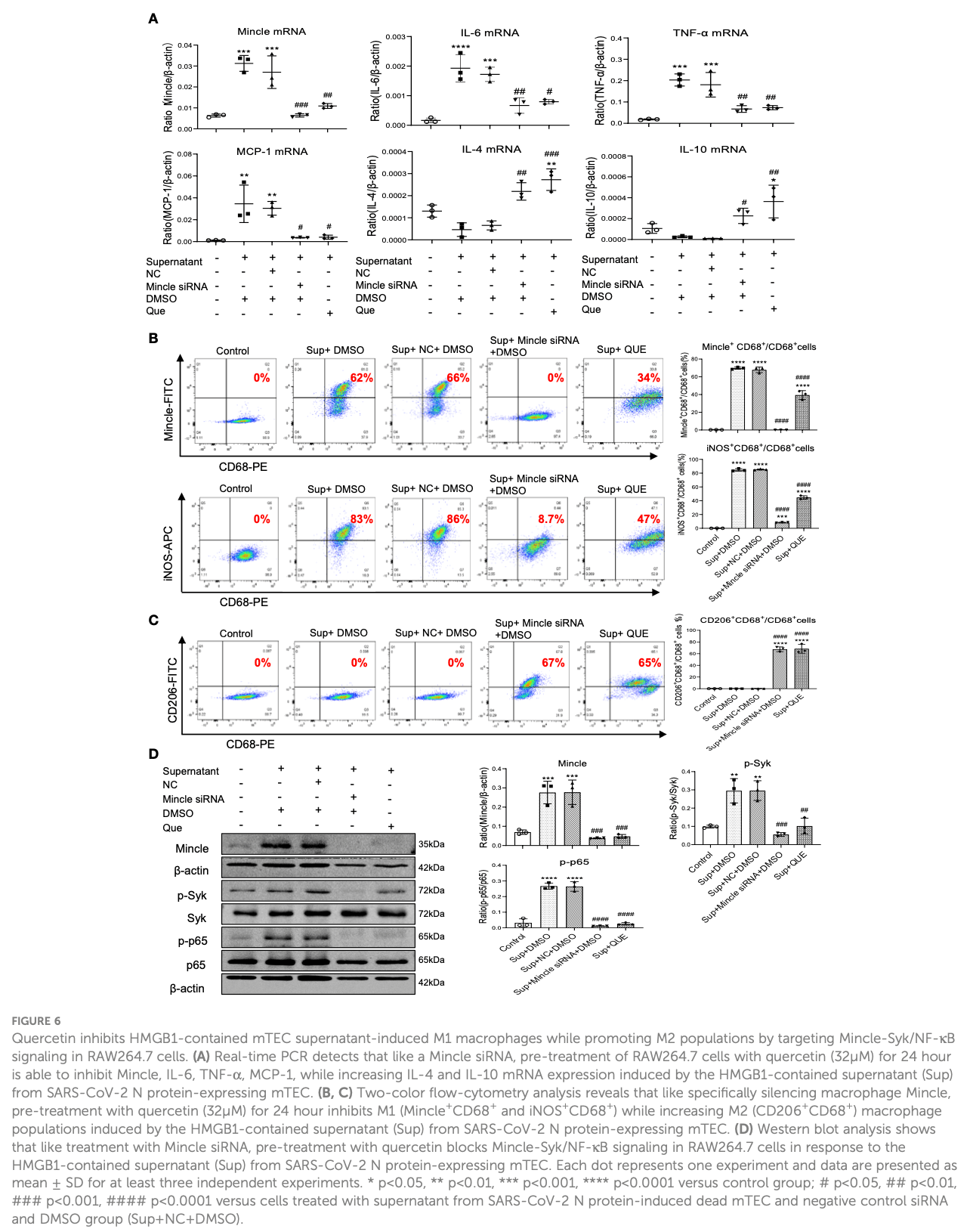
SARS-CoV-2 N protein induced acute kidney injury in diabetic db/db mice is associated with a Mincle-dependent M1 macrophage activation
et al., Frontiers in Immunology, doi:10.3389/fimmu.2023.1264447, Nov 2023
Quercetin for COVID-19
27th treatment shown to reduce risk in
July 2021, now with p = 0.002 from 12 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro and mouse study showing that quercetin may ameliorate COVID-19 associated acute kidney injury through modulation of macrophage polarization by blocking the Mincle/Syk/NF-kB pathway. Authors suggest that the SARS-CoV-2 N protein can exacerbate kidney injury in diabetic mice by promoting M1 proinflammatory macrophage activation via the Mincle-Syk/NF-kB pathway. Treatment with the flavonoid quercetin was found to inhibit N protein-induced acute kidney injury by suppressing Mincle signaling and switching macrophages from the M1 to M2 anti-inflammatory phenotype.
Bioavailability. Quercetin has low bioavailability and studies typically use advanced formulations to improve bioavailability which may be required to reach therapeutic concentrations.
91 preclinical studies support the efficacy of quercetin for COVID-19:
In silico studies predict inhibition of SARS-CoV-2, or minimization of side effects, with quercetin or metabolites via binding to the spikeA,11,12,18,19,32,34,35,37,40,48,49,51,52,75 (and specifically the receptor binding domainB,8), MproC,7,8,11,12,16,18,20,22,24,26,28,30,33,34,37,40,44,46-48,52-55,72 , RNA-dependent RNA polymeraseD,8,10-12,18,42 , PLproE,12,47,55 , ACE2F,27,32,33,37,38,47,51 , TMPRSS2G,32, nucleocapsidH,12, helicaseI,12,39,44 , endoribonucleaseJ,49, NSP16/10K,15, cathepsin LL,36, Wnt-3M,32, FZDN,32, LRP6O,32, ezrinP,50, ADRPQ,48, NRP1R,51, EP300S,25, PTGS2T,33, HSP90AA1U,25,33 , matrix metalloproteinase 9V,41, IL-6W,31,45 , IL-10X,31, VEGFAY,45, and RELAZ,45 proteins, and inhibition of spike-ACE2 interactionAA,9.
In vitro studies demonstrate inhibition of the MproC,24,58,63,71 protein, and inhibition of spike-ACE2 interactionAA,59.
In vitro studies demonstrate efficacy in Calu-3AB,62, A549AC,31, HEK293-ACE2+AD,70, Huh-7AE,35, Caco-2AF,61, Vero E6AG,29,52,61 , mTECAH,64, RAW264.7AI,64, and HLMECAJ,9 cells.
Animal studies demonstrate efficacy in K18-hACE2 miceAK,67, db/db miceAL,64,74 , BALB/c miceAM,73, and rats29.
Quercetin reduced proinflammatory cytokines and protected lung and kidney tissue against LPS-induced damage in mice73, inhibits LPS-induced cytokine storm by modulating key inflammatory and antioxidant pathways in macrophages14, may block ACE2-spike interaction and NLRP3 inflammasome, limiting viral entry and inflammation5, upregulates the SIRT1/AMPK axis to inhibit oxidative injury and accelerate viral clearance76, inhibits SARS-CoV-2 ORF3a ion channel activity, which contributes to viral pathogenicity and cytotoxicity66, may alleviate COVID-19 ARDS via inhibition of EGFR and JAK2 inflammatory targets1, and may destabilize the Spike protein, IL-6R, and integrins via conserved residues, blocking viral entry, hyperinflammation, and platelet aggregation77.
1.
Gupta et al., Harnessing phytoconstituents to treat COVID-19 triggered acute respiratory distress syndrome: Insights from network pharmacology, and molecular modeling, Phytochemistry Letters, doi:10.1016/j.phytol.2025.104105.
2.
Sun et al., Feasibility of the inhibitor development for SARS-CoV-2: a systematic approach for drug design, Journal of Molecular Modeling, doi:10.1007/s00894-025-06541-2.
3.
Torabfam et al., Improving quercetin solubility via structural modification enhances dual-target coronavirus entry: an integrated in-vitro and in-silico study, Scientific Reports, doi:10.1038/s41598-025-27374-2.
4.
Abdelhameed et al., Phytochemical and antiviral investigation of Cynanchum acutum L. extract and derived semi-synthetic analogs targeting SARS-CoV-2 main protease, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-025-00907-2.
5.
Manikyam et al., INP-Guided Network Pharmacology Discloses Multi-Target Therapeutic Strategy Against Cytokine and IgE Storms in the SARS-CoV-2 NB.1.8.1 Variant, Research Square, doi:10.21203/rs.3.rs-6819274/v1.
6.
Makoana et al., Integration of metabolomics and chemometrics with in-silico and in-vitro approaches to unravel SARS-Cov-2 inhibitors from South African plants, PLOS ONE, doi:10.1371/journal.pone.0320415.
7.
Bano et al., Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability, Viruses, doi:10.3390/v17030402.
8.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
9.
Moharram et al., Secondary metabolites of Alternaria alternate appraisal of their SARS-CoV-2 inhibitory and anti-inflammatory potentials, PLOS ONE, doi:10.1371/journal.pone.0313616.
10.
Metwaly et al., Integrated study of Quercetin as a potent SARS-CoV-2 RdRp inhibitor: Binding interactions, MD simulations, and In vitro assays, PLOS ONE, doi:10.1371/journal.pone.0312866.
11.
Al balawi et al., Assessing multi-target antiviral and antioxidant activities of natural compounds against SARS-CoV-2: an integrated in vitro and in silico study, Bioresources and Bioprocessing, doi:10.1186/s40643-024-00822-z.
12.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
13.
Pan et al., Decoding the mechanism of Qingjie formula in the prevention of COVID-19 based on network pharmacology and molecular docking, Heliyon, doi:10.1016/j.heliyon.2024.e39167.
14.
Xu et al., Quercetin inhibited LPS-induced cytokine storm by interacting with the AKT1-FoxO1 and Keap1-Nrf2 signaling pathway in macrophages, Scientific Reports, doi:10.1038/s41598-024-71569-y.
15.
Tamil Selvan et al., Computational Investigations to Identify Potent Natural Flavonoid Inhibitors of the Nonstructural Protein (NSP) 16/10 Complex Against Coronavirus, Cureus, doi:10.7759/cureus.68098.
16.
Sunita et al., Characterization of Phytochemical Inhibitors of the COVID-19 Primary Protease Using Molecular Modelling Approach, Asian Journal of Microbiology and Biotechnology, doi:10.56557/ajmab/2024/v9i28800.
17.
Wu et al., Biomarkers Prediction and Immune Landscape in Covid-19 and “Brain Fog”, Elsevier BV, doi:10.2139/ssrn.4897774.
18.
Raman et al., Phytoconstituents of Citrus limon (Lemon) as Potential Inhibitors Against Multi Targets of SARS‐CoV‐2 by Use of Molecular Modelling and In Vitro Determination Approaches, ChemistryOpen, doi:10.1002/open.202300198.
19.
Asad et al., Exploring the antiviral activity of Adhatoda beddomei bioactive compounds in interaction with coronavirus spike protein, Archives of Medical Reports, 1:1, archmedrep.com/index.php/amr/article/view/3.
20.
Irfan et al., Phytoconstituents of Artemisia Annua as potential inhibitors of SARS CoV2 main protease: an in silico study, BMC Infectious Diseases, doi:10.1186/s12879-024-09387-w.
21.
Yuan et al., Network pharmacology and molecular docking reveal the mechanisms of action of Panax notoginseng against post-COVID-19 thromboembolism, Review of Clinical Pharmacology and Pharmacokinetics - International Edition, doi:10.61873/DTFA3974.
22.
Nalban et al., Targeting COVID-19 (SARS-CoV-2) main protease through phytochemicals of Albizia lebbeck: molecular docking, molecular dynamics simulation, MM–PBSA free energy calculations, and DFT analysis, Journal of Proteins and Proteomics, doi:10.1007/s42485-024-00136-w.
23.
Zhou et al., Bioinformatics and system biology approaches to determine the connection of SARS-CoV-2 infection and intrahepatic cholangiocarcinoma, PLOS ONE, doi:10.1371/journal.pone.0300441.
24.
Waqas et al., Discovery of Novel Natural Inhibitors Against SARS-CoV-2 Main Protease: A Rational Approach to Antiviral Therapeutics, Current Medicinal Chemistry, doi:10.2174/0109298673292839240329081008.
25.
Hasanah et al., Decoding the therapeutic potential of empon-empon: a bioinformatics expedition unraveling mechanisms against COVID-19 and atherosclerosis, International Journal of Applied Pharmaceutics, doi:10.22159/ijap.2024v16i2.50128.
26.
Shaik et al., Computational identification of selected bioactive compounds from Cedrus deodara as inhibitors against SARS-CoV-2 main protease: a pharmacoinformatics study, Indian Drugs, doi:10.53879/id.61.02.13859.
27.
Wang et al., Investigating the Mechanism of Qu Du Qiang Fei 1 Hao Fang Formula against Coronavirus Disease 2019 Based on Network Pharmacology Method, World Journal of Traditional Chinese Medicine, doi:10.4103/2311-8571.395061.
28.
Singh et al., Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 M Pro protein - An in-silico and cell-based approach, Research Square, doi:10.21203/rs.3.rs-3888947/v1.
29.
El-Megharbel et al., Chemical and spectroscopic characterization of (Artemisinin/Quercetin/ Zinc) novel mixed ligand complex with assessment of its potent high antiviral activity against SARS-CoV-2 and antioxidant capacity against toxicity induced by acrylamide in male rats, PeerJ, doi:10.7717/peerj.15638.
30.
Akinwumi et al., Evaluation of therapeutic potentials of some bioactive compounds in selected African plants targeting main protease (Mpro) in SARS-CoV-2: a molecular docking study, Egyptian Journal of Medical Human Genetics, doi:10.1186/s43042-023-00456-4.
31.
Yang et al., Active ingredient and mechanistic analysis of traditional Chinese medicine formulas for the prevention and treatment of COVID-19: Insights from bioinformatics and in vitro experiments, Medicine, doi:10.1097/MD.0000000000036238.
32.
Chandran et al., Molecular docking analysis of quercetin with known CoVid-19 targets, Bioinformation, doi:10.6026/973206300191081.
33.
Qin et al., Exploring the bioactive compounds of Feiduqing formula for the prevention and management of COVID-19 through network pharmacology and molecular docking, Medical Data Mining, doi:10.53388/MDM202407003.
34.
Moschovou et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, International Journal of Molecular Sciences, doi:10.3390/ijms242115894.
35.
Pan (B) et al., Quercetin: A promising drug candidate against the potential SARS-CoV-2-Spike mutants with high viral infectivity, Computational and Structural Biotechnology Journal, doi:10.1016/j.csbj.2023.10.029.
36.
Ahmed et al., Evaluation of the Effect of Zinc, Quercetin, Bromelain and Vitamin C on COVID-19 Patients, International Journal of Diabetes Management, doi:10.61797/ijdm.v2i2.259.
37.
Thapa et al., In-silico Approach for Predicting the Inhibitory Effect of Home Remedies on Severe Acute Respiratory Syndrome Coronavirus-2, Makara Journal of Science, doi:10.7454/mss.v27i3.1609.
38.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
39.
Singh (B) et al., Flavonoids as Potent Inhibitor of SARS-CoV-2 Nsp13 Helicase: Grid Based Docking Approach, Middle East Research Journal of Pharmaceutical Sciences, doi:10.36348/merjps.2023.v03i04.001.
40.
Mandal et al., In silico anti-viral assessment of phytoconstituents in a traditional (Siddha Medicine) polyherbal formulation – Targeting Mpro and pan-coronavirus post-fusion Spike protein, Journal of Traditional and Complementary Medicine, doi:10.1016/j.jtcme.2023.07.004.
41.
Sai Ramesh et al., Computational analysis of the phytocompounds of Mimusops elengi against spike protein of SARS CoV2 – An Insilico model, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2023.125553.
42.
Corbo et al., Inhibitory potential of phytochemicals on five SARS-CoV-2 proteins: in silico evaluation of endemic plants of Bosnia and Herzegovina, Biotechnology & Biotechnological Equipment, doi:10.1080/13102818.2023.2222196.
43.
Azmi et al., Utilization of quercetin flavonoid compounds in onion (Allium cepa L.) as an inhibitor of SARS-CoV-2 spike protein against ACE2 receptors, 11th International Seminar on New Paradigm and Innovation on Natural Sciences and its Application, doi:10.1063/5.0140285.
44.
Alanzi et al., Structure-based virtual identification of natural inhibitors of SARS-CoV-2 and its Delta and Omicron variant proteins, Future Virology, doi:10.2217/fvl-2022-0184.
45.
Yang (B) et al., In silico evidence implicating novel mechanisms of Prunella vulgaris L. as a potential botanical drug against COVID-19-associated acute kidney injury, Frontiers in Pharmacology, doi:10.3389/fphar.2023.1188086.
46.
Wang (B) et al., Computational Analysis of Lianhua Qingwen as an Adjuvant Treatment in Patients with COVID-19, Society of Toxicology Conference, 2023, www.researchgate.net/publication/370491709_Y_Wang_A_E_Tan_O_Chew_A_Hsueh_and_D_E_Johnson_2023_Computational_Analysis_of_Lianhua_Qingwen_as_an_Adjuvant_Treatment_in_Patients_with_COVID-19_Toxicologist_1921_507.
47.
Ibeh et al., Computational studies of potential antiviral compounds from some selected Nigerian medicinal plants against SARS-CoV-2 proteins, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2023.101230.
48.
Nguyen et al., The Potential of Ameliorating COVID-19 and Sequelae From Andrographis paniculata via Bioinformatics, Bioinformatics and Biology Insights, doi:10.1177/11779322221149622.
49.
Alavi et al., Interaction of Epigallocatechin Gallate and Quercetin with Spike Glycoprotein (S-Glycoprotein) of SARS-CoV-2: In Silico Study, Biomedicines, doi:10.3390/biomedicines10123074.
50.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
51.
Şimşek et al., In silico identification of SARS-CoV-2 cell entry inhibitors from selected natural antivirals, Journal of Molecular Graphics and Modelling, doi:10.1016/j.jmgm.2021.108038.
52.
Kandeil et al., Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2, Pathogens, doi:10.3390/pathogens10060758.
53.
Rehman et al., Natural Compounds as Inhibitors of SARS-CoV-2 Main Protease (3CLpro): A Molecular Docking and Simulation Approach to Combat COVID-19, Current Pharmaceutical Design, doi:10.2174/1381612826999201116195851.
54.
Sekiou et al., In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2, ChemRxiv, doi:10.26434/chemrxiv.12181404.v1.
55.
Zhang et al., In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus, Journal of Integrative Medicine, doi:10.1016/j.joim.2020.02.005.
56.
Sisti et al., Evaluation of respiratory virus transmissibility and resilience from fomites: the case of 11 SARS-CoV-2 clinical isolates, Applied and Environmental Microbiology, doi:10.1128/aem.00774-25.
57.
Spinelli et al., Amphibian‐Derived Peptides as Natural Inhibitors of SARS‐CoV‐2 Main Protease (Mpro): A Combined In Vitro and In Silico Approach, Chemistry & Biodiversity, doi:10.1002/cbdv.202403202.
58.
Aguilera-Rodriguez et al., Inhibition of SARS-CoV-2 3CLpro by chemically modified tyrosinase from Agaricus bisporus, RSC Medicinal Chemistry, doi:10.1039/D4MD00289J.
59.
Emam et al., Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors, AMB Express, doi:10.1186/s13568-024-01739-8.
60.
Fang et al., Development of nanoparticles incorporated with quercetin and ACE2-membrane as a novel therapy for COVID-19, Journal of Nanobiotechnology, doi:10.1186/s12951-024-02435-2.
61.
Roy et al., Quercetin inhibits SARS-CoV-2 infection and prevents syncytium formation by cells co-expressing the viral spike protein and human ACE2, Virology Journal, doi:10.1186/s12985-024-02299-w.
62.
DiGuilio et al., Quercetin improves and protects Calu-3 airway epithelial barrier function, Frontiers in Cell and Developmental Biology, doi:10.3389/fcell.2023.1271201.
63.
Zhang (B) et al., Discovery of the covalent SARS‐CoV‐2 Mpro inhibitors from antiviral herbs via integrating target‐based high‐throughput screening and chemoproteomic approaches, Journal of Medical Virology, doi:10.1002/jmv.29208.
64.
Wu (B) et al., SARS-CoV-2 N protein induced acute kidney injury in diabetic db/db mice is associated with a Mincle-dependent M1 macrophage activation, Frontiers in Immunology, doi:10.3389/fimmu.2023.1264447.
65.
Xu (B) et al., Bioactive compounds from Huashi Baidu decoction possess both antiviral and anti-inflammatory effects against COVID-19, Proceedings of the National Academy of Sciences, doi:10.1073/pnas.2301775120.
66.
Fam et al., Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites, Scientific Reports, doi:10.1038/s41598-023-31764-9.
67.
Aguado et al., Senolytic therapy alleviates physiological human brain aging and COVID-19 neuropathology, bioRxiv, doi:10.1101/2023.01.17.524329.
68.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
69.
Munafò et al., Quercetin and Luteolin Are Single-digit Micromolar Inhibitors of the SARS-CoV-2 RNA-dependent RNA Polymerase, Research Square, doi:10.21203/rs.3.rs-1149846/v1.
70.
Singh (C) et al., The spike protein of SARS-CoV-2 virus induces heme oxygenase-1: Pathophysiologic implications, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, doi:10.1016/j.bbadis.2021.166322.
71.
Bahun et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594.
72.
Abian et al., Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.07.235.
73.
Shaker et al., Anti-cytokine Storm Activity of Fraxin, Quercetin, and their Combination on Lipopolysaccharide-Induced Cytokine Storm in Mice: Implications in COVID-19, Iranian Journal of Medical Sciences, doi:10.30476/ijms.2023.98947.3102.
74.
Wu (C) et al., Treatment with Quercetin inhibits SARS-CoV-2 N protein-induced acute kidney injury by blocking Smad3-dependent G1 cell cycle arrest, Molecular Therapy, doi:10.1016/j.ymthe.2022.12.002.
75.
Azmi (B) et al., The role of vitamin D receptor and IL‐6 in COVID‐19, Molecular Genetics & Genomic Medicine, doi:10.1002/mgg3.2172.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The receptor binding domain is a specific region of the spike protein that binds ACE2 and is a major target of neutralizing antibodies. Focusing on the precise binding site allows highly specific disruption of viral attachment with reduced potential for off-target effects.
c.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
d.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
e.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
f.
The angiotensin converting enzyme 2 (ACE2) protein is a host cell transmembrane protein that serves as the cellular receptor for the SARS-CoV-2 spike protein. ACE2 is expressed on many cell types, including epithelial cells in the lungs, and allows the virus to enter and infect host cells. Inhibition may affect ACE2's physiological function in blood pressure control.
g.
Transmembrane protease serine 2 (TMPRSS2) is a host cell protease that primes the spike protein, facilitating cellular entry. TMPRSS2 activity helps enable cleavage of the spike protein required for membrane fusion and virus entry. Inhibition may especially protect respiratory epithelial cells, buy may have physiological effects.
h.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
i.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
j.
The endoribonuclease, also known as NendoU or nsp15, cleaves specific sequences in viral RNA which may help the virus evade detection by the host immune system. Inhibition may hinder the virus's ability to mask itself from the immune system, facilitating a stronger immune response.
k.
The NSP16/10 complex consists of non-structural proteins 16 and 10, forming a 2'-O-methyltransferase that modifies the viral RNA cap structure. This modification helps the virus evade host immune detection by mimicking host mRNA, making NSP16/10 a promising antiviral target.
l.
Cathepsin L is a host lysosomal cysteine protease that can prime the spike protein through an alternative pathway when TMPRSS2 is unavailable. Dual targeting of cathepsin L and TMPRSS2 may maximize disruption of alternative pathways for virus entry.
m.
Wingless-related integration site (Wnt) ligand 3 is a host signaling molecule that activates the Wnt signaling pathway, which is important in development, cell growth, and tissue repair. Some studies suggest that SARS-CoV-2 infection may interfere with the Wnt signaling pathway, and that Wnt3a is involved in SARS-CoV-2 entry.
n.
The frizzled (FZD) receptor is a host transmembrane receptor that binds Wnt ligands, initiating the Wnt signaling cascade. FZD serves as a co-receptor, along with ACE2, in some proposed mechanisms of SARS-CoV-2 infection. The virus may take advantage of this pathway as an alternative entry route.
o.
Low-density lipoprotein receptor-related protein 6 is a cell surface co-receptor essential for Wnt signaling. LRP6 acts in tandem with FZD for signal transduction and has been discussed as a potential co-receptor for SARS-CoV-2 entry.
p.
The ezrin protein links the cell membrane to the cytoskeleton (the cell's internal support structure) and plays a role in cell shape, movement, adhesion, and signaling. Drugs that occupy the same spot on ezrin where the viral spike protein would bind may hindering viral attachment, and drug binding could further stabilize ezrin, strengthening its potential natural capacity to impede viral fusion and entry.
q.
The Adipocyte Differentiation-Related Protein (ADRP, also known as Perilipin 2 or PLIN2) is a lipid droplet protein regulating the storage and breakdown of fats in cells. SARS-CoV-2 may hijack the lipid handling machinery of host cells and ADRP may play a role in this process. Disrupting ADRP's interaction with the virus may hinder the virus's ability to use lipids for replication and assembly.
r.
Neuropilin-1 (NRP1) is a cell surface receptor with roles in blood vessel development, nerve cell guidance, and immune responses. NRP1 may function as a co-receptor for SARS-CoV-2, facilitating viral entry into cells. Blocking NRP1 may disrupt an alternative route of viral entry.
s.
EP300 (E1A Binding Protein P300) is a transcriptional coactivator involved in several cellular processes, including growth, differentiation, and apoptosis, through its acetyltransferase activity that modifies histones and non-histone proteins. EP300 facilitates viral entry into cells and upregulates inflammatory cytokine production.
t.
Prostaglandin G/H synthase 2 (PTGS2, also known as COX-2) is an enzyme crucial for the production of inflammatory molecules called prostaglandins. PTGS2 plays a role in the inflammatory response that can become severe in COVID-19 and inhibitors (like some NSAIDs) may have benefits in dampening harmful inflammation, but note that prostaglandins have diverse physiological functions.
u.
Heat Shock Protein 90 Alpha Family Class A Member 1 (HSP90AA1) is a chaperone protein that helps other proteins fold correctly and maintains their stability. HSP90AA1 plays roles in cell signaling, survival, and immune responses. HSP90AA1 may interact with numerous viral proteins, but note that it has diverse physiological functions.
v.
Matrix metalloproteinase 9 (MMP9), also called gelatinase B, is a zinc-dependent enzyme that breaks down collagen and other components of the extracellular matrix. MMP9 levels increase in severe COVID-19. Overactive MMP9 can damage lung tissue and worsen inflammation. Inhibition of MMP9 may prevent excessive tissue damage and help regulate the inflammatory response.
w.
The interleukin-6 (IL-6) pro-inflammatory cytokine (signaling molecule) has a complex role in the immune response and may trigger and perpetuate inflammation. Elevated IL-6 levels are associated with severe COVID-19 cases and cytokine storm. Anti-IL-6 therapies may be beneficial in reducing excessive inflammation in severe COVID-19 cases.
x.
The interleukin-10 (IL-10) anti-inflammatory cytokine helps regulate and dampen immune responses, preventing excessive inflammation. IL-10 levels can also be elevated in severe COVID-19. IL-10 could either help control harmful inflammation or potentially contribute to immune suppression.
y.
Vascular Endothelial Growth Factor A (VEGFA) promotes the growth of new blood vessels (angiogenesis) and has roles in inflammation and immune responses. VEGFA may contribute to blood vessel leakiness and excessive inflammation associated with severe COVID-19.
z.
RELA is a transcription factor subunit of NF-kB and is a key regulator of inflammation, driving pro-inflammatory gene expression. SARS-CoV-2 may hijack and modulate NF-kB pathways.
aa.
The interaction between the SARS-CoV-2 spike protein and the human ACE2 receptor is a primary method of viral entry, inhibiting this interaction can prevent the virus from attaching to and entering host cells, halting infection at an early stage.
ab.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
ac.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
ad.
HEK293-ACE2+ is a human embryonic kidney cell line engineered for high ACE2 expression and SARS-CoV-2 susceptibility.
ae.
Huh-7 cells were derived from a liver tumor (hepatoma).
af.
Caco-2 cells come from a colorectal adenocarcinoma (cancer). They are valued for their ability to form a polarized cell layer with properties similar to the intestinal lining.
ag.
Vero E6 is an African green monkey kidney cell line with low/no ACE2 expression and high SARS-CoV-2 susceptibility. The cell line is easy to maintain and supports robust viral replication, however the monkey origin may not accurately represent human responses.
ah.
mTEC is a mouse tubular epithelial cell line.
ai.
RAW264.7 is a mouse macrophage cell line.
aj.
HLMEC (Human Lung Microvascular Endothelial Cells) are primary endothelial cells derived from the lung microvasculature. They are used to study endothelial function, inflammation, and viral interactions, particularly in the context of lung infections such as SARS-CoV-2. HLMEC express ACE2 and are susceptible to SARS-CoV-2 infection, making them a relevant model for studying viral entry and endothelial responses in the lung.
ak.
A mouse model expressing the human ACE2 receptor under the control of the K18 promoter.
al.
A mouse model of obesity and severe insulin resistance leading to type 2 diabetes due to a mutation in the leptin receptor gene that impairs satiety signaling.
am.
A mouse model commonly used in infectious disease and cancer research due to higher immune response and susceptibility to infection.
Wu et al., 3 Nov 2023, peer-reviewed, 12 authors.
Contact: hylan@cuhk.edu.hk, yuxueqing@gdph.org.cn, wangxiaoqin@hbhtcm.com.
SARS-CoV-2 N protein induced acute kidney injury in diabetic db/db mice is associated with a Mincle-dependent M1 macrophage activation
Frontiers in Immunology, doi:10.3389/fimmu.2023.1264447
Cytokine storm" is common in critically ill COVID-19 patients, however, mechanisms remain largely unknown. Here, we reported that overexpression of SARS-CoV-2 N protein in diabetic db/db mice significantly increased tubular death and the release of HMGB1, one of the damage-associated molecular patterns (DAMPs), to trigger M1 proinflammatory macrophage activation and production of IL-6, TNF-a, and MCP-1 via a Mincle-Syk/NF-kB-dependent mechanism. This was further confirmed in vitro that overexpression of SARS-CoV-2 N protein caused the release of HMGB1 from injured tubular cells under high AGE conditions, which resulted in M1 macrophage activation and production of proinflammatory cytokines via a Mincle-Syk/NF-kB-dependent mechanism. This was further evidenced by specifically silencing macrophage Mincle to block HMGB1-induced M1 macrophage activation and production of IL-6, TNF-a, and MCP-1 in vitro. Importantly, we also uncovered that treatment with quercetin largely improved SARS-CoV-2 N protein-induced AKI in db/db mice. Mechanistically, we found that quercetin treatment significantly inhibited the release of a DAMP molecule HMGB1 and inactivated M1 pro-inflammatory macrophage while promoting reparative M2 macrophage responses by
Ethics statement The animal study was approved by Animal Experimentation Ethics Committee at the Chinese University of Hong Kong. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmadian, Khatibi, Soofiyani, Abediazar, Shoja et al., Covid-19 and kidney injury: Pathophysiology and molecular mechanisms, Rev Med Virol, doi:10.1002/rmv.2176
Bernini, Velotti, Natural polyphenols as immunomodulators to rescue immune response homeostasis: quercetin as a research model against severe COVID-19, Molecules, doi:10.3390/molecules26195803
Brown, Sensing necrosis with mincle, Nat Immunol, doi:10.1038/ni1008-1099
Chan, Chaudhary, Saha, Chauhan, Vaid et al., AKI in hospitalized patients with COVID-19, J Am Soc Nephrol, doi:10.1681/ASN.2020050615
Chen, Huang, Quan, Liu, Wang et al., HMGB1 as a potential biomarker and therapeutic target for severe COVID-19, Heliyon, doi:10.1016/j.heliyon.2020.e05672
Cheng, Luo, Wang, Zhang, Wang et al., Kidney disease is associated with in-hospital death of patients with COVID-19, Kidney Int, doi:10.1016/j.kint.2020.03.005
Cummings, Baldwin, Abrams, Jacobson, Meyer et al., Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study, Lancet, doi:10.1016/S0140-6736(20)31189-2
Diao, Wang, Wang, Feng, Zhang et al., Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection, Nat Commun, doi:10.1038/s41467-021-22781-1
Diniz, Souza, Duarte, Sousa, Mechanistic aspects and therapeutic potential of quercetin against COVID-19-associated acute kidney injury, Molecules, doi:10.3390/molecules25235772
Fukao, Nagasawa, Nihei, Hiki, Naito et al., COVID-19-induced acute renal tubular injury associated with elevation of serum inflammatory cytokine, Clin Exp Nephrol, doi:10.1007/s10157-021-02101-z
Gabarre, Dumas, Dupont, Darmon, Azoulay et al., Acute kidney injury in critically ill patients with COVID-19, Intensive Care Med, doi:10.1007/s00134-020-06153-9
Gao, Ding, Dong, Zhang, Azkur et al., Risk factors for severe and critically ill COVID-19 patients: A review, Allergy, doi:10.1111/all.14657
Gradin, Andersson, Luther, Anderberg, Rubertsson et al., Urinary cytokines correlate with acute kidney injury in critically ill COVID-19 patients, Cytokine, doi:10.1016/j.cyto.2021.155589
Gu, Zhang, Cen, Wu, Lu et al., Quercetin as a potential treatment for COVID-19-induced acute kidney injury: Based on network pharmacology and molecular docking study, PloS One, doi:10.1371/journal.pone.0245209
Gupta, Coca, Chan, Melamed, Brenner et al., AKI treated with renal replacement therapy in critically ill patients with COVID-19, J Am Soc Nephrol, doi:10.1681/ASN.2020060897
Inoue, M1 macrophage triggered by Mincle leads to a deterioration of acute kidney injury, Kidney Int, doi:10.1016/j.kint.2016.11.026
Izzedine, Jhaveri, Acute kidney injury in patients with COVID-19: an update on the pathophysiology, Nephrol Dial Transplant, doi:10.1093/ndt/gfaa184
Jiang, Chen, Shao, Lu, Zhou, HMGB1 silencing in macrophages prevented their functional skewing and ameliorated EAM development: Nuclear HMGB1 may be a checkpoint molecule of macrophage reprogramming, Int Immunopharmacol, doi:10.1016/j.intimp.2018.01.013
Kingeter, Lin, C-type lectin receptor-induced NF-kB activation in innate immune and inflammatory responses, Cell Mol Immunol, doi:10.1038/cmi.2011.58
Lan, Mu, Nikolic-Paterson, Atkins, A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens, J Histochem Cytochem, doi:10.1177/43.1.7822770
Legrand, Bell, Forni, Joannidis, Koyner et al., Pathophysiology of COVID-19-associated acute kidney injury, Nat Rev Nephrol, doi:10.1038/s41581-021-00452-0
Li, Gong, Zhong, Wang, Guo et al., Neutralization of the extracellular HMGB1 released by ischaemic damaged renal cells protects against renal ischaemia-reperfusion injury, Nephrol Dial Transplant, doi:10.1093/ndt/gfq466
Li, Liu, Meng, Yin, Gao et al., Critical roles of cytokine storm and secondary bacterial infection in acute kidney injury development in COVID-19: A multi-center retrospective cohort study, J Med Virol, doi:10.1002/jmv.27234
Liang, Chen, Wu, Huang, Wei, SARS-CoV-2 N protein induces acute kidney injury in diabetic mice via the Smad3-Ripk3/MLKL necroptosis pathway, Signal Transduct Target Ther, doi:10.1038/s41392-023-01410-x
Liu, Li, Liu, Liang, Wang et al., Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients, EBioMedicine, doi:10.1016/j.ebiom.2020.102763
Lu, Wu, Liu, Ruan, Zhang et al., Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization, Biochem Pharmacol, doi:10.1016/j.bcp.2018.05.007
Lv, Tang, Li, You, Li et al., The pattern recognition receptor, Mincle, is essential for maintaining the M1 macrophage phenotype in acute renal inflammation, Kidney Int, doi:10.1016/j.kint.2016.10.020
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet, doi:10.1016/S0140-6736(20)30628-0
Munafo, Donati, Brindani, Ottonello, Armirotti et al., Quercetin and luteolin are single-digit micromolar inhibitors of the SARS-CoV-2 RNA-dependent RNA polymerase, Sci Rep, doi:10.1038/s41598-022-14664-2
Paludan, Mogensen, Innate immunological pathways in COVID-19 pathogenesis, Sci Immunol, doi:10.1126/sciimmunol.abm5505
Pan, Shen, Yu, Ge, Chen et al., SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation, Nat Commun, doi:10.1038/s41467-021-25015-6
Parthasarathy, Martinelli, Vollmann, Best, Therien, The impact of DAMP-mediated inflammation in severe COVID-19 and related disorders, Biochem Pharmacol, doi:10.1016/j.bcp.2021.114847
Pierro, Derosa, Maffioli, Bertuccioli, Togni et al., Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: A prospective, randomized, controlled, and open-label study, Int J Gen Med, doi:10.2147/IJGM.S318720
Pierro, Iqtadar, Khan, Mumtaz, Chaudhry et al., Potential clinical benefits of quercetin in the early stage of COVID-19: results of a second, pilot, randomized, controlled and open-label clinical trial, Int J Gen Med, doi:10.2147/IJGM.S318949
Tan, Wang, Deng, Zhong, Yan et al., Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-kB signaling maintained macrophage inflammation, Phytother Res, doi:10.1002/ptr.6507
Tang, Nikolic-Paterson, Lan, Macrophages: versatile players in renal inflammation and fibrosis, Nat Rev Nephrol, doi:10.1038/s41581-019-0110-2
Tian, Zhang, Tang, Guo, Dong et al., HMGB1 exacerbates renal tubulointerstitial fibrosis through facilitating M1 macrophage phenotype at the early stage of obstructive injury, Am J Physiol Renal Physiol, doi:10.1152/ajprenal.00484.2014
Wang, Chen, Hu, Pan, Liang et al., SARS-coV-2 N protein induces acute kidney injury via smad3-dependent G1 cell cycle arrest mechanism, Adv Sci (Weinh), doi:10.1002/advs.202103248
Wang, Yang, Li, Huang, Jiang et al., Specific cytokines in the inflammatory cytokine storm of patients with COVID-19-associated acute respiratory distress syndrome and extrapulmonary multiple-organ dysfunction, Virol J, doi:10.1186/s12985-021-01588-y
Wu, Ma, Cai, Zhuang, Zhao, RNA-induced liquid phase separation of SARS-CoV-2 nucleocapsid protein facilitates NF-kB hyper-activation and inflammation, Signal Transduct Target Ther, doi:10.1038/s41392-021-00575-7
Wu, Ma, Wang, Corpuz, Panchapakesan et al., HMGB1 contributes to kidney ischemia reperfusion injury, J Am Soc Nephrol, doi:10.1681/ASN.2009101048
Wu, Wang, Liang, Chen, Wei et al., Treatment with quercetin inhibits SARS-CoV-2 N protein-induced acute kidney injury by blocking Smad3-dependent G1 cell-cycle arrest, Mol Ther, doi:10.1016/j.ymthe.2022.12.002
Yanai, Ban, Taniguchi, High-mobility group box family of proteins: ligand and sensor for innate immunity, Trends Immunol, doi:10.1016/j.it.2012.10.005
Yang, Xie, Tu, Fu, Xu et al., The signal pathways and treatment of cytokine storm in COVID-19, Signal Transduct Target Ther, doi:10.1038/s41392-021-00679-0
Zheng, Zhao, Yang, Acute kidney injury in COVID-19: the chinese experience, Semin Nephrol, doi:10.1016/j.semnephrol.2020.09.001
DOI record:
{
"DOI": "10.3389/fimmu.2023.1264447",
"ISSN": [
"1664-3224"
],
"URL": "http://dx.doi.org/10.3389/fimmu.2023.1264447",
"abstract": "<jats:p>“Cytokine storm” is common in critically ill COVID-19 patients, however, mechanisms remain largely unknown. Here, we reported that overexpression of SARS-CoV-2 N protein in diabetic db/db mice significantly increased tubular death and the release of HMGB1, one of the damage-associated molecular patterns (DAMPs), to trigger M1 proinflammatory macrophage activation and production of IL-6, TNF-α, and MCP-1 via a Mincle-Syk/NF-κB-dependent mechanism. This was further confirmed <jats:italic>in vitro</jats:italic> that overexpression of SARS-CoV-2 N protein caused the release of HMGB1 from injured tubular cells under high AGE conditions, which resulted in M1 macrophage activation and production of proinflammatory cytokines via a Mincle-Syk/NF-κB-dependent mechanism. This was further evidenced by specifically silencing macrophage Mincle to block HMGB1-induced M1 macrophage activation and production of IL-6, TNF-α, and MCP-1 <jats:italic>in vitro</jats:italic>. Importantly, we also uncovered that treatment with quercetin largely improved SARS-CoV-2 N protein-induced AKI in db/db mice. Mechanistically, we found that quercetin treatment significantly inhibited the release of a DAMP molecule HMGB1 and inactivated M1 pro-inflammatory macrophage while promoting reparative M2 macrophage responses by suppressing Mincle-Syk/NF-κB signaling <jats:italic>in vivo</jats:italic> and <jats:italic>in vitro</jats:italic>. In conclusion, SARS-CoV-2 N protein-induced AKI in db/db mice is associated with Mincle-dependent M1 macrophage activation. Inhibition of this pathway may be a mechanism through which quercetin inhibits COVID-19-associated AKI.</jats:p>",
"alternative-id": [
"10.3389/fimmu.2023.1264447"
],
"author": [
{
"affiliation": [],
"family": "Wu",
"given": "Wenjing",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wang",
"given": "Wenbiao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liang",
"given": "Liying",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Junzhe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sun",
"given": "Sifan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wei",
"given": "Biao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhong",
"given": "Yu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Xiao-Ru",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Jian",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Xiaoqin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yu",
"given": "Xueqing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lan",
"given": "Hui-Yao",
"sequence": "additional"
}
],
"container-title": "Frontiers in Immunology",
"container-title-short": "Front. Immunol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
11,
3
]
],
"date-time": "2023-11-03T09:49:45Z",
"timestamp": 1699004985000
},
"deposited": {
"date-parts": [
[
2023,
11,
3
]
],
"date-time": "2023-11-03T09:49:47Z",
"timestamp": 1699004987000
},
"indexed": {
"date-parts": [
[
2023,
11,
4
]
],
"date-time": "2023-11-04T00:45:34Z",
"timestamp": 1699058734304
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
11,
3
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
3
]
],
"date-time": "2023-11-03T00:00:00Z",
"timestamp": 1698969600000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2023.1264447/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
11,
3
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
3
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1016/j.semnephrol.2020.09.001",
"article-title": "Acute kidney injury in COVID-19: the chinese experience",
"author": "Zheng",
"doi-asserted-by": "publisher",
"journal-title": "Semin Nephrol",
"key": "B1",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1681/ASN.2020050615",
"article-title": "AKI in hospitalized patients with COVID-19",
"author": "Chan",
"doi-asserted-by": "publisher",
"journal-title": "J Am Soc Nephrol",
"key": "B2",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)31189-2",
"article-title": "Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study",
"author": "Cummings",
"doi-asserted-by": "publisher",
"journal-title": "Lancet",
"key": "B3",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.kint.2020.03.005",
"article-title": "Kidney disease is associated with in-hospital death of patients with COVID-19",
"author": "Cheng",
"doi-asserted-by": "publisher",
"journal-title": "Kidney Int",
"key": "B4",
"volume": "97",
"year": "2020"
},
{
"DOI": "10.1111/all.14657",
"article-title": "Risk factors for severe and critically ill COVID-19 patients: A review",
"author": "Gao",
"doi-asserted-by": "publisher",
"journal-title": "Allergy",
"key": "B5",
"volume": "76",
"year": "2021"
},
{
"DOI": "10.1681/ASN.2020060897",
"article-title": "AKI treated with renal replacement therapy in critically ill patients with COVID-19",
"author": "Gupta",
"doi-asserted-by": "publisher",
"journal-title": "J Am Soc Nephrol",
"key": "B6",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1007/s10157-021-02101-z",
"article-title": "COVID-19-induced acute renal tubular injury associated with elevation of serum inflammatory cytokine",
"author": "Fukao",
"doi-asserted-by": "publisher",
"journal-title": "Clin Exp Nephrol",
"key": "B7",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1038/s41392-021-00679-0",
"article-title": "The signal pathways and treatment of cytokine storm in COVID-19",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "255",
"journal-title": "Signal Transduct Target Ther",
"key": "B8",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1038/s41581-021-00452-0",
"article-title": "Pathophysiology of COVID-19-associated acute kidney injury",
"author": "Legrand",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Nephrol",
"key": "B9",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"article-title": "COVID- 19: consider cytokine storm syndromes and immunosuppression",
"author": "Mehta",
"doi-asserted-by": "publisher",
"journal-title": "Lancet",
"key": "B10",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.ebiom.2020.102763",
"article-title": "Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients",
"author": "Liu",
"doi-asserted-by": "publisher",
"journal-title": "EBioMedicine",
"key": "B11",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1016/j.cyto.2021.155589",
"article-title": "Urinary cytokines correlate with acute kidney injury in critically ill COVID-19 patients",
"author": "Gradin",
"doi-asserted-by": "publisher",
"journal-title": "Cytokine",
"key": "B12",
"volume": "146",
"year": "2021"
},
{
"DOI": "10.1186/s12985-021-01588-y",
"article-title": "Specific cytokines in the inflammatory cytokine storm of patients with COVID-19-associated acute respiratory distress syndrome and extrapulmonary multiple-organ dysfunction",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "117",
"journal-title": "Virol J",
"key": "B13",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1002/jmv.27234",
"article-title": "Critical roles of cytokine storm and secondary bacterial infection in acute kidney injury development in COVID-19: A multi- center retrospective cohort study",
"author": "Li",
"doi-asserted-by": "publisher",
"journal-title": "J Med Virol",
"key": "B14",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1007/s00134-020-06153-9",
"article-title": "Acute kidney injury in critically ill patients with COVID-19",
"author": "Gabarre",
"doi-asserted-by": "publisher",
"journal-title": "Intensive Care Med",
"key": "B15",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1038/s41467-021-22781-1",
"article-title": "Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection",
"author": "Diao",
"doi-asserted-by": "publisher",
"first-page": "2506",
"journal-title": "Nat Commun",
"key": "B16",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1126/sciimmunol.abm5505",
"article-title": "Innate immunological pathways in COVID-19 pathogenesis",
"author": "Paludan",
"doi-asserted-by": "publisher",
"journal-title": "Sci Immunol",
"key": "B17",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1038/s41581-019-0110-2",
"article-title": "Macrophages: versatile players in renal inflammation and fibrosis",
"author": "Tang",
"doi-asserted-by": "publisher",
"journal-title": "Nat Rev Nephrol",
"key": "B18",
"volume": "15",
"year": "2019"
},
{
"DOI": "10.1038/ni1008-1099",
"article-title": "Sensing necrosis with mincle",
"author": "Brown",
"doi-asserted-by": "publisher",
"journal-title": "Nat Immunol",
"key": "B19",
"volume": "9",
"year": "2008"
},
{
"DOI": "10.1038/cmi.2011.58",
"article-title": "C-type lectin receptor-induced NF-κB activation in innate immune and inflammatory responses",
"author": "Kingeter",
"doi-asserted-by": "publisher",
"journal-title": "Cell Mol Immunol",
"key": "B20",
"volume": "9",
"year": "2012"
},
{
"DOI": "10.1016/j.bcp.2021.114847",
"article-title": "The impact of DAMP-mediated inflammation in severe COVID-19 and related disorders",
"author": "Parthasarathy",
"doi-asserted-by": "publisher",
"journal-title": "Biochem Pharmacol",
"key": "B21",
"volume": "195",
"year": "2022"
},
{
"DOI": "10.1016/j.heliyon.2020.e05672",
"article-title": "HMGB1 as a potential biomarker and therapeutic target for severe COVID-19",
"author": "Chen",
"doi-asserted-by": "publisher",
"journal-title": "Heliyon",
"key": "B22",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1093/ndt/gfaa184",
"article-title": "Acute kidney injury in patients with COVID-19: an update on the pathophysiology",
"author": "Izzedine",
"doi-asserted-by": "publisher",
"journal-title": "Nephrol Dial Transplant",
"key": "B23",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.1016/j.ymthe.2022.12.002",
"article-title": "Treatment with quercetin inhibits SARS-CoV-2 N protein-induced acute kidney injury by blocking Smad3-dependent G1 cell-cycle arrest",
"author": "Wu",
"doi-asserted-by": "publisher",
"journal-title": "Mol Ther",
"key": "B24",
"volume": "31",
"year": "2023"
},
{
"DOI": "10.1002/advs.202103248",
"article-title": "SARS-coV-2 N protein induces acute kidney injury via smad3-dependent G1 cell cycle arrest mechanism",
"author": "Wang",
"doi-asserted-by": "publisher",
"journal-title": "Adv Sci (Weinh)",
"key": "B25",
"volume": "9",
"year": "2022"
},
{
"DOI": "10.1038/s41392-023-01410-x",
"article-title": "SARS-CoV-2 N protein induces acute kidney injury in diabetic mice via the Smad3-Ripk3/MLKL necroptosis pathway",
"author": "Liang",
"doi-asserted-by": "publisher",
"first-page": "147",
"journal-title": "Signal Transduct Target Ther",
"key": "B26",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.2147/IJGM.S318949",
"article-title": "Potential clinical benefits of quercetin in the early stage of COVID-19: results of a second, pilot, randomized, controlled and open-label clinical trial",
"author": "Di Pierro",
"doi-asserted-by": "publisher",
"first-page": "2807",
"journal-title": "Int J Gen Med",
"key": "B27",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.2147/IJGM.S318720",
"article-title": "Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: A prospective, randomized, controlled, and open-label study",
"author": "Di Pierro",
"doi-asserted-by": "publisher",
"first-page": "2359",
"journal-title": "Int J Gen Med",
"key": "B28",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0245209",
"article-title": "Quercetin as a potential treatment for COVID-19-induced acute kidney injury: Based on network pharmacology and molecular docking study",
"author": "Gu",
"doi-asserted-by": "publisher",
"journal-title": "PloS One",
"key": "B29",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1038/s41598-022-14664-2",
"article-title": "Quercetin and luteolin are single-digit micromolar inhibitors of the SARS-CoV-2 RNA-dependent RNA polymerase",
"author": "Munafo",
"doi-asserted-by": "publisher",
"first-page": "10571",
"journal-title": "Sci Rep",
"key": "B30",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1002/ptr.6507",
"article-title": "Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-κB signaling maintained macrophage inflammation",
"author": "Tan",
"doi-asserted-by": "publisher",
"journal-title": "Phytother Res",
"key": "B31",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1177/43.1.7822770",
"article-title": "A novel, simple, reliable, and sensitive method for multiple immunoenzyme staining: use of microwave oven heating to block antibody crossreactivity and retrieve antigens",
"author": "Lan",
"doi-asserted-by": "publisher",
"first-page": "97",
"journal-title": "J Histochem Cytochem",
"key": "B32",
"volume": "43",
"year": "1995"
},
{
"DOI": "10.1002/rmv.2176",
"article-title": "Covid-19 and kidney injury: Pathophysiology and molecular mechanisms",
"author": "Ahmadian",
"doi-asserted-by": "publisher",
"journal-title": "Rev Med Virol",
"key": "B33",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.1016/j.kint.2016.10.020",
"article-title": "The pattern recognition receptor, Mincle, is essential for maintaining the M1 macrophage phenotype in acute renal inflammation",
"author": "Lv",
"doi-asserted-by": "publisher",
"first-page": "587",
"journal-title": "Kidney Int",
"key": "B34",
"volume": "91",
"year": "2017"
},
{
"DOI": "10.1038/s41467-021-25015-6",
"article-title": "SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation",
"author": "Pan",
"doi-asserted-by": "publisher",
"first-page": "4664",
"journal-title": "Nat Commun",
"key": "B35",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41392-021-00575-7",
"article-title": "RNA-induced liquid phase separation of SARS-CoV-2 nucleocapsid protein facilitates NF-κB hyper-activation and inflammation",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "167",
"journal-title": "Signal Transduct Target Ther",
"key": "B36",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/j.it.2012.10.005",
"article-title": "High-mobility group box family of proteins: ligand and sensor for innate immunity",
"author": "Yanai",
"doi-asserted-by": "publisher",
"journal-title": "Trends Immunol",
"key": "B37",
"volume": "33",
"year": "2012"
},
{
"DOI": "10.1681/ASN.2009101048",
"article-title": "HMGB1 contributes to kidney ischemia reperfusion injury",
"author": "Wu",
"doi-asserted-by": "publisher",
"journal-title": "J Am Soc Nephrol",
"key": "B38",
"volume": "21",
"year": "2010"
},
{
"DOI": "10.1093/ndt/gfq466",
"article-title": "Neutralization of the extracellular HMGB1 released by ischaemic damaged renal cells protects against renal ischaemia- reperfusion injury",
"author": "Li",
"doi-asserted-by": "publisher",
"journal-title": "Nephrol Dial Transplant",
"key": "B39",
"volume": "26",
"year": "2011"
},
{
"DOI": "10.1152/ajprenal.00484.2014",
"article-title": "HMGB1 exacerbates renal tubulointerstitial fibrosis through facilitating M1 macrophage phenotype at the early stage of obstructive injury",
"author": "Tian",
"doi-asserted-by": "publisher",
"journal-title": "Am J Physiol Renal Physiol",
"key": "B40",
"volume": "308",
"year": "2015"
},
{
"DOI": "10.1016/j.intimp.2018.01.013",
"article-title": "HMGB1 silencing in macrophages prevented their functional skewing and ameliorated EAM development: Nuclear HMGB1 may be a checkpoint molecule of macrophage reprogramming",
"author": "Jiang",
"doi-asserted-by": "publisher",
"journal-title": "Int Immunopharmacol",
"key": "B41",
"volume": "56",
"year": "2018"
},
{
"DOI": "10.1016/j.kint.2016.11.026",
"article-title": "M1 macrophage triggered by Mincle leads to a deterioration of acute kidney injury",
"author": "Inoue",
"doi-asserted-by": "publisher",
"journal-title": "Kidney Int",
"key": "B42",
"volume": "91",
"year": "2017"
},
{
"DOI": "10.3390/molecules26195803",
"article-title": "Natural polyphenols as immunomodulators to rescue immune response homeostasis: quercetin as a research model against severe COVID-19",
"author": "Bernini",
"doi-asserted-by": "publisher",
"first-page": "5803",
"journal-title": "Molecules",
"key": "B43",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.3390/molecules25235772",
"article-title": "Mechanistic aspects and therapeutic potential of quercetin against COVID-19-associated acute kidney injury",
"author": "Diniz",
"doi-asserted-by": "publisher",
"first-page": "5772",
"journal-title": "Molecules",
"key": "B44",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1016/j.bcp.2018.05.007",
"article-title": "Quercetin ameliorates kidney injury and fibrosis by modulating M1/M2 macrophage polarization",
"author": "Lu",
"doi-asserted-by": "publisher",
"journal-title": "Biochem Pharmacol",
"key": "B45",
"volume": "154",
"year": "2018"
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fimmu.2023.1264447/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Immunology",
"Immunology and Allergy"
],
"subtitle": [],
"title": "SARS-CoV-2 N protein induced acute kidney injury in diabetic db/db mice is associated with a Mincle-dependent M1 macrophage activation",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "14"
}
