Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study
et al., International Journal of General Medicine, doi:10.2147/IJGM.S318720, NCT04578158, Jun 2021
Quercetin for COVID-19
27th treatment shown to reduce risk in
July 2021, now with p = 0.002 from 12 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
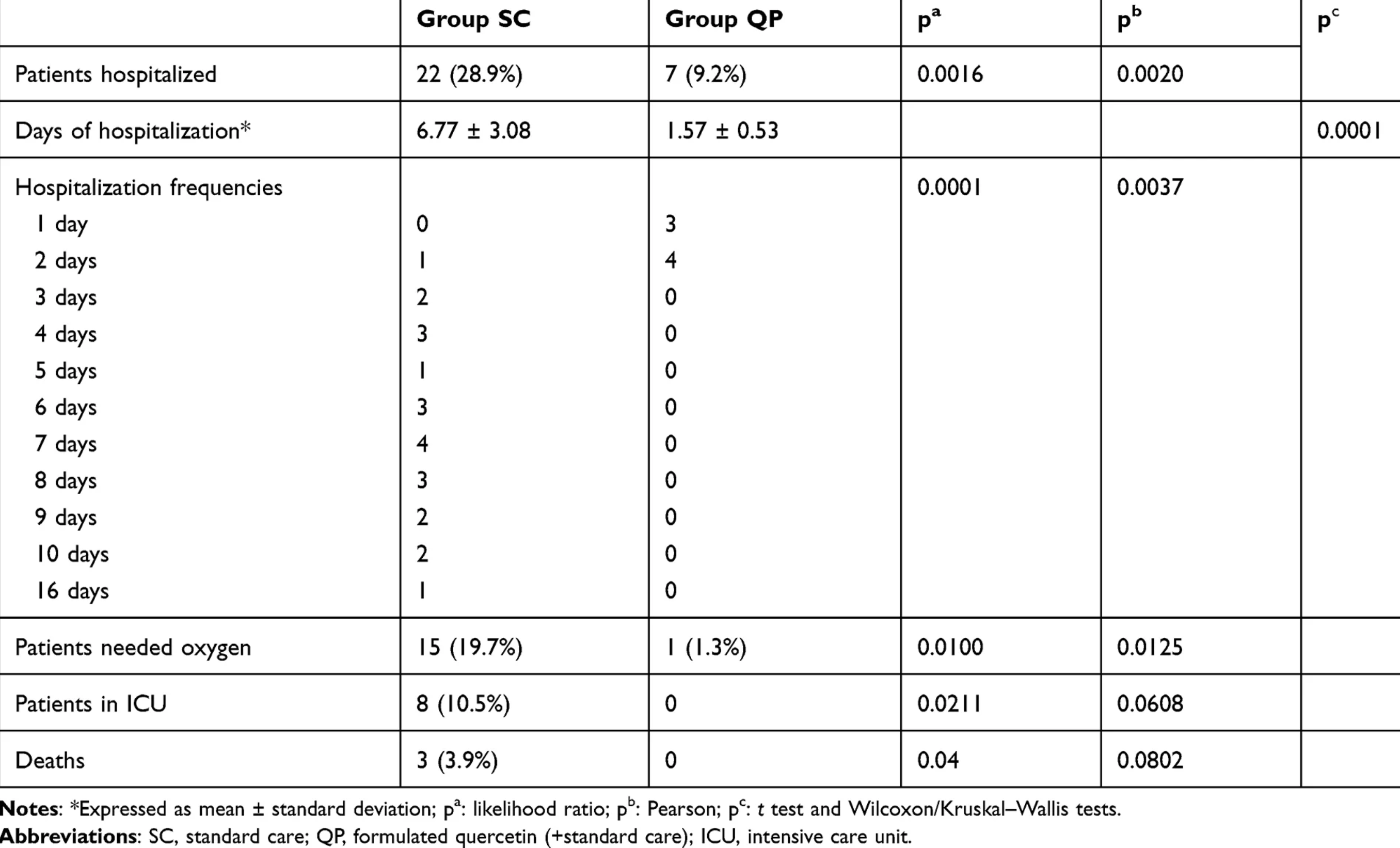
RCT 152 outpatients in Pakistan, 76 treated with quercetin phytosome, showing lower mortality, ICU admission, and hospitalization with treatment.
Bioavailability. Quercetin has low bioavailability and studies typically use advanced formulations to improve bioavailability which may be required to reach therapeutic concentrations.
This is the 3rd of 11 COVID-19 RCTs for quercetin, which collectively show efficacy with p=0.0023.
This is the 3rd of 12 COVID-19 controlled studies for quercetin, which collectively show efficacy with p=0.002.
|
risk of death, 85.7% lower, RR 0.14, p = 0.25, treatment 0 of 76 (0.0%), control 3 of 76 (3.9%), NNT 25, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 94.1% lower, RR 0.06, p = 0.006, treatment 0 of 76 (0.0%), control 8 of 76 (10.5%), NNT 9.5, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of hospitalization, 68.2% lower, RR 0.32, p = 0.003, treatment 7 of 76 (9.2%), control 22 of 76 (28.9%), NNT 5.1.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Di Pierro et al., 8 Jun 2021, Randomized Controlled Trial, Pakistan, peer-reviewed, 19 authors, study period September 2020 - March 2021, trial NCT04578158 (history).
Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study
International Journal of General Medicine, doi:10.2147/ijgm.s318720
Background: Quercetin, a well-known naturally occurring polyphenol, has recently been shown by molecular docking, in vitro and in vivo studies to be a possible anti-COVID-19 candidate. Quercetin has strong antioxidant, anti-inflammatory, immunomodulatory, and antiviral properties, and it is characterized by a very high safety profile, exerted in animals and in humans. Like most other polyphenols, quercetin shows a very low rate of oral absorption and its clinical use is considered by most of modest utility. Quercetin in a delivery-food grade system with sunflower phospholipids (Quercetin Phytosome ® , QP) increases its oral absorption up to 20-fold. Methods: In the present prospective, randomized, controlled, and open-label study, a daily dose of 1000 mg of QP was investigated for 30 days in 152 COVID-19 outpatients to disclose its adjuvant effect in treating the early symptoms and in preventing the severe outcomes of the disease.
Results: The results revealed a reduction in frequency and length of hospitalization, in need of non-invasive oxygen therapy, in progression to intensive care units and in number of deaths. The results also confirmed the very high safety profile of quercetin and suggested possible anti-fatigue and pro-appetite properties. Conclusion: QP is a safe agent and in combination with standard care, when used in early stage of viral infection, could aid in improving the early symptoms and help in preventing the severity of COVID-19 disease. It is suggested that a double-blind, placebo-controlled study should be urgently carried out to confirm the results of our study.
International Journal of General Medicine
Dovepress
DovePress International Journal of General Medicine 2021:14
References
Abian, Ortega-Alarcon, Jimenez-Alesanco, Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening, Int J Biol Macromol, doi:10.1016/j.ijbiomac.2020.07.235
Almatroodi, Alsahli, Almatroudi, Potential therapeutic targets of quercetin, a plant flavonol, and its role in the therapy of various types of cancer through the modulation of various cell signaling pathways, Molecules, doi:10.3390/molecules26051315
Aschwanden, Five reasons why COVID herd immunity is probably impossible, Nature, doi:10.1038/d41586-021-00728-2
Batiha, Beshbishy, Ikram, The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: quercetin, Foods, doi:10.3390/foods9030374
Brito, Lima, Cordeiro, Da, Nizer, Effectiveness of supplementation with quercetin-type flavonols for treatment of viral lower respiratory tract infections: systematic review and meta-analysis of preclinical studies, Phytother Res, doi:10.1002/ptr.7122
David, Arulmoli, Parasuraman, Overviews of biological importance of quercetin: a bioactive flavonoid, Pharmacogn Rev, doi:10.4103/0973-7847.194044
Derosa, Maffioli, Angelo, Pierro, A role for quercetin in coronavirus disease 2019 (COVID-19), Phytother Res, doi:10.1002/ptr.6887
Gao, Liu, Wang, Liu, Xu et al., Preparation of a chemically stable quercetin formulation using nanosuspension technology, Int J Pharm, doi:10.1016/j.ijpharm.2010.11.009
Gautam, Madathil, Tahani, Medium-term outcome of severe to critically ill patients with SARS-CoV-2 infection, Clin Infect Dis, doi:10.1093/cid/ciab341
Heinz, Henson, Austin, Nieman, Quercetin supplementation and upper respiratory tract infection: a randomized community clinical trial, Pharmacol Res, doi:10.1016/j.phrs.2010.05.001
Karki, Verma, Trozzi, Tao, Kraka et al., Predicting Potential SARS-COV-2 drugs-in depth drug database screening using deep neural network framework SSnet, classical virtual screening and docking, Int J Mol Sci, doi:10.3390/ijms22041573
Khaerunnisa, Kurniawan, Awaluddin, Suhartati, Soetjipto, Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study, doi:10.20944/preprints.202003.0226.v1
Kim, Ryu, Social distancing attitudes, national context, and health outcomes during the COVID-19 pandemic: findings from a global survey, Prev Med, doi:10.1016/j.ypmed.2021.106544
Lindinger-Sternart, Kaur, Widyaningsih, Patel, COVID-19 phobia across the world: impact of resilience on COVID-19 phobia in different nations, Couns Psychother Res, doi:10.1002/capr.12387
Luo, Zhang, Luo, Treatment efficacy analysis of traditional Chinese medicine for novel coronavirus pneumonia (COVID-19): an empirical study from Wuhan, Hubei Province, China, Chin Med, doi:10.1186/s13020-020-00317-x
Mckee, Sternberg, Stange, Laufer, Naujokat, Candidate drugs against SARS-CoV-2 and COVID-19, Pharmacol Res, doi:10.1016/j.phrs.2020.104859
Mirtaleb, Mirtaleb, Nosrati, Heshmatnia, Falak et al., Potential therapeutic agents to COVID-19: an update review on antiviral therapy, immunotherapy, and cell therapy, Biomed Pharmacother, doi:10.1016/j.biopha.2021.111518
Nayak, Mishra, Naik, Swapnarekha, Cengiz et al., An impact study of COVID-19 on six different industries: automobile, energy and power, agriculture, education, travel and tourism and consumer electronics, Expert Syst, doi:10.1111/exsy.12677
Pal, Squitti, Picozza, Zinc and COVID-19: basis of current clinical Trials, Biol Trace Elem Res, doi:10.1007/s12011-020-02437-9
Pandey, Rane, Chatterjee, Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: an in-silico study for drug development, J Biomol Struct Dyn, doi:10.1080/07391102.2020.1796811
Patel, Mistry, Shinde, Syed, Singh et al., Pro-inflammatory cytokine regulation of P-glycoprotein in the developing blood-brain barrier, Eur J Med Chem, doi:10.1371/journal.pone.0043022
Pawar, Pal, Molecular and functional resemblance of dexamethasone and quercetin: a paradigm worth exploring in dexamethasone-nonresponsive COVID-19 patients, Phytother Res, doi:10.1002/ptr.6886
Pierro, Khan, Bertuccioli, Quercetin Phytosome ® as a potential candidate for managing COVID-19, Minerva Gastroenterol Dietol, doi:10.23736/S1121-421X.20.02771-3
Riva, Ronchi, Petrangolini, Bosisio, Allegrini, Improved oral absorption of quercetin from quercetin Phytosome ® , a new delivery system based on food grade lecithin, Eur J Drug Metab Pharmacokinet, doi:10.1007/s13318-018-0517-3
Smith, Smith, Repurposing therapeutics for COVID-19: supercomputer-based docking to the SARS-CoV-2 viral spike protein and viral spike protein-human ACE2 interface, ChemRxiv, doi:10.26434/chemrxiv.11871402.v3
Wang, Sun, Mao, The biological activities, chemical stability, metabolism, and delivery systems of quercetin: a review, Trends Food Sci Technol, doi:10.1016/j.tifs.2016.07.004
Williamson, Kerimi, Testing of natural products in clinical trials targeting the SARS-CoV-2 (Covid-19) viral spike protein-angiotensin converting enzyme-2 (ACE2) interaction, Biochem Pharmacol, doi:10.1016/j.bcp.2020.114123
Xu, Hu, Wang, Cui, Antioxidant activities of quercetin and its complexes for medicinal application, Molecules, doi:10.3390/molecules24061123
Zhang, Wu, Zhang, Deng, Peng, In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus, J Integr Med, doi:10.1016/j.joim.2020.02.005
DOI record:
{
"DOI": "10.2147/ijgm.s318720",
"ISSN": [
"1178-7074"
],
"URL": "http://dx.doi.org/10.2147/IJGM.S318720",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6654-8675",
"affiliation": [],
"authenticated-orcid": true,
"family": "Di Pierro",
"given": "Francesco",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-3573-4760",
"affiliation": [],
"authenticated-orcid": true,
"family": "Derosa",
"given": "Giuseppe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maffioli",
"given": "Pamela",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3922-9115",
"affiliation": [],
"authenticated-orcid": true,
"family": "Bertuccioli",
"given": "Alexander",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Togni",
"given": "Stefano",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2819-943X",
"affiliation": [],
"authenticated-orcid": true,
"family": "Riva",
"given": "Antonella",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4380-9577",
"affiliation": [],
"authenticated-orcid": true,
"family": "Allegrini",
"given": "Pietro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Amjad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Saeed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khan",
"given": "Bilal Ahmad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Altaf",
"given": "Naireen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zahid",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ujjan",
"given": "Ikram Din",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nigar",
"given": "Roohi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Khushk",
"given": "Mehwish Imam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Phulpoto",
"given": "Maryam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lail",
"given": "Amanullah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Devrajani",
"given": "Bikha Ram",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Sagheer",
"sequence": "additional"
}
],
"container-title": "International Journal of General Medicine",
"container-title-short": "IJGM",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
6,
8
]
],
"date-time": "2021-06-08T00:02:37Z",
"timestamp": 1623110557000
},
"deposited": {
"date-parts": [
[
2021,
6,
8
]
],
"date-time": "2021-06-08T00:02:39Z",
"timestamp": 1623110559000
},
"indexed": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T06:35:18Z",
"timestamp": 1711694118902
},
"is-referenced-by-count": 108,
"issued": {
"date-parts": [
[
2021,
6
]
]
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/3.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
6,
1
]
],
"date-time": "2021-06-01T00:00:00Z",
"timestamp": 1622505600000
}
}
],
"link": [
{
"URL": "https://www.dovepress.com/getfile.php?fileID=70294",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.dovepress.com/getfile.php?fileID=70294",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "2359-2366",
"prefix": "10.2147",
"published": {
"date-parts": [
[
2021,
6
]
]
},
"published-online": {
"date-parts": [
[
2021,
6
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1016/j.ypmed.2021.106544",
"author": "Kim",
"doi-asserted-by": "publisher",
"first-page": "106544",
"journal-title": "Prev Med",
"key": "ref1",
"volume": "148",
"year": "2021"
},
{
"DOI": "10.1002/capr.12387",
"author": "Lindinger-Sternart",
"doi-asserted-by": "publisher",
"first-page": "290",
"journal-title": "Couns Psychother Res",
"key": "ref2",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1111/exsy.12677",
"author": "Nayak",
"doi-asserted-by": "publisher",
"journal-title": "Expert Syst",
"key": "ref3",
"year": "2021"
},
{
"DOI": "10.1038/d41586-021-00728-2",
"author": "Aschwanden",
"doi-asserted-by": "publisher",
"first-page": "520",
"journal-title": "Nature",
"key": "ref4",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.1016/j.biopha.2021.111518",
"author": "Mirtaleb",
"doi-asserted-by": "publisher",
"first-page": "111518",
"journal-title": "Biomed Pharmacother",
"key": "ref5",
"volume": "138",
"year": "2021"
},
{
"DOI": "10.3390/ijms22041573",
"author": "Karki",
"doi-asserted-by": "publisher",
"first-page": "1573",
"journal-title": "Int J Mol Sci",
"key": "ref6",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.phrs.2020.104859",
"author": "McKee",
"doi-asserted-by": "publisher",
"first-page": "104859",
"journal-title": "Pharmacol Res",
"key": "ref7",
"volume": "157",
"year": "2020"
},
{
"DOI": "10.1016/j.joim.2020.02.005",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "152",
"journal-title": "J Integr Med",
"key": "ref8",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.26434/chemrxiv.11871402.v3",
"author": "Smith",
"doi-asserted-by": "publisher",
"journal-title": "ChemRxiv",
"key": "ref9",
"year": "2020"
},
{
"DOI": "10.1016/j.bcp.2020.114123",
"author": "Williamson",
"doi-asserted-by": "publisher",
"first-page": "114123",
"journal-title": "Biochem Pharmacol",
"key": "ref10",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1080/07391102.2020.1796811",
"author": "Pandey",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "J Biomol Struct Dyn",
"key": "ref11",
"year": "2020"
},
{
"DOI": "10.20944/preprints.202003.0226.v1",
"author": "Khaerunnisa",
"doi-asserted-by": "publisher",
"journal-title": "Preprints",
"key": "ref12",
"year": "2020"
},
{
"DOI": "10.1016/j.ijbiomac.2020.07.235",
"author": "Abian",
"doi-asserted-by": "publisher",
"first-page": "1693",
"journal-title": "Int J Biol Macromol",
"key": "ref13",
"volume": "164",
"year": "2020"
},
{
"DOI": "10.4103/0973-7847.194044",
"author": "Anand David",
"doi-asserted-by": "publisher",
"first-page": "84",
"journal-title": "Pharmacogn Rev",
"key": "ref14",
"volume": "10",
"year": "2016"
},
{
"DOI": "10.1016/j.ijpharm.2010.11.009",
"author": "Gao",
"doi-asserted-by": "publisher",
"first-page": "231",
"journal-title": "Int J Pharm",
"key": "ref15",
"volume": "404",
"year": "2011"
},
{
"DOI": "10.1016/j.tifs.2016.07.004",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "21",
"journal-title": "Trends Food Sci Technol",
"key": "ref16",
"volume": "56",
"year": "2016"
},
{
"DOI": "10.1007/s13318-018-0517-3",
"author": "Riva",
"doi-asserted-by": "publisher",
"first-page": "169",
"journal-title": "Eur J Drug Metab Pharmacokinet",
"key": "ref17",
"volume": "44",
"year": "2019"
},
{
"DOI": "10.1002/ptr.6887",
"author": "Derosa",
"doi-asserted-by": "publisher",
"first-page": "1230",
"journal-title": "Phytother Res",
"key": "ref18",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.23736/S1121-421X.20.02771-3",
"author": "Di Pierro",
"doi-asserted-by": "publisher",
"journal-title": "Minerva Gastroenterol Dietol",
"key": "ref19",
"year": "2020"
},
{
"DOI": "10.3390/foods9030374",
"author": "Batiha",
"doi-asserted-by": "publisher",
"first-page": "374",
"journal-title": "Foods",
"key": "ref20",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciab341",
"author": "Gautam",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "ref21",
"year": "2021"
},
{
"DOI": "10.3390/molecules26051315",
"author": "Almatroodi",
"doi-asserted-by": "publisher",
"first-page": "1315",
"journal-title": "Molecules",
"key": "ref22",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1016/j.ejmech.2018.06.053",
"author": "Patel",
"doi-asserted-by": "publisher",
"first-page": "889",
"journal-title": "Eur J Med Chem",
"key": "ref23",
"volume": "155",
"year": "2018"
},
{
"DOI": "10.1371/journal.pone.0043022",
"author": "Iqbal",
"doi-asserted-by": "publisher",
"first-page": "e43022",
"journal-title": "PLoS One",
"key": "ref24",
"volume": "7",
"year": "2012"
},
{
"DOI": "10.1002/ptr.6886",
"author": "Pawar",
"doi-asserted-by": "publisher",
"first-page": "3085",
"journal-title": "Phytother Res",
"key": "ref25",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1007/s12011-020-02437-9",
"author": "Pal",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Biol Trace Elem Res",
"key": "ref26",
"year": "2020"
},
{
"DOI": "10.3390/molecules24061123",
"author": "Xu",
"doi-asserted-by": "publisher",
"first-page": "1123",
"journal-title": "Molecules",
"key": "ref27",
"volume": "24",
"year": "2019"
},
{
"DOI": "10.1016/j.phrs.2010.05.001",
"author": "Heinz",
"doi-asserted-by": "publisher",
"first-page": "237",
"journal-title": "Pharmacol Res",
"key": "ref28",
"volume": "62",
"year": "2010"
},
{
"DOI": "10.1186/s13020-020-00317-x",
"author": "Luo",
"doi-asserted-by": "publisher",
"first-page": "34",
"journal-title": "Chin Med",
"key": "ref29",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1002/ptr.7122",
"author": "Brito",
"doi-asserted-by": "publisher",
"journal-title": "Phytother Res",
"key": "ref30",
"year": "2021"
}
],
"reference-count": 30,
"references-count": 30,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.dovepress.com/possible-therapeutic-effects-of-adjuvant-quercetin-supplementation-aga-peer-reviewed-fulltext-article-IJGM"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Possible Therapeutic Effects of Adjuvant Quercetin Supplementation Against Early-Stage COVID-19 Infection: A Prospective, Randomized, Controlled, and Open-Label Study",
"type": "journal-article",
"volume": "Volume 14"
}
