Phytochemical and antiviral investigation of Cynanchum acutum L. extract and derived semi-synthetic analogs targeting SARS-CoV-2 main protease
et al., Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-025-00907-2, Oct 2025
Quercetin for COVID-19
27th treatment shown to reduce risk in
July 2021, now with p = 0.002 from 12 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
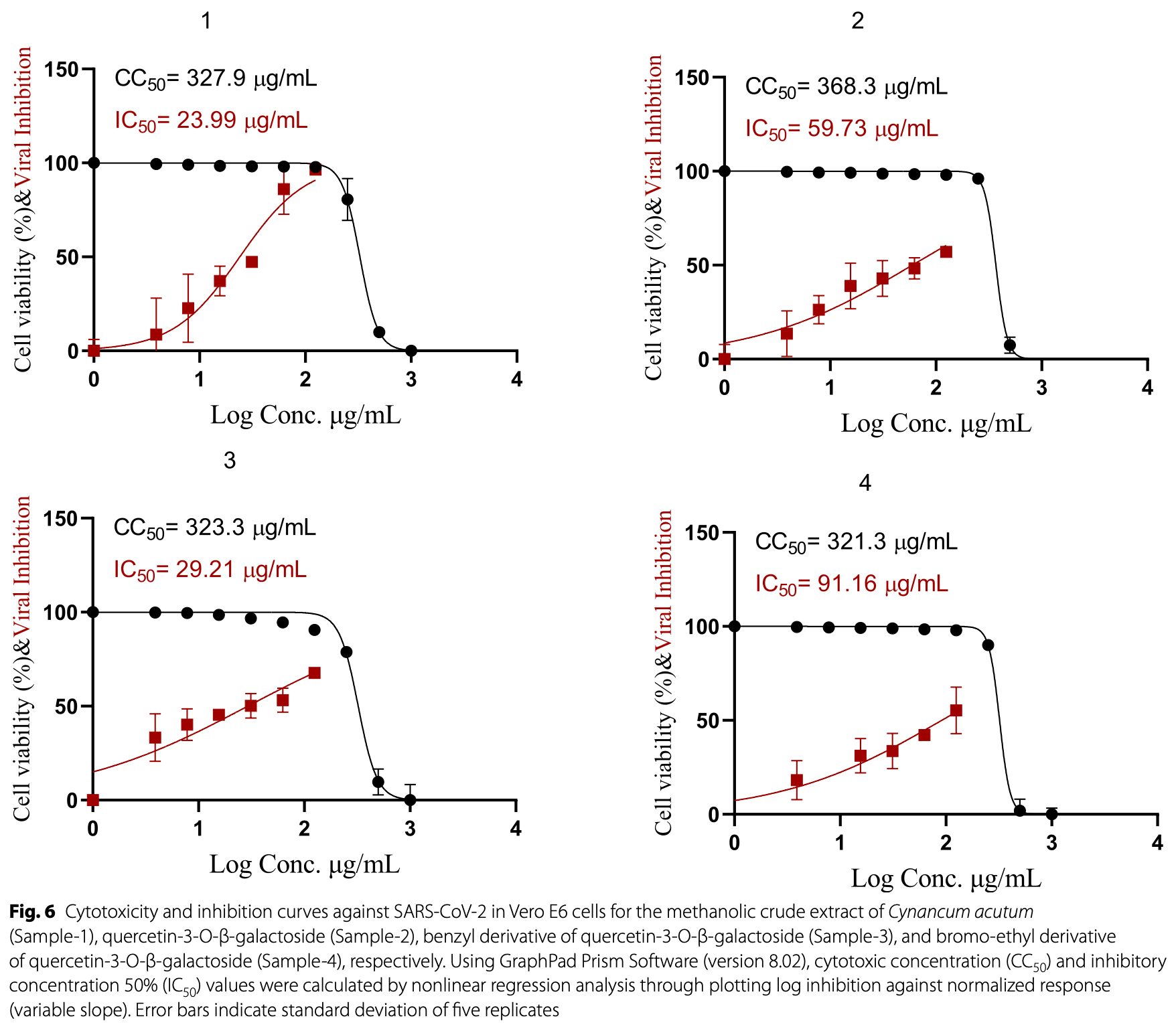
In vitro study showing that Cynanchum acutum extract and its quercetin-3-O-β-galactoside derivatives inhibit SARS-CoV-2 infection in Vero E6 cells. LC-MS/MS analysis identified 46 phytochemicals in the extract, with 20 being flavonoids including quercetin and its glycosides. Molecular docking analysis showed strong binding scores with the SARS-CoV-2 main protease (Mpro) of -7.72, -8.34, and -7.22 kcal/mol for quercetin-3-O-β-galactoside, its benzyl derivative, and bromo-ethyl derivative respectively.
Bioavailability. Quercetin has low bioavailability and studies typically use advanced formulations to improve bioavailability which may be required to reach therapeutic concentrations.
91 preclinical studies support the efficacy of quercetin for COVID-19:
In silico studies predict inhibition of SARS-CoV-2, or minimization of side effects, with quercetin or metabolites via binding to the spikeA,11,12,18,19,32,34,35,37,40,48,49,51,52,75 (and specifically the receptor binding domainB,8), MproC,7,8,11,12,16,18,20,22,24,26,28,30,33,34,37,40,44,46-48,52-55,72 , RNA-dependent RNA polymeraseD,8,10-12,18,42 , PLproE,12,47,55 , ACE2F,27,32,33,37,38,47,51 , TMPRSS2G,32, nucleocapsidH,12, helicaseI,12,39,44 , endoribonucleaseJ,49, NSP16/10K,15, cathepsin LL,36, Wnt-3M,32, FZDN,32, LRP6O,32, ezrinP,50, ADRPQ,48, NRP1R,51, EP300S,25, PTGS2T,33, HSP90AA1U,25,33 , matrix metalloproteinase 9V,41, IL-6W,31,45 , IL-10X,31, VEGFAY,45, and RELAZ,45 proteins, and inhibition of spike-ACE2 interactionAA,9.
In vitro studies demonstrate inhibition of the MproC,24,58,63,71 protein, and inhibition of spike-ACE2 interactionAA,59.
In vitro studies demonstrate efficacy in Calu-3AB,62, A549AC,31, HEK293-ACE2+AD,70, Huh-7AE,35, Caco-2AF,61, Vero E6AG,29,52,61 , mTECAH,64, RAW264.7AI,64, and HLMECAJ,9 cells.
Animal studies demonstrate efficacy in K18-hACE2 miceAK,67, db/db miceAL,64,74 , BALB/c miceAM,73, and rats29.
Quercetin reduced proinflammatory cytokines and protected lung and kidney tissue against LPS-induced damage in mice73, inhibits LPS-induced cytokine storm by modulating key inflammatory and antioxidant pathways in macrophages14, may block ACE2-spike interaction and NLRP3 inflammasome, limiting viral entry and inflammation5, upregulates the SIRT1/AMPK axis to inhibit oxidative injury and accelerate viral clearance76, inhibits SARS-CoV-2 ORF3a ion channel activity, which contributes to viral pathogenicity and cytotoxicity66, may alleviate COVID-19 ARDS via inhibition of EGFR and JAK2 inflammatory targets1, and may destabilize the Spike protein, IL-6R, and integrins via conserved residues, blocking viral entry, hyperinflammation, and platelet aggregation77.
1.
Gupta et al., Harnessing phytoconstituents to treat COVID-19 triggered acute respiratory distress syndrome: Insights from network pharmacology, and molecular modeling, Phytochemistry Letters, doi:10.1016/j.phytol.2025.104105.
2.
Sun et al., Feasibility of the inhibitor development for SARS-CoV-2: a systematic approach for drug design, Journal of Molecular Modeling, doi:10.1007/s00894-025-06541-2.
3.
Torabfam et al., Improving quercetin solubility via structural modification enhances dual-target coronavirus entry: an integrated in-vitro and in-silico study, Scientific Reports, doi:10.1038/s41598-025-27374-2.
4.
Abdelhameed et al., Phytochemical and antiviral investigation of Cynanchum acutum L. extract and derived semi-synthetic analogs targeting SARS-CoV-2 main protease, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-025-00907-2.
5.
Manikyam et al., INP-Guided Network Pharmacology Discloses Multi-Target Therapeutic Strategy Against Cytokine and IgE Storms in the SARS-CoV-2 NB.1.8.1 Variant, Research Square, doi:10.21203/rs.3.rs-6819274/v1.
6.
Makoana et al., Integration of metabolomics and chemometrics with in-silico and in-vitro approaches to unravel SARS-Cov-2 inhibitors from South African plants, PLOS ONE, doi:10.1371/journal.pone.0320415.
7.
Bano et al., Biochemical Screening of Phytochemicals and Identification of Scopoletin as a Potential Inhibitor of SARS-CoV-2 Mpro, Revealing Its Biophysical Impact on Structural Stability, Viruses, doi:10.3390/v17030402.
8.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
9.
Moharram et al., Secondary metabolites of Alternaria alternate appraisal of their SARS-CoV-2 inhibitory and anti-inflammatory potentials, PLOS ONE, doi:10.1371/journal.pone.0313616.
10.
Metwaly et al., Integrated study of Quercetin as a potent SARS-CoV-2 RdRp inhibitor: Binding interactions, MD simulations, and In vitro assays, PLOS ONE, doi:10.1371/journal.pone.0312866.
11.
Al balawi et al., Assessing multi-target antiviral and antioxidant activities of natural compounds against SARS-CoV-2: an integrated in vitro and in silico study, Bioresources and Bioprocessing, doi:10.1186/s40643-024-00822-z.
12.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
13.
Pan et al., Decoding the mechanism of Qingjie formula in the prevention of COVID-19 based on network pharmacology and molecular docking, Heliyon, doi:10.1016/j.heliyon.2024.e39167.
14.
Xu et al., Quercetin inhibited LPS-induced cytokine storm by interacting with the AKT1-FoxO1 and Keap1-Nrf2 signaling pathway in macrophages, Scientific Reports, doi:10.1038/s41598-024-71569-y.
15.
Tamil Selvan et al., Computational Investigations to Identify Potent Natural Flavonoid Inhibitors of the Nonstructural Protein (NSP) 16/10 Complex Against Coronavirus, Cureus, doi:10.7759/cureus.68098.
16.
Sunita et al., Characterization of Phytochemical Inhibitors of the COVID-19 Primary Protease Using Molecular Modelling Approach, Asian Journal of Microbiology and Biotechnology, doi:10.56557/ajmab/2024/v9i28800.
17.
Wu et al., Biomarkers Prediction and Immune Landscape in Covid-19 and “Brain Fog”, Elsevier BV, doi:10.2139/ssrn.4897774.
18.
Raman et al., Phytoconstituents of Citrus limon (Lemon) as Potential Inhibitors Against Multi Targets of SARS‐CoV‐2 by Use of Molecular Modelling and In Vitro Determination Approaches, ChemistryOpen, doi:10.1002/open.202300198.
19.
Asad et al., Exploring the antiviral activity of Adhatoda beddomei bioactive compounds in interaction with coronavirus spike protein, Archives of Medical Reports, 1:1, archmedrep.com/index.php/amr/article/view/3.
20.
Irfan et al., Phytoconstituents of Artemisia Annua as potential inhibitors of SARS CoV2 main protease: an in silico study, BMC Infectious Diseases, doi:10.1186/s12879-024-09387-w.
21.
Yuan et al., Network pharmacology and molecular docking reveal the mechanisms of action of Panax notoginseng against post-COVID-19 thromboembolism, Review of Clinical Pharmacology and Pharmacokinetics - International Edition, doi:10.61873/DTFA3974.
22.
Nalban et al., Targeting COVID-19 (SARS-CoV-2) main protease through phytochemicals of Albizia lebbeck: molecular docking, molecular dynamics simulation, MM–PBSA free energy calculations, and DFT analysis, Journal of Proteins and Proteomics, doi:10.1007/s42485-024-00136-w.
23.
Zhou et al., Bioinformatics and system biology approaches to determine the connection of SARS-CoV-2 infection and intrahepatic cholangiocarcinoma, PLOS ONE, doi:10.1371/journal.pone.0300441.
24.
Waqas et al., Discovery of Novel Natural Inhibitors Against SARS-CoV-2 Main Protease: A Rational Approach to Antiviral Therapeutics, Current Medicinal Chemistry, doi:10.2174/0109298673292839240329081008.
25.
Hasanah et al., Decoding the therapeutic potential of empon-empon: a bioinformatics expedition unraveling mechanisms against COVID-19 and atherosclerosis, International Journal of Applied Pharmaceutics, doi:10.22159/ijap.2024v16i2.50128.
26.
Shaik et al., Computational identification of selected bioactive compounds from Cedrus deodara as inhibitors against SARS-CoV-2 main protease: a pharmacoinformatics study, Indian Drugs, doi:10.53879/id.61.02.13859.
27.
Wang et al., Investigating the Mechanism of Qu Du Qiang Fei 1 Hao Fang Formula against Coronavirus Disease 2019 Based on Network Pharmacology Method, World Journal of Traditional Chinese Medicine, doi:10.4103/2311-8571.395061.
28.
Singh et al., Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 M Pro protein - An in-silico and cell-based approach, Research Square, doi:10.21203/rs.3.rs-3888947/v1.
29.
El-Megharbel et al., Chemical and spectroscopic characterization of (Artemisinin/Quercetin/ Zinc) novel mixed ligand complex with assessment of its potent high antiviral activity against SARS-CoV-2 and antioxidant capacity against toxicity induced by acrylamide in male rats, PeerJ, doi:10.7717/peerj.15638.
30.
Akinwumi et al., Evaluation of therapeutic potentials of some bioactive compounds in selected African plants targeting main protease (Mpro) in SARS-CoV-2: a molecular docking study, Egyptian Journal of Medical Human Genetics, doi:10.1186/s43042-023-00456-4.
31.
Yang et al., Active ingredient and mechanistic analysis of traditional Chinese medicine formulas for the prevention and treatment of COVID-19: Insights from bioinformatics and in vitro experiments, Medicine, doi:10.1097/MD.0000000000036238.
32.
Chandran et al., Molecular docking analysis of quercetin with known CoVid-19 targets, Bioinformation, doi:10.6026/973206300191081.
33.
Qin et al., Exploring the bioactive compounds of Feiduqing formula for the prevention and management of COVID-19 through network pharmacology and molecular docking, Medical Data Mining, doi:10.53388/MDM202407003.
34.
Moschovou et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, International Journal of Molecular Sciences, doi:10.3390/ijms242115894.
35.
Pan (B) et al., Quercetin: A promising drug candidate against the potential SARS-CoV-2-Spike mutants with high viral infectivity, Computational and Structural Biotechnology Journal, doi:10.1016/j.csbj.2023.10.029.
36.
Ahmed et al., Evaluation of the Effect of Zinc, Quercetin, Bromelain and Vitamin C on COVID-19 Patients, International Journal of Diabetes Management, doi:10.61797/ijdm.v2i2.259.
37.
Thapa et al., In-silico Approach for Predicting the Inhibitory Effect of Home Remedies on Severe Acute Respiratory Syndrome Coronavirus-2, Makara Journal of Science, doi:10.7454/mss.v27i3.1609.
38.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
39.
Singh (B) et al., Flavonoids as Potent Inhibitor of SARS-CoV-2 Nsp13 Helicase: Grid Based Docking Approach, Middle East Research Journal of Pharmaceutical Sciences, doi:10.36348/merjps.2023.v03i04.001.
40.
Mandal et al., In silico anti-viral assessment of phytoconstituents in a traditional (Siddha Medicine) polyherbal formulation – Targeting Mpro and pan-coronavirus post-fusion Spike protein, Journal of Traditional and Complementary Medicine, doi:10.1016/j.jtcme.2023.07.004.
41.
Sai Ramesh et al., Computational analysis of the phytocompounds of Mimusops elengi against spike protein of SARS CoV2 – An Insilico model, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2023.125553.
42.
Corbo et al., Inhibitory potential of phytochemicals on five SARS-CoV-2 proteins: in silico evaluation of endemic plants of Bosnia and Herzegovina, Biotechnology & Biotechnological Equipment, doi:10.1080/13102818.2023.2222196.
43.
Azmi et al., Utilization of quercetin flavonoid compounds in onion (Allium cepa L.) as an inhibitor of SARS-CoV-2 spike protein against ACE2 receptors, 11th International Seminar on New Paradigm and Innovation on Natural Sciences and its Application, doi:10.1063/5.0140285.
44.
Alanzi et al., Structure-based virtual identification of natural inhibitors of SARS-CoV-2 and its Delta and Omicron variant proteins, Future Virology, doi:10.2217/fvl-2022-0184.
45.
Yang (B) et al., In silico evidence implicating novel mechanisms of Prunella vulgaris L. as a potential botanical drug against COVID-19-associated acute kidney injury, Frontiers in Pharmacology, doi:10.3389/fphar.2023.1188086.
46.
Wang (B) et al., Computational Analysis of Lianhua Qingwen as an Adjuvant Treatment in Patients with COVID-19, Society of Toxicology Conference, 2023, www.researchgate.net/publication/370491709_Y_Wang_A_E_Tan_O_Chew_A_Hsueh_and_D_E_Johnson_2023_Computational_Analysis_of_Lianhua_Qingwen_as_an_Adjuvant_Treatment_in_Patients_with_COVID-19_Toxicologist_1921_507.
47.
Ibeh et al., Computational studies of potential antiviral compounds from some selected Nigerian medicinal plants against SARS-CoV-2 proteins, Informatics in Medicine Unlocked, doi:10.1016/j.imu.2023.101230.
48.
Nguyen et al., The Potential of Ameliorating COVID-19 and Sequelae From Andrographis paniculata via Bioinformatics, Bioinformatics and Biology Insights, doi:10.1177/11779322221149622.
49.
Alavi et al., Interaction of Epigallocatechin Gallate and Quercetin with Spike Glycoprotein (S-Glycoprotein) of SARS-CoV-2: In Silico Study, Biomedicines, doi:10.3390/biomedicines10123074.
50.
Chellasamy et al., Docking and molecular dynamics studies of human ezrin protein with a modelled SARS-CoV-2 endodomain and their interaction with potential invasion inhibitors, Journal of King Saud University - Science, doi:10.1016/j.jksus.2022.102277.
51.
Şimşek et al., In silico identification of SARS-CoV-2 cell entry inhibitors from selected natural antivirals, Journal of Molecular Graphics and Modelling, doi:10.1016/j.jmgm.2021.108038.
52.
Kandeil et al., Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2, Pathogens, doi:10.3390/pathogens10060758.
53.
Rehman et al., Natural Compounds as Inhibitors of SARS-CoV-2 Main Protease (3CLpro): A Molecular Docking and Simulation Approach to Combat COVID-19, Current Pharmaceutical Design, doi:10.2174/1381612826999201116195851.
54.
Sekiou et al., In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2, ChemRxiv, doi:10.26434/chemrxiv.12181404.v1.
55.
Zhang et al., In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus, Journal of Integrative Medicine, doi:10.1016/j.joim.2020.02.005.
56.
Sisti et al., Evaluation of respiratory virus transmissibility and resilience from fomites: the case of 11 SARS-CoV-2 clinical isolates, Applied and Environmental Microbiology, doi:10.1128/aem.00774-25.
57.
Spinelli et al., Amphibian‐Derived Peptides as Natural Inhibitors of SARS‐CoV‐2 Main Protease (Mpro): A Combined In Vitro and In Silico Approach, Chemistry & Biodiversity, doi:10.1002/cbdv.202403202.
58.
Aguilera-Rodriguez et al., Inhibition of SARS-CoV-2 3CLpro by chemically modified tyrosinase from Agaricus bisporus, RSC Medicinal Chemistry, doi:10.1039/D4MD00289J.
59.
Emam et al., Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors, AMB Express, doi:10.1186/s13568-024-01739-8.
60.
Fang et al., Development of nanoparticles incorporated with quercetin and ACE2-membrane as a novel therapy for COVID-19, Journal of Nanobiotechnology, doi:10.1186/s12951-024-02435-2.
61.
Roy et al., Quercetin inhibits SARS-CoV-2 infection and prevents syncytium formation by cells co-expressing the viral spike protein and human ACE2, Virology Journal, doi:10.1186/s12985-024-02299-w.
62.
DiGuilio et al., Quercetin improves and protects Calu-3 airway epithelial barrier function, Frontiers in Cell and Developmental Biology, doi:10.3389/fcell.2023.1271201.
63.
Zhang (B) et al., Discovery of the covalent SARS‐CoV‐2 Mpro inhibitors from antiviral herbs via integrating target‐based high‐throughput screening and chemoproteomic approaches, Journal of Medical Virology, doi:10.1002/jmv.29208.
64.
Wu (B) et al., SARS-CoV-2 N protein induced acute kidney injury in diabetic db/db mice is associated with a Mincle-dependent M1 macrophage activation, Frontiers in Immunology, doi:10.3389/fimmu.2023.1264447.
65.
Xu (B) et al., Bioactive compounds from Huashi Baidu decoction possess both antiviral and anti-inflammatory effects against COVID-19, Proceedings of the National Academy of Sciences, doi:10.1073/pnas.2301775120.
66.
Fam et al., Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites, Scientific Reports, doi:10.1038/s41598-023-31764-9.
67.
Aguado et al., Senolytic therapy alleviates physiological human brain aging and COVID-19 neuropathology, bioRxiv, doi:10.1101/2023.01.17.524329.
68.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
69.
Munafò et al., Quercetin and Luteolin Are Single-digit Micromolar Inhibitors of the SARS-CoV-2 RNA-dependent RNA Polymerase, Research Square, doi:10.21203/rs.3.rs-1149846/v1.
70.
Singh (C) et al., The spike protein of SARS-CoV-2 virus induces heme oxygenase-1: Pathophysiologic implications, Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, doi:10.1016/j.bbadis.2021.166322.
71.
Bahun et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594.
72.
Abian et al., Structural stability of SARS-CoV-2 3CLpro and identification of quercetin as an inhibitor by experimental screening, International Journal of Biological Macromolecules, doi:10.1016/j.ijbiomac.2020.07.235.
73.
Shaker et al., Anti-cytokine Storm Activity of Fraxin, Quercetin, and their Combination on Lipopolysaccharide-Induced Cytokine Storm in Mice: Implications in COVID-19, Iranian Journal of Medical Sciences, doi:10.30476/ijms.2023.98947.3102.
74.
Wu (C) et al., Treatment with Quercetin inhibits SARS-CoV-2 N protein-induced acute kidney injury by blocking Smad3-dependent G1 cell cycle arrest, Molecular Therapy, doi:10.1016/j.ymthe.2022.12.002.
75.
Azmi (B) et al., The role of vitamin D receptor and IL‐6 in COVID‐19, Molecular Genetics & Genomic Medicine, doi:10.1002/mgg3.2172.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The receptor binding domain is a specific region of the spike protein that binds ACE2 and is a major target of neutralizing antibodies. Focusing on the precise binding site allows highly specific disruption of viral attachment with reduced potential for off-target effects.
c.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
d.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
e.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
f.
The angiotensin converting enzyme 2 (ACE2) protein is a host cell transmembrane protein that serves as the cellular receptor for the SARS-CoV-2 spike protein. ACE2 is expressed on many cell types, including epithelial cells in the lungs, and allows the virus to enter and infect host cells. Inhibition may affect ACE2's physiological function in blood pressure control.
g.
Transmembrane protease serine 2 (TMPRSS2) is a host cell protease that primes the spike protein, facilitating cellular entry. TMPRSS2 activity helps enable cleavage of the spike protein required for membrane fusion and virus entry. Inhibition may especially protect respiratory epithelial cells, buy may have physiological effects.
h.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
i.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
j.
The endoribonuclease, also known as NendoU or nsp15, cleaves specific sequences in viral RNA which may help the virus evade detection by the host immune system. Inhibition may hinder the virus's ability to mask itself from the immune system, facilitating a stronger immune response.
k.
The NSP16/10 complex consists of non-structural proteins 16 and 10, forming a 2'-O-methyltransferase that modifies the viral RNA cap structure. This modification helps the virus evade host immune detection by mimicking host mRNA, making NSP16/10 a promising antiviral target.
l.
Cathepsin L is a host lysosomal cysteine protease that can prime the spike protein through an alternative pathway when TMPRSS2 is unavailable. Dual targeting of cathepsin L and TMPRSS2 may maximize disruption of alternative pathways for virus entry.
m.
Wingless-related integration site (Wnt) ligand 3 is a host signaling molecule that activates the Wnt signaling pathway, which is important in development, cell growth, and tissue repair. Some studies suggest that SARS-CoV-2 infection may interfere with the Wnt signaling pathway, and that Wnt3a is involved in SARS-CoV-2 entry.
n.
The frizzled (FZD) receptor is a host transmembrane receptor that binds Wnt ligands, initiating the Wnt signaling cascade. FZD serves as a co-receptor, along with ACE2, in some proposed mechanisms of SARS-CoV-2 infection. The virus may take advantage of this pathway as an alternative entry route.
o.
Low-density lipoprotein receptor-related protein 6 is a cell surface co-receptor essential for Wnt signaling. LRP6 acts in tandem with FZD for signal transduction and has been discussed as a potential co-receptor for SARS-CoV-2 entry.
p.
The ezrin protein links the cell membrane to the cytoskeleton (the cell's internal support structure) and plays a role in cell shape, movement, adhesion, and signaling. Drugs that occupy the same spot on ezrin where the viral spike protein would bind may hindering viral attachment, and drug binding could further stabilize ezrin, strengthening its potential natural capacity to impede viral fusion and entry.
q.
The Adipocyte Differentiation-Related Protein (ADRP, also known as Perilipin 2 or PLIN2) is a lipid droplet protein regulating the storage and breakdown of fats in cells. SARS-CoV-2 may hijack the lipid handling machinery of host cells and ADRP may play a role in this process. Disrupting ADRP's interaction with the virus may hinder the virus's ability to use lipids for replication and assembly.
r.
Neuropilin-1 (NRP1) is a cell surface receptor with roles in blood vessel development, nerve cell guidance, and immune responses. NRP1 may function as a co-receptor for SARS-CoV-2, facilitating viral entry into cells. Blocking NRP1 may disrupt an alternative route of viral entry.
s.
EP300 (E1A Binding Protein P300) is a transcriptional coactivator involved in several cellular processes, including growth, differentiation, and apoptosis, through its acetyltransferase activity that modifies histones and non-histone proteins. EP300 facilitates viral entry into cells and upregulates inflammatory cytokine production.
t.
Prostaglandin G/H synthase 2 (PTGS2, also known as COX-2) is an enzyme crucial for the production of inflammatory molecules called prostaglandins. PTGS2 plays a role in the inflammatory response that can become severe in COVID-19 and inhibitors (like some NSAIDs) may have benefits in dampening harmful inflammation, but note that prostaglandins have diverse physiological functions.
u.
Heat Shock Protein 90 Alpha Family Class A Member 1 (HSP90AA1) is a chaperone protein that helps other proteins fold correctly and maintains their stability. HSP90AA1 plays roles in cell signaling, survival, and immune responses. HSP90AA1 may interact with numerous viral proteins, but note that it has diverse physiological functions.
v.
Matrix metalloproteinase 9 (MMP9), also called gelatinase B, is a zinc-dependent enzyme that breaks down collagen and other components of the extracellular matrix. MMP9 levels increase in severe COVID-19. Overactive MMP9 can damage lung tissue and worsen inflammation. Inhibition of MMP9 may prevent excessive tissue damage and help regulate the inflammatory response.
w.
The interleukin-6 (IL-6) pro-inflammatory cytokine (signaling molecule) has a complex role in the immune response and may trigger and perpetuate inflammation. Elevated IL-6 levels are associated with severe COVID-19 cases and cytokine storm. Anti-IL-6 therapies may be beneficial in reducing excessive inflammation in severe COVID-19 cases.
x.
The interleukin-10 (IL-10) anti-inflammatory cytokine helps regulate and dampen immune responses, preventing excessive inflammation. IL-10 levels can also be elevated in severe COVID-19. IL-10 could either help control harmful inflammation or potentially contribute to immune suppression.
y.
Vascular Endothelial Growth Factor A (VEGFA) promotes the growth of new blood vessels (angiogenesis) and has roles in inflammation and immune responses. VEGFA may contribute to blood vessel leakiness and excessive inflammation associated with severe COVID-19.
z.
RELA is a transcription factor subunit of NF-kB and is a key regulator of inflammation, driving pro-inflammatory gene expression. SARS-CoV-2 may hijack and modulate NF-kB pathways.
aa.
The interaction between the SARS-CoV-2 spike protein and the human ACE2 receptor is a primary method of viral entry, inhibiting this interaction can prevent the virus from attaching to and entering host cells, halting infection at an early stage.
ab.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
ac.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
ad.
HEK293-ACE2+ is a human embryonic kidney cell line engineered for high ACE2 expression and SARS-CoV-2 susceptibility.
ae.
Huh-7 cells were derived from a liver tumor (hepatoma).
af.
Caco-2 cells come from a colorectal adenocarcinoma (cancer). They are valued for their ability to form a polarized cell layer with properties similar to the intestinal lining.
ag.
Vero E6 is an African green monkey kidney cell line with low/no ACE2 expression and high SARS-CoV-2 susceptibility. The cell line is easy to maintain and supports robust viral replication, however the monkey origin may not accurately represent human responses.
ah.
mTEC is a mouse tubular epithelial cell line.
ai.
RAW264.7 is a mouse macrophage cell line.
aj.
HLMEC (Human Lung Microvascular Endothelial Cells) are primary endothelial cells derived from the lung microvasculature. They are used to study endothelial function, inflammation, and viral interactions, particularly in the context of lung infections such as SARS-CoV-2. HLMEC express ACE2 and are susceptible to SARS-CoV-2 infection, making them a relevant model for studying viral entry and endothelial responses in the lung.
ak.
A mouse model expressing the human ACE2 receptor under the control of the K18 promoter.
al.
A mouse model of obesity and severe insulin resistance leading to type 2 diabetes due to a mutation in the leptin receptor gene that impairs satiety signaling.
am.
A mouse model commonly used in infectious disease and cancer research due to higher immune response and susceptibility to infection.
Abdelhameed et al., 30 Oct 2025, peer-reviewed, 14 authors.
Contact: gehan_ibrahim@pharm.suez.edu.eg, ssahmed@kau.edu.sa.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Phytochemical and antiviral investigation of Cynanchum acutum L. extract and derived semi-synthetic analogs targeting SARS-CoV-2 main protease
Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-025-00907-2
Background The genus Cynanchum, family Apocynaceae is a group of climbing vines that have long been in folk medicine used as antitussives, analgesics, anticonvulsants, expectorants, diuretics, antifebriles, and tonics.
Results Cynanchum acutum crude extract was investigated to determine its chemical composition through LC-ESI-TOF-MS/MS technique, where 46 hits were observed. Among these compounds, quercetin-3-O-β-galactoside was previously reported within the plant as a major component. This compound was isolated and purified using different chromatographic techniques, and its concentration was estimated using high-performance thin-layer chromatography (HPTLC). Two semi-synthetic derivatives were synthesized from this compound, namely 7-benzyl-and 7-bromoethyl quercetin-3-O-β-galactosides. Both analogs, which are more hydrophobic, were developed as an attempt to improve the physiochemical properties and, in turn, the pharmacokinetics of the parent compound. Our study also includes the determination of antiviral activity against COVID-19 of Cynanchum acutum crude extract along with quercetin-3-O-β-galactoside in addition to the two semi-synthesized derivatives. The antiviral assay revealed that the synthetic benzyl derivative of quercetin-3-O-β-galactoside demonstrated promising activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The potential molecular aspects of the parent and semi-synthetic analogs were highlighted through molecular modeling simulation of docking the compounds at the viral main protease (Mpro) binding pocket. In silico findings demonstrated significant affinity and residue-wise binding interactions in relation to the co-crystallized small molecule Mpro inhibitor.
Conclusion Collectively, our study adds to the current knowledge of SARS-CoV-2 pharmacotherapy by introducing drug-like small molecules with potential activity profiles.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s43094-025-00907-2 .
Additional file1 Author contributions R.
Declarations Ethics approval and consent to participate The study protocol was approved by the ethical committee of the Faculty of Pharmacy at Suez Canal University (approval number: 202010M2).
Competing interests The authors declare that they have no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Abdelhameed, Ibrahim, Elfaky, Habib, Mahamed et al., Antioxidant and anti-inflammatory activity of Cynanchum acutum L. isolated flavonoids using experimentally induced type 2 diabetes mellitus: biological and in silico investigation for nf-κb pathway/mir-146a expression modulation, Antioxidants
Abouzeid, Ibrahim, Sammour, Phytochemical, insecticidal and molluscicidal investigations of the aerial parts of Cynanchum acutum L. growing in Egypt, Bull Fac Pharm Cairo Univ
Administration, Coronavirus (COVID-19) drugs in date last updated
Agrawal, Agrawal, Blunden, Quercetin: antiviral significance and possible COVID-19 integrative considerations, Nat Prod Commun
Ahmed, Ka, Yang, Liu Pingping, Insecticidal constituents and activity of alkaloids from Cynanchum mongolicum 15. El-Demerdash A, Dawidar AM, Keshk EM, Abdel-Mogib M, Rev Latinoam Quim
Ali, Bashmil, Cottrell, Suleria, Dunshea, Lc-ms/ ms-qtof screening and identification of phenolic compounds from Australian grown herbs and their antioxidant potential, Antioxidants
Allen, Greiner, Wishart, Competitive fragmentation modeling of ESI-MS/MS spectra for putative metabolite identification, Metabolomics
Araf, Akter, Yd, Fatemi, Parvez et al., Omicron variant of SARS-CoV-2: genomics, transmissibility, and responses to current COVID-19 vaccines, J Med Virol
Awaad, Phytochemical and biological activities of Cynanchum acutum growing in Egypt, Bull Fac Pharm Cairo Univ
Bajpai, Esmay, In vitro studies in drug discovery and development: an analysis of study objectives and application of good laboratory practices (GLP), Drug Metab Rev
Bonaccorsi, Caristi, Gargiulli, Leuzzi, Flavonol glucoside profile of southern Italian red onion (Allium cepa L.), J Agric Food Chem
Boulos, Flora of Egypt VII Al Hadara Publishing Cairo, Egypt
Branch, Guidelines from the international conference on harmonisation (ICH), J Pharm Biomed Anal
Bruce, Guy, Rezzi, Ross, Quantitative measurement of betaine and free choline in plasma, cereals and cereal products by isotope dilution LC-MS/MS, J Agric Food Chem
Cai, Yin, Liu, Zhao, Characterization and identification of in vitro metabolites of (-)-epicatechin using ultra-high performance liquid chromatography-mass spectrometry, Trop J Pharm Res
Cai, Zhang, Zhu, Zhu, Wang et al., Risk of reinfection and severity with the predominant BA. 5 Omicron subvariant China, from December 2022 to, Emerg Microbes Infect
Cantos, Espin, Tomás-Barberán, Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC-DAD-MS-MS, J Agric Food Chem
Chaves, Fintelman-Rodrigues, Wang, Sacramento, Temerozo et al., Commercially available flavonols are better SARS-CoV-2 inhibitors than isoflavone and flavones, Viruses
Chen, Lai, Kao, The constituents of Cynanchum taiwanianum, J Chin Chem Soc
Cocuron, Ross, Alonso, Liquid chromatography tandem mass spectrometry quantification of 13C-labeling in sugars, Metabolites
Dabeek, Marra, Dietary quercetin and kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans, Nutrients
De Bois Maquillé, Wund, Renaudin, Gautier, Jardy et al., Determination of gluconate in nuclear waste by high-performance liquid chromatography: comparison of pulsed amperometric detection and electrospray mass spectrometry detection, J Radioanal Nuclear Chem
Devaraj, Krishna, Viswanatha, Simultaneous determination of quercetin, rutin and kaempferol in the leaf extracts of Moringa oleifera Lam. and Raphinus sativus Linn. by liquid chromatographytandem mass spectrometry. Zhong xi yi jie he, xue bao J Chin Integr Med
Dinda, Dinda, Dinda, Ghosh, Das, Anti-SARS-CoV-2, antioxidant and immunomodulatory potential of dietary flavonol quercetin: focus on molecular targets and clinical efficacy, Eur J Med Chem Rep
Eberhardt, Santos-Martins, Tillack, Forli, Autodock vina 1.2. 0: new docking methods, expanded force field, and python bindings, J Chem Inf Model
El-Meligy, Zain, Ahmed, Protective role of Cynanchum acutum L. extracts on carbon tetrachloride-induced hepatotoxicity in rat, Int J Chem Appl Biol Sci
El-Naggar, Hassan, Elkaeed, Alesawy, Aa, Design, synthesis, and SAR studies of novel 4-methoxyphenyl pyrazole and pyrimidine derivatives as potential dual tyrosine kinase inhibitors targeting both EGFR and VEGFR-2, Bioorg Chem
Eltamany, Goda, Nafie, Abu-Elsaoud, Hareeri et al., Comparative assessment of the antioxidant and anticancer activities of Plicosepalus acacia and Plicosepalus curviflorus: metabolomic profiling and in silico studies, Antioxidants, doi:10.3390/antiox11071249
Eltamany, Nafie, Khodeer, El-Tanahy, Kader et al., Rubia tinctorum root extracts: chemical profile and management of type II diabetes mellitus, RSC Adv
Engels, Gräter, Esquivel, Jiménez, Gänzle et al., Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra high-performance liquid chromatography/electrospray ionization mass spectrometry, Food Res Int
Fang, Yu, Prior, LC/MS/MS characterization of phenolic constituents in dried plums, J Agric Food Chem
Fawzy, Abdallah, Marzouk, Soliman, Sleem, Antidiabetic and antioxidant activities of major flavonoids of Cynanchum acutum L. (Asclepiadaceae) growing in Egypt, Z Naturforsch C
Fernández-Fernández, López-Martínez, Romero-González, Vidal, Flores et al., Simple LC-MS determination of citric and malic acids in fruits and vegetables, Chromatographia
Ferreira, De, Junior, Da Silva, Castro et al., Distribution of metabolites in galled and non-galled leaves of Clusia lanceolata and its antioxidant activity, Rev Bras
Fiori, Amadesi, Fanelli, Tropeano, Rugolo et al., Cellular and mitochondrial determination of low molecular mass organic acids by LC-MS/MS, J Pharm Biomed Anal
Flieger, Bandouchova, Cerny, Chudíčková, Kolarik et al., Vitamin B2 as a virulence factor in Pseudogymnoascus destructans skin infection, Sci Rep
Gammatantrawet, Nguyễn, Susawaengsup, Ramli, Tongkoom et al., Phytochemistry of medicinal herbs belongs to asclepiadaceae family for therapeutic applications: a critical review, Mol Biotechnol
Gao, Song, Claff, Woodson, Sylvester et al., Discovery and crystallographic studies of nonpeptidic piperazine derivatives as covalent SARS-CoV-2 main protease inhibitors, J Med Chem
Geenen, Guallar-Hoyas, Michopoulos, Kenna, Kolaja et al., HPLC-MS/MS methods for the quantitative analysis of 5-oxoproline (pyroglutamate) in rat plasma and hepatic cell line culture medium, J Pharm Biomed Anal
Giordano, Rapisarda, Donati, Rotilio, Development and validation of an LC-MS/MS analysis for simultaneous determination of delphinidin-3-glucoside, cyanidin-3-glucoside and cyanidin-3-(6-malonylglucoside) in human plasma and urine after blood orange juice administration, J Sep Sci
Goda, Nafie, Awad, Kader, Ibrahim et al., In vitro and in vivo studies of anti-lung cancer activity of Artemesia judaica L. crude extract combined with LC-MS/MS metabolic profiling, docking simulation and HPLC-DAD quantification, Antioxidants
Han, Zhou, Yang, Zhou, Deng et al., Ethnobotany, phytochemistry and pharmacological effects of plants in genus Cynanchum Linn. (Asclepiadaceae), Molecules
Heneidak, Grayer, Kite, Simmonds, Flavonoid glycosides from Egyptian species of the tribe Asclepiadeae (Apocynaceae, subfamily Asclepiadoideae), Biochem Syst Ecol
Hi, Kutkat, Sweelam, Eldehna, Mostafa et al., Investigating the potential anti-SARS-CoV-2 and anti-MERS-CoV activities of Yellow necklacepod among three selected medicinal plants: extraction, isolation, identification, in vitro, modes of action, and molecular docking studies, Metabolites
Higdon, Wahl, Jones, Rosen, Truelove et al., A systematic review of coronavirus disease 2019 vaccine efficacy and effectiveness against severe acute respiratory syndrome coronavirus 2 infection and disease, Open Forum Infect Dis, doi:10.1093/ofid/ofac138
Ho, Shen, -H, Chen, Chen, Lu et al., Therapeutic implications of quercetin and its derived-products in COVID-19 protection and prophylactic, Heliyon
Hu, Xiong, Zhu, Zhang, Zhang et al., The SARS-CoV-2 main protease (Mpro): structure, function, and emerging therapies for COVID-19, MedComm
Inamadugu, Damaramadugu, Mullangi, Ponneri, Simultaneous determination of niacin and its metabolites-nicotinamide, nicotinuric acid and N-methyl-2-pyridone-5-carboxamide-in human plasma by LC-MS/MS and its application to a human pharmacokinetic study, Biomed Chromatogr
Islam, Yoo, Lee, Ms, Park et al., Simultaneous quantitation of five flavonoid glycosides in herba Epimedii by high-performance liquid chromatography-tandem mass spectrometry, Phytochem Anal
Jang, Kim, Lee, Jeong, Bak et al., Characterization and quantification of flavonoid glycosides in the Prunus genus by UPLC-DAD-QTOF/MS, Saudi J Biol Sci
Kadhi, Melchini, Mithen, Saha, Development of a LC-MS/MS method for the simultaneous detection of tricarboxylic acid cycle intermediates in a range of biological matrices, J Anal Methods Chem
Kim, Oh, Park, Anti-viral effect of herbal medicine Korean traditional Cynanchum paniculatum (BGE.) kitag extracts, Afr J Tradit Complement Altern Med
Kneller, Phillips, Weiss, Pant, Zhang et al., Unusual zwitterionic catalytic site of SARS-CoV-2 main protease revealed by neutron crystallography, J Biol Chem
Konda, Iguchi, Harigaya, Takayanagi, Ogura et al., Hancokinol, a novel triterpene, from Cynanchum hancokianum, Tetrahedron Lett
Kontoyianni, Mcclellan, Sokol, Evaluation of docking performance: comparative data on docking algorithms, J Med Chem
Kronenberger, Laufer, Pillaiyar, COVID-19 therapeutics: smallmolecule drug development targeting SARS-CoV-2 main protease, Drug Discov Today
Lang, Yagar, Eggers, Hofmann, Quantitative investigation of trigonelline, nicotinic acid, and nicotinamide in foods, urine, and plasma by means of LC-MS/MS and stable isotope dilution analysis, J Agric Food Chem
Lds, Mendez, LC/ESI-MS method applied to characterization of flavonoids glycosides in B. forficata subsp. pruinosa, Quim Nova
Lin, Xu, Huang, Xu, Li et al., Qualitative and quantitative analysis of phenolic acids, flavonoids and iridoid glycosides in Yinhua Kanggan tablet by UPLC-QqQ-MS/MS, Molecules
Ling, Ren, Mallery, Ugalde, Pei et al., A rapid and sensitive LC-MS/MS method for quantification of four anthocyanins and its application in a clinical pharmacology study of a bioadhesive black raspberry gel, J Chromatogr B
Lopes-Lutz, Dettmann, Nimalaratne, Schieber, Characterization and quantification of polyphenols in Amazon grape (Pourouma cecropiifolia Martius), Molecules
Mackinnon, Craft, Analysis of betaines from marine algae using LC-MS-MS natural products from marine algae: methods and protocols
Mangiavacchi, Botwina, Menichetti, Bagnoli, Rosati et al., Seleno-functionalization of quercetin improves the non-covalent Abdelhameed et al, Future Journal of Pharmaceutical Sciences
Mazzoni, Giampieri, Suarez, Gasparrini, Mezzetti et al., Isolation of strawberry anthocyanin-rich fractions and their mechanisms of action against murine breast cancer cell lines, Food Funct
Mehanna, Hazem, Badr, Abo-Elmatty, Ms, Plicosepalus acacia extract and its major constituents, methyl gallate and quercetin, potentiate therapeutic angiogenesis in diabetic hind limb ischemia: HPTLC quantification and LC-MS/ MS metabolic profiling, Antioxidants
Mona, Quercetin-3,4'-O-di-beta-glucoside In date last updated
Montoro, Tuberoso, Perrone, Piacente, Cabras et al., Characterisation by liquid chromatography-electrospray tandem mass spectrometry of anthocyanins in extracts of Myrtus communis L. berries used for the preparation of myrtle liqueur, J Chromatogr A
Mostafa, Kandeil, Elshaier, Kutkat, Moatasim et al., FDA-approved drugs with potent in vitro antiviral activity against severe acute respiratory syndrome coronavirus 2, Pharmaceuticals
Olennikov, Chirikova, Kashchenko, Nikolaev, Kim et al., Bioactive phenolics of the genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS profile of the Siberian species and their inhibitory potential against α-amylase and α-glucosidase, Front Pharmacol
Razgonova, Zakharenko, Pikula, Manakov, Ercişli et al., LC-MS/MS Screening of phenolic compounds in wild and cultivated grapes vitis amurensis rupr, Molecules
Revelou, Kokotou, Constantinou-Kokotou, Identification of auxin metabolites in Brassicaceae by ultra-performance liquid chromatography coupled with high-resolution mass spectrometry, Molecules
Salerno, Casale, Calandruccio, Procopio, Characterization of flavonoids in Citrus bergamia (Bergamot) polyphenolic fraction by liquid chromatography-high resolution mass spectrometry (LC/HRMS), PharmaNutrition
Sayed, Basam, El-Naggar, Marzouk, El-Hawary, LC-MS/MS and GC-MS profiling as well as the antimicrobial effect of leaves of selected Yucca species introduced to Egypt, Sci Rep
Scigelova, Hornshaw, Giannakopulos, Fourier transform mass spectrometry, Molecular & Cellular Proteomics
Shahat, Shafeek, Husseiny, Claeys, Apers et al., A new flavonoid from Carrichtera annua, Nat Prod Sci
Sharma, Sharma, Gupta, Sinha, Simultaneous determination of epicatechin, syringic acid, quercetin-3-O-galactoside and quercitrin in the leaves of Rhododendron species by using a validated HPTLC method, J Food Compos Anal
Shin, Lee, Lee, Chung, Bae et al., Comparison of anthocyanin content in seed coats of black soybean [Glycine max (L.) Merr.] cultivars using liquid chromatography coupled to tandem mass spectrometry, Food Sci Biotechnol
Silvestro, Tarcomnicu, Dulea, Attili, Ciuca et al., Confirmation of diosmetin 3-O-glucuronide as major metabolite of diosmin in humans, using micro-liquid-chromatography-mass spectrometry and ion mobility mass spectrometry, Anal Bioanal Chem
Song, Zhang, Fu, Mo, Zhang et al., Screening for selective inhibitors of xanthine oxidase from Flos Chrysanthemum using ultrafiltration LC-MS combined with enzyme channel blocking, J Chromatogr B
Stöggl, Huck, Bonn, Structural elucidation of catechin and epicatechin in sorrel leaf extracts using liquid-chromatography coupled to diode array-, fluorescence-, and mass spectrometric detection, J Sep Sci
Sun, Lin, Lester, Wang, Harnly, Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/ HRMS n, J Agric Food Chem
Sánchez-Ilárduya, Sánchez-Fernández, Viloria-Bernal, López-Márquez, Berrueta et al., Mass spectrometry fragmentation pattern of coloured flavanol-anthocyanin and anthocyanin-flavanol derivatives in aged red wines of Rioja, Aust J Grape Wine Res
Sánchez-Rabaneda, Jáuregui, Lamuela-Raventós, Viladomat, Bastida et al., Qualitative analysis of phenolic compounds in apple pomace using liquid chromatography coupled to mass spectrometry in tandem mode, Rapid Commun Mass Spectrom
Tine, Yang, Renucci, Costa, Wélé et al., LC-MS/ MS analysis of flavonoid compounds from Zanthoxylum zanthoxyloides extracts and their antioxidant activities, Nat Prod Commun
Tsimogiannis, Samiotaki, Panayotou, Oreopoulou, Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/ MS, Molecules
Täckholm, Students' flora of Egypt, 2nd Cairo University
Wang, Halquist, Sweet, Simultaneous determination of gallic acid and gentisic acid in organic anion transporter expressing cells by liquid chromatography-tandem mass spectrometry, J Chromatogr B
Wang, Ng, Natural products with hypoglycemic, hypotensive, hypocholesterolemic, antiatherosclerotic and antithrombotic activities, Life Sci
Yan, Zhang, Liu, Huang, Yan et al., Secopregnane steroidal glycosides from the roots of Cynanchum atratum and their anti-TMV activity, Fitoterapia
Yang, Yang, Ding, Liu, Lou et al., The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor, Proc Natl Acad Sci U S A
Yildiz, Sen, Erenler, Demirtas, Behcet, Bioactivity-guided isolation of flavonoids from Cynanchum acutum L. subsp. sibiricum (willd.) Rech. f. and investigation of their antiproliferative activity, Nat Prod Res
Youssef, El-Swaify, Dalain, Cytotoxic activity of methanol extract of Cynanchum acutum L. seeds on human cancer cell lines, Latin Am J Pharm
Zhang, Sun, Shi, Wu, Jia et al., Four new indole alkaloids from the roots of Isatis tinctoria, Nat Prod Res
Zhao, Shen, He, Li, Mu et al., Carbohydrates from Cynanchum otophyllum, Carbohydr Res
Zhong, Robinson, Warner, Barrow, Dunshea et al., LC-ESI-QTOF-MS/MS characterization of seaweed phenolics and their antioxidant potential, Mar Drugs
Šibul, Orčić, Berežni, Anačkov, Mimica-Dukić, HPLC-MS/ MS profiling of wild-growing scentless chamomile, Acta Chromatogr
DOI record:
{
"DOI": "10.1186/s43094-025-00907-2",
"ISSN": [
"2314-7253"
],
"URL": "http://dx.doi.org/10.1186/s43094-025-00907-2",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>\n The genus\n <jats:italic>Cynanchum</jats:italic>\n , family Apocynaceae is a group of climbing vines that have long been in folk medicine used as antitussives, analgesics, anticonvulsants, expectorants, diuretics, antifebriles, and tonics.\n </jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>\n <jats:italic>Cynanchum acutum</jats:italic>\n crude extract was investigated to determine its chemical composition through LC-ESI-TOF-MS/MS technique, where 46 hits were observed. Among these compounds, quercetin-3-\n <jats:italic>O</jats:italic>\n -\n <jats:italic>β</jats:italic>\n -galactoside was previously reported within the plant as a major component. This compound was isolated and purified using different chromatographic techniques, and its concentration was estimated using high-performance thin-layer chromatography (HPTLC). Two semi-synthetic derivatives were synthesized from this compound, namely 7-benzyl- and 7-bromoethyl quercetin-3-\n <jats:italic>O</jats:italic>\n -\n <jats:italic>β</jats:italic>\n -galactosides. Both analogs, which are more hydrophobic, were developed as an attempt to improve the physiochemical properties and, in turn, the pharmacokinetics of the parent compound. Our study also includes the determination of antiviral activity against COVID-19 of\n <jats:italic>Cynanchum acutum</jats:italic>\n crude extract along with quercetin-3-\n <jats:italic>O</jats:italic>\n -\n <jats:italic>β</jats:italic>\n -galactoside in addition to the two semi-synthesized derivatives. The antiviral assay revealed that the synthetic benzyl derivative of quercetin-3-\n <jats:italic>O</jats:italic>\n -\n <jats:italic>β</jats:italic>\n -galactoside demonstrated promising activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The potential molecular aspects of the parent and semi-synthetic analogs were highlighted through molecular modeling simulation of docking the compounds at the viral main protease (Mpro) binding pocket. In silico findings demonstrated significant affinity and residue-wise binding interactions in relation to the co-crystallized small molecule Mpro inhibitor.\n </jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Collectively, our study adds to the current knowledge of SARS-CoV-2 pharmacotherapy by introducing drug-like small molecules with potential activity profiles.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Graphical abstract</jats:title>\n </jats:sec>",
"alternative-id": [
"907"
],
"article-number": "149",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "11 July 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "21 October 2025"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "30 October 2025"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The study protocol was approved by the ethical committee of the Faculty of Pharmacy at Suez Canal University (approval number: 202010M2)."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The authors declare that they have no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Abdelhameed",
"given": "Reda F. A.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ibrahim",
"given": "Ahmed K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Darwish",
"given": "Khaled M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Atta",
"given": "Asmaa M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shabayek",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Idriss",
"given": "Mona Timan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ibrahim",
"given": "Amany K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmed",
"given": "Safwat A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Badr",
"given": "Jihan M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nashawi",
"given": "Asma Ahmad",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Murshid",
"given": "Samar S. A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lashkar",
"given": "Manar O.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-3799-0581",
"affiliation": [],
"authenticated-orcid": false,
"family": "Elhady",
"given": "Sameh S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Habib",
"given": "Eman S.",
"sequence": "additional"
}
],
"container-title": "Future Journal of Pharmaceutical Sciences",
"container-title-short": "Futur J Pharm Sci",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2025,
10,
30
]
],
"date-time": "2025-10-30T08:34:18Z",
"timestamp": 1761813258000
},
"deposited": {
"date-parts": [
[
2025,
10,
30
]
],
"date-time": "2025-10-30T10:02:58Z",
"timestamp": 1761818578000
},
"funder": [
{
"DOI": "10.13039/501100004054",
"award": [
"GPIP-1625-249-2024"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100004054",
"id-type": "DOI"
}
],
"name": "King Abdulaziz University"
}
],
"indexed": {
"date-parts": [
[
2025,
10,
30
]
],
"date-time": "2025-10-30T10:04:54Z",
"timestamp": 1761818694603,
"version": "build-2065373602"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2025,
10,
30
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
30
]
],
"date-time": "2025-10-30T00:00:00Z",
"timestamp": 1761782400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
10,
30
]
],
"date-time": "2025-10-30T00:00:00Z",
"timestamp": 1761782400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s43094-025-00907-2.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s43094-025-00907-2/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s43094-025-00907-2.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2025,
10,
30
]
]
},
"published-online": {
"date-parts": [
[
2025,
10,
30
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/S0024-3205(99)00253-2",
"author": "HX Wang",
"doi-asserted-by": "publisher",
"first-page": "2663",
"journal-title": "Life Sci",
"key": "907_CR1",
"unstructured": "Wang HX, Ng TB (1999) Natural products with hypoglycemic, hypotensive, hypocholesterolemic, antiatherosclerotic and antithrombotic activities. Life Sci 65:2663–2677",
"volume": "65",
"year": "1999"
},
{
"key": "907_CR2",
"unstructured": "Täckholm V (1974) Students’ flora of Egypt, 2nd Cairo University, Egypt 888"
},
{
"key": "907_CR3",
"unstructured": "Boulos L (2000) Flora of Egypt VII Al Hadara Publishing Cairo, Egypt"
},
{
"DOI": "10.1515/znc-2008-9-1008",
"author": "GA Fawzy",
"doi-asserted-by": "publisher",
"first-page": "658",
"journal-title": "Z Naturforsch C",
"key": "907_CR4",
"unstructured": "Fawzy GA, Abdallah HM, Marzouk MSA, Soliman FM, Sleem AA (2008) Antidiabetic and antioxidant activities of major flavonoids of Cynanchum acutum L. (Asclepiadaceae) growing in Egypt. Z Naturforsch C 63:658–662",
"volume": "63",
"year": "2008"
},
{
"author": "A Youssef",
"first-page": "1997",
"journal-title": "Latin Am J Pharm",
"key": "907_CR5",
"unstructured": "Youssef A, El-Swaify Z, Al-saraireh Y, Dalain S (2018) Cytotoxic activity of methanol extract of Cynanchum acutum L. seeds on human cancer cell lines. Latin Am J Pharm 37:1997–2003",
"volume": "37",
"year": "2018"
},
{
"author": "A Awaad",
"first-page": "153",
"journal-title": "Bull Fac Pharm Cairo Univ",
"key": "907_CR6",
"unstructured": "Awaad A (2000) Phytochemical and biological activities of Cynanchum acutum growing in Egypt. Bull Fac Pharm Cairo Univ 38:153–162",
"volume": "38",
"year": "2000"
},
{
"author": "AHS AbouZeid",
"first-page": "235",
"journal-title": "Bull Fac Pharm Cairo Univ",
"key": "907_CR7",
"unstructured": "AbouZeid AHS, Ibrahim NA, Sammour EA (2001) Phytochemical, insecticidal and molluscicidal investigations of the aerial parts of Cynanchum acutum L. growing in Egypt. Bull Fac Pharm Cairo Univ 39:235–245",
"volume": "39",
"year": "2001"
},
{
"DOI": "10.4103/2348-0734.124349",
"author": "RM El-Meligy",
"doi-asserted-by": "publisher",
"journal-title": "Int J Chem Appl Biol Sci",
"key": "907_CR8",
"unstructured": "El-Meligy RM, Zain ME, Ahmed FA (2014) Protective role of Cynanchum acutum L. extracts on carbon tetrachloride-induced hepatotoxicity in rat. Int J Chem Appl Biol Sci 1:9",
"volume": "1",
"year": "2014"
},
{
"DOI": "10.1016/j.fitote.2014.03.027",
"author": "Y Yan",
"doi-asserted-by": "publisher",
"first-page": "50",
"journal-title": "Fitoterapia",
"key": "907_CR9",
"unstructured": "Yan Y, Zhang J-X, Liu K-X, Huang T, Yan C, Huang L-J et al (2014) Seco-pregnane steroidal glycosides from the roots of Cynanchum atratum and their anti-TMV activity. Fitoterapia 97:50–63",
"volume": "97",
"year": "2014"
},
{
"DOI": "10.21010/ajtcam.v14i3.21",
"author": "W Kim",
"doi-asserted-by": "publisher",
"first-page": "194",
"journal-title": "Afr J Tradit Complement Altern Med",
"key": "907_CR10",
"unstructured": "Kim W, Oh T-S, Park Y-J (2017) Anti-viral effect of herbal medicine Korean traditional Cynanchum paniculatum (BGE.) kitag extracts. Afr J Tradit Complement Altern Med 14:194–198",
"volume": "14",
"year": "2017"
},
{
"DOI": "10.1016/j.carres.2004.04.016",
"author": "Y-B Zhao",
"doi-asserted-by": "publisher",
"first-page": "1967",
"journal-title": "Carbohydr Res",
"key": "907_CR11",
"unstructured": "Zhao Y-B, Shen Y-M, He H-P, Li Y-M, Mu Q-Z, Hao X-J (2004) Carbohydrates from Cynanchum otophyllum. Carbohydr Res 339:1967–1972",
"volume": "339",
"year": "2004"
},
{
"DOI": "10.1016/S0040-4039(00)98059-6",
"author": "Y Konda",
"doi-asserted-by": "publisher",
"first-page": "5315",
"journal-title": "Tetrahedron Lett",
"key": "907_CR12",
"unstructured": "Konda Y, Iguchi M, Harigaya Y, Takayanagi H, Ogura H, Li X et al (1990) Hancokinol, a novel triterpene, from Cynanchum hancokianum. Tetrahedron Lett 31:5315–5318",
"volume": "31",
"year": "1990"
},
{
"key": "907_CR13",
"unstructured": "Ahmed FH, Ka E (1990) Lupeol long-chain fatty acid esters and other lipid constituents from cynanchum actum L fam asclepiadaceae"
},
{
"DOI": "10.3390/molecules200917483",
"doi-asserted-by": "crossref",
"key": "907_CR14",
"unstructured": "Ge Yang, G. Y., Liu PingPing, L. P., Yang Rui, Y. R., Zhang Liu, Z. L., Chen HongXing, C. H., Camara, I. et al. (2015) Insecticidal constituents and activity of alkaloids from Cynanchum mongolicum"
},
{
"author": "A El-Demerdash",
"first-page": "65",
"journal-title": "Rev Latinoam Quim",
"key": "907_CR15",
"unstructured": "El-Demerdash A, Dawidar AM, Keshk EM, Abdel-Mogib M (2009) Coumarins from Cynanchum acutum. Rev Latinoam Quim 37:65–69",
"volume": "37",
"year": "2009"
},
{
"DOI": "10.1016/j.bse.2006.03.001",
"author": "S Heneidak",
"doi-asserted-by": "publisher",
"first-page": "575",
"journal-title": "Biochem Syst Ecol",
"key": "907_CR16",
"unstructured": "Heneidak S, Grayer RJ, Kite GC, Simmonds MSJ (2006) Flavonoid glycosides from Egyptian species of the tribe Asclepiadeae (Apocynaceae, subfamily Asclepiadoideae). Biochem Syst Ecol 34:575–584",
"volume": "34",
"year": "2006"
},
{
"DOI": "10.1080/22221751.2023.2292071",
"author": "J Cai",
"doi-asserted-by": "publisher",
"journal-title": "Emerg Microbes Infect",
"key": "907_CR17",
"unstructured": "Cai J, Zhang H, Zhu K, Zhu F, Wang Y, Wang S et al (2024) Risk of reinfection and severity with the predominant BA. 5 Omicron subvariant China, from December 2022 to January 2023. Emerg Microbes Infect 13:2292071",
"volume": "13",
"year": "2024"
},
{
"DOI": "10.1093/ofid/ofac138",
"author": "MM Higdon",
"doi-asserted-by": "publisher",
"journal-title": "Open Forum Infect Dis",
"key": "907_CR18",
"unstructured": "Higdon MM, Wahl B, Jones CB, Rosen JG, Truelove SA, Baidya A et al (2022) A systematic review of coronavirus disease 2019 vaccine efficacy and effectiveness against severe acute respiratory syndrome coronavirus 2 infection and disease. Open Forum Infect Dis. https://doi.org/10.1093/ofid/ofac138",
"year": "2022"
},
{
"key": "907_CR19",
"unstructured": "Administration, U. S. F. a. D. (2023) Coronavirus (COVID-19) drugs in date last updated, https://www.fda.gov/drugs/emergency-preparedness-drugs/coronavirus-covid-19-drugs,"
},
{
"DOI": "10.1002/jmv.27588",
"author": "Y Araf",
"doi-asserted-by": "publisher",
"first-page": "1825",
"journal-title": "J Med Virol",
"key": "907_CR20",
"unstructured": "Araf Y, Akter F, Tang Yd, Fatemi R, Parvez MSA, Zheng C et al (2022) Omicron variant of SARS-CoV-2: genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol 94:1825–1832",
"volume": "94",
"year": "2022"
},
{
"author": "B Dinda",
"journal-title": "Eur J Med Chem Rep",
"key": "907_CR21",
"unstructured": "Dinda B, Dinda M, Dinda S, Ghosh PS, Das SK (2024) Anti-SARS-CoV-2, antioxidant and immunomodulatory potential of dietary flavonol quercetin: focus on molecular targets and clinical efficacy. Eur J Med Chem Rep 10:100125",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.3390/antiox10111713",
"author": "RFA Abdelhameed",
"doi-asserted-by": "publisher",
"journal-title": "Antioxidants",
"key": "907_CR22",
"unstructured": "Abdelhameed RFA, Ibrahim AK, Elfaky MA, Habib ES, Mahamed MI, Mehanna ET et al (2021) Antioxidant and anti-inflammatory activity of Cynanchum acutum L. isolated flavonoids using experimentally induced type 2 diabetes mellitus: biological and in silico investigation for nf-κb pathway/mir-146a expression modulation. Antioxidants 10:1713",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1039/D0RA03442H",
"author": "EE Eltamany",
"doi-asserted-by": "publisher",
"first-page": "24159",
"journal-title": "RSC Adv",
"key": "907_CR23",
"unstructured": "Eltamany EE, Nafie MS, Khodeer DM, El-Tanahy AHH, Abdel-Kader MS, Badr JM et al (2020) Rubia tinctorum root extracts: chemical profile and management of type II diabetes mellitus. RSC Adv 10:24159–24168",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.jpba.2005.02.037",
"author": "SK Branch",
"doi-asserted-by": "publisher",
"first-page": "798",
"journal-title": "J Pharm Biomed Anal",
"key": "907_CR24",
"unstructured": "Branch SK (2005) Guidelines from the international conference on harmonisation (ICH). J Pharm Biomed Anal 38:798–805",
"volume": "38",
"year": "2005"
},
{
"DOI": "10.1016/j.jfca.2009.11.003",
"author": "N Sharma",
"doi-asserted-by": "publisher",
"first-page": "214",
"journal-title": "J Food Compos Anal",
"key": "907_CR25",
"unstructured": "Sharma N, Sharma UK, Gupta AP, Sinha AK (2010) Simultaneous determination of epicatechin, syringic acid, quercetin-3-O-galactoside and quercitrin in the leaves of Rhododendron species by using a validated HPTLC method. J Food Compos Anal 23:214–219",
"volume": "23",
"year": "2010"
},
{
"DOI": "10.3390/ph13120443",
"author": "A Mostafa",
"doi-asserted-by": "publisher",
"journal-title": "Pharmaceuticals",
"key": "907_CR26",
"unstructured": "Mostafa A, Kandeil A, Amm Elshaier Y, Kutkat O, Moatasim Y, Rashad AA et al (2020) FDA-approved drugs with potent in vitro antiviral activity against severe acute respiratory syndrome coronavirus 2. Pharmaceuticals 13:443",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1021/acs.jcim.1c00203",
"author": "J Eberhardt",
"doi-asserted-by": "publisher",
"first-page": "3891",
"journal-title": "J Chem Inf Model",
"key": "907_CR27",
"unstructured": "Eberhardt J, Santos-Martins D, Tillack AF, Forli S (2021) Autodock vina 1.2. 0: new docking methods, expanded force field, and python bindings. J Chem Inf Model 61:3891–3898",
"volume": "61",
"year": "2021"
},
{
"DOI": "10.1080/14786419.2017.1289201",
"author": "I Yildiz",
"doi-asserted-by": "publisher",
"first-page": "2629",
"journal-title": "Nat Prod Res",
"key": "907_CR28",
"unstructured": "Yildiz I, Sen O, Erenler R, Demirtas I, Behcet L (2017) Bioactivity–guided isolation of flavonoids from Cynanchum acutum L. subsp. sibiricum (willd.) Rech. f. and investigation of their antiproliferative activity. Nat Prod Res 31:2629–2633",
"volume": "31",
"year": "2017"
},
{
"DOI": "10.1002/jccs.199100067",
"author": "ZS Chen",
"doi-asserted-by": "publisher",
"first-page": "393",
"journal-title": "J Chin Chem Soc",
"key": "907_CR29",
"unstructured": "Chen ZS, Lai JS, Kao YH (1991) The constituents of Cynanchum taiwanianum. J Chin Chem Soc 38:393–396",
"volume": "38",
"year": "1991"
},
{
"DOI": "10.3390/molecules23051194",
"author": "L Han",
"doi-asserted-by": "publisher",
"journal-title": "Molecules",
"key": "907_CR30",
"unstructured": "Han L, Zhou X, Yang M, Zhou L, Deng X, Wei S et al (2018) Ethnobotany, phytochemistry and pharmacological effects of plants in genus Cynanchum Linn. (Asclepiadaceae). Molecules 23:1194",
"volume": "23",
"year": "2018"
},
{
"DOI": "10.1007/s12033-024-01122-9",
"author": "N Gammatantrawet",
"doi-asserted-by": "publisher",
"first-page": "885",
"journal-title": "Mol Biotechnol",
"key": "907_CR31",
"unstructured": "Gammatantrawet N, Nguyễn CT, Susawaengsup C, Ramli ANM, Tongkoom K, Chatsungnoen T et al (2025) Phytochemistry of medicinal herbs belongs to asclepiadaceae family for therapeutic applications: a critical review. Mol Biotechnol 67:885–909",
"volume": "67",
"year": "2025"
},
{
"author": "O Al Kadhi",
"first-page": "5391832",
"issue": "1",
"journal-title": "J Anal Methods Chem",
"key": "907_CR32",
"unstructured": "Al Kadhi O, Melchini A, Mithen R, Saha S (2017) Development of a LC-MS/MS method for the simultaneous detection of tricarboxylic acid cycle intermediates in a range of biological matrices. J Anal Methods Chem 2017(1):5391832",
"volume": "2017",
"year": "2017"
},
{
"DOI": "10.1365/s10337-010-1611-0",
"author": "R Fernández-Fernández",
"doi-asserted-by": "publisher",
"first-page": "55",
"journal-title": "Chromatographia",
"key": "907_CR33",
"unstructured": "Fernández-Fernández R, López-Martínez JC, Romero-González R, Martínez-Vidal JL, Alarcón Flores MI, Garrido Frenich A (2010) Simple LC–MS determination of citric and malic acids in fruits and vegetables. Chromatographia 72:55–62",
"volume": "72",
"year": "2010"
},
{
"DOI": "10.1016/j.jpba.2017.11.071",
"author": "J Fiori",
"doi-asserted-by": "publisher",
"first-page": "33",
"journal-title": "J Pharm Biomed Anal",
"key": "907_CR34",
"unstructured": "Fiori J, Amadesi E, Fanelli F, Tropeano CV, Rugolo M, Gotti R (2018) Cellular and mitochondrial determination of low molecular mass organic acids by LC–MS/MS. J Pharm Biomed Anal 150:33–38",
"volume": "150",
"year": "2018"
},
{
"DOI": "10.1007/s10967-015-4076-7",
"author": "L de Bois Maquillé",
"doi-asserted-by": "publisher",
"first-page": "213",
"journal-title": "J Radioanal Nuclear Chem",
"key": "907_CR35",
"unstructured": "de Bois Maquillé L, Wund P, Renaudin L, Gautier C, Jardy A, Vial J et al (2015) Determination of gluconate in nuclear waste by high-performance liquid chromatography: comparison of pulsed amperometric detection and electrospray mass spectrometry detection. J Radioanal Nuclear Chem 306:213–220",
"volume": "306",
"year": "2015"
},
{
"DOI": "10.1016/j.jpba.2011.06.001",
"author": "S Geenen",
"doi-asserted-by": "publisher",
"first-page": "655",
"journal-title": "J Pharm Biomed Anal",
"key": "907_CR36",
"unstructured": "Geenen S, Guallar-Hoyas C, Michopoulos F, Kenna JG, Kolaja KL, Westerhoff HV et al (2011) HPLC–MS/MS methods for the quantitative analysis of 5-oxoproline (pyroglutamate) in rat plasma and hepatic cell line culture medium. J Pharm Biomed Anal 56:655–663",
"volume": "56",
"year": "2011"
},
{
"DOI": "10.4314/tjpr.v16i12.24",
"author": "RJ Cai",
"doi-asserted-by": "publisher",
"first-page": "2985",
"journal-title": "Trop J Pharm Res",
"key": "907_CR37",
"unstructured": "Cai RJ, Yin XL, Liu J, Zhao GZ (2017) Characterization and identification of in vitro metabolites of (-)-epicatechin using ultra-high performance liquid chromatography-mass spectrometry. Trop J Pharm Res 16:2985–2990",
"volume": "16",
"year": "2017"
},
{
"DOI": "10.1002/jssc.200301694",
"author": "WM Stöggl",
"doi-asserted-by": "publisher",
"first-page": "524",
"journal-title": "J Sep Sci",
"key": "907_CR38",
"unstructured": "Stöggl WM, Huck CW, Bonn GK (2004) Structural elucidation of catechin and epicatechin in sorrel leaf extracts using liquid-chromatography coupled to diode array-, fluorescence-, and mass spectrometric detection. J Sep Sci 27:524–528",
"volume": "27",
"year": "2004"
},
{
"DOI": "10.1021/jf903930k",
"author": "SJ Bruce",
"doi-asserted-by": "publisher",
"first-page": "2055",
"journal-title": "J Agric Food Chem",
"key": "907_CR39",
"unstructured": "Bruce SJ, Guy PA, Rezzi S, Ross AB (2010) Quantitative measurement of betaine and free choline in plasma, cereals and cereal products by isotope dilution LC-MS/MS. J Agric Food Chem 58:2055–2061",
"volume": "58",
"year": "2010"
},
{
"DOI": "10.1007/978-1-4939-2684-8_17",
"doi-asserted-by": "crossref",
"key": "907_CR40",
"unstructured": "MacKinnon SL, Craft C (2015) Analysis of betaines from marine algae using LC-MS-MS natural products from marine algae: methods and protocols 267–275"
},
{
"DOI": "10.3390/antiox10111770",
"author": "A Ali",
"doi-asserted-by": "publisher",
"journal-title": "Antioxidants",
"key": "907_CR41",
"unstructured": "Ali A, Bashmil YM, Cottrell JJ, Suleria HAR, Dunshea FR (2021) Lc-ms/ms-qtof screening and identification of phenolic compounds from Australian grown herbs and their antioxidant potential. Antioxidants 10:1770",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.3390/metabo10010030",
"author": "J-C Cocuron",
"doi-asserted-by": "publisher",
"first-page": "30",
"issue": "1",
"journal-title": "Metabolites",
"key": "907_CR42",
"unstructured": "Cocuron J-C, Ross Z, Alonso AP (2020) Liquid chromatography tandem mass spectrometry quantification of 13C-labeling in sugars. Metabolites 10(1):30",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1021/jf802838s",
"author": "R Lang",
"doi-asserted-by": "publisher",
"first-page": "11114",
"journal-title": "J Agric Food Chem",
"key": "907_CR43",
"unstructured": "Lang R, Yagar EF, Eggers R, Hofmann T (2008) Quantitative investigation of trigonelline, nicotinic acid, and nicotinamide in foods, urine, and plasma by means of LC-MS/MS and stable isotope dilution analysis. J Agric Food Chem 56:11114–11121",
"volume": "56",
"year": "2008"
},
{
"DOI": "10.1002/bmc.1406",
"author": "JK Inamadugu",
"doi-asserted-by": "publisher",
"first-page": "1059",
"journal-title": "Biomed Chromatogr",
"key": "907_CR44",
"unstructured": "Inamadugu JK, Damaramadugu R, Mullangi R, Ponneri V (2010) Simultaneous determination of niacin and its metabolites—nicotinamide, nicotinuric acid and N-methyl-2-pyridone-5-carboxamide—in human plasma by LC-MS/MS and its application to a human pharmacokinetic study. Biomed Chromatogr 24:1059–1074",
"volume": "24",
"year": "2010"
},
{
"DOI": "10.1021/jf0201327",
"author": "N Fang",
"doi-asserted-by": "publisher",
"first-page": "3579",
"journal-title": "J Agric Food Chem",
"key": "907_CR45",
"unstructured": "Fang N, Yu S, Prior RL (2002) LC/MS/MS characterization of phenolic constituents in dried plums. J Agric Food Chem 50:3579–3585",
"volume": "50",
"year": "2002"
},
{
"DOI": "10.3390/molecules26123650",
"author": "M Razgonova",
"doi-asserted-by": "publisher",
"first-page": "3650",
"issue": "12",
"journal-title": "Molecules",
"key": "907_CR46",
"unstructured": "Razgonova M, Zakharenko A, Pikula K, Manakov Y, ErcİŞLİ S, Derbush I et al (2021) LC-MS/MS Screening of phenolic compounds in wild and cultivated grapes vitis amurensis rupr. Molecules 26(12):3650",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1021/jf401802n",
"author": "J Sun",
"doi-asserted-by": "publisher",
"first-page": "10960",
"journal-title": "J Agric Food Chem",
"key": "907_CR47",
"unstructured": "Sun J, Xiao Z, Lin L-Z, Lester GE, Wang Q, Harnly JM et al (2013) Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMS n. J Agric Food Chem 61:10960–10970",
"volume": "61",
"year": "2013"
},
{
"DOI": "10.1038/srep33200",
"author": "M Flieger",
"doi-asserted-by": "publisher",
"journal-title": "Sci Rep",
"key": "907_CR48",
"unstructured": "Flieger M, Bandouchova H, Cerny J, Chudíčková M, Kolarik M, Kovacova V et al (2016) Vitamin B2 as a virulence factor in Pseudogymnoascus destructans skin infection. Sci Rep 6:33200",
"volume": "6",
"year": "2016"
},
{
"DOI": "10.3390/molecules200712209",
"author": "Y Lin",
"doi-asserted-by": "publisher",
"first-page": "12209",
"journal-title": "Molecules",
"key": "907_CR49",
"unstructured": "Lin Y, Xu W, Huang M, Xu W, Li H, Ye M et al (2015) Qualitative and quantitative analysis of phenolic acids, flavonoids and iridoid glycosides in Yinhua Kanggan tablet by UPLC-QqQ-MS/MS. Molecules 20:12209–12228",
"volume": "20",
"year": "2015"
},
{
"DOI": "10.3390/antiox10111701",
"author": "AR Abdel-Hamed",
"doi-asserted-by": "publisher",
"journal-title": "Antioxidants",
"key": "907_CR50",
"unstructured": "Abdel-Hamed AR, Mehanna ET, Hazem RM, Badr JM, Abo-Elmatty DM, Abdel-Kader MS et al (2021) Plicosepalus acacia extract and its major constituents, methyl gallate and quercetin, potentiate therapeutic angiogenesis in diabetic hind limb ischemia: HPTLC quantification and LC-MS/MS metabolic profiling. Antioxidants 10:1701",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1038/s41598-020-74440-y",
"author": "AM El Sayed",
"doi-asserted-by": "publisher",
"journal-title": "Sci Rep",
"key": "907_CR51",
"unstructured": "El Sayed AM, Basam SM, El-Naggar E-MBA, Marzouk HS, El-Hawary S (2020) LC–MS/MS and GC–MS profiling as well as the antimicrobial effect of leaves of selected Yucca species introduced to Egypt. Sci Rep 10:17778",
"volume": "10",
"year": "2020"
},
{
"key": "907_CR52",
"unstructured": "(MoNA), M. o. N. A. (2024) Quercetin-3,4'-O-di-beta-glucoside In date last updated, https://mona.fiehnlab.ucdavis.edu/spectra/browse?query=exists(compound.metaData.name:%27InChIKey%27%20and%20compound.metaData.value:%27RPVIQWDFJPYNJM-DEFKTLOSSA-N%27),"
},
{
"author": "Y Tine",
"journal-title": "Nat Prod Commun",
"key": "907_CR53",
"unstructured": "Tine Y, Yang Y, Renucci F, Costa J, Wélé A, Paolini J (2017) LC-MS/MS analysis of flavonoid compounds from Zanthoxylum zanthoxyloides extracts and their antioxidant activities. Nat Prod Commun 12:1934578X1701201213",
"volume": "12",
"year": "2017"
},
{
"author": "AA Shahat",
"first-page": "122",
"journal-title": "Nat Prod Sci",
"key": "907_CR54",
"unstructured": "Shahat AA, Abdel-Shafeek KA, Husseiny HA, Claeys M, Apers S, Pieters L (2006) A new flavonoid from Carrichtera annua. Nat Prod Sci 12:122–124",
"volume": "12",
"year": "2006"
},
{
"DOI": "10.1016/j.phanu.2015.10.001",
"author": "R Salerno",
"doi-asserted-by": "publisher",
"first-page": "S1",
"journal-title": "PharmaNutrition",
"key": "907_CR55",
"unstructured": "Salerno R, Casale F, Calandruccio C, Procopio A (2016) Characterization of flavonoids in Citrus bergamia (Bergamot) polyphenolic fraction by liquid chromatography–high resolution mass spectrometry (LC/HRMS). PharmaNutrition 4:S1–S7",
"volume": "4",
"year": "2016"
},
{
"DOI": "10.1016/j.jchromb.2009.10.026",
"author": "Y Ling",
"doi-asserted-by": "publisher",
"first-page": "4027",
"journal-title": "J Chromatogr B",
"key": "907_CR56",
"unstructured": "Ling Y, Ren C, Mallery SR, Ugalde CM, Pei P, Saradhi UVRV et al (2009) A rapid and sensitive LC–MS/MS method for quantification of four anthocyanins and its application in a clinical pharmacology study of a bioadhesive black raspberry gel. J Chromatogr B 877:4027–4034",
"volume": "877",
"year": "2009"
},
{
"DOI": "10.1074/mcp.O111.009431",
"doi-asserted-by": "crossref",
"key": "907_CR57",
"unstructured": "Scigelova M, Hornshaw M, Giannakopulos A, and Makarov A (2011) Fourier transform mass spectrometry Molecular & Cellular Proteomics 10"
},
{
"DOI": "10.1016/j.chroma.2005.11.055",
"author": "P Montoro",
"doi-asserted-by": "publisher",
"first-page": "232",
"journal-title": "J Chromatogr A",
"key": "907_CR58",
"unstructured": "Montoro P, Tuberoso CIG, Perrone A, Piacente S, Cabras P, Pizza C (2006) Characterisation by liquid chromatography-electrospray tandem mass spectrometry of anthocyanins in extracts of Myrtus communis L. berries used for the preparation of myrtle liqueur. J Chromatogr A 1112:232–240",
"volume": "1112",
"year": "2006"
},
{
"DOI": "10.3390/md18060331",
"author": "B Zhong",
"doi-asserted-by": "publisher",
"first-page": "331",
"journal-title": "Mar Drugs",
"key": "907_CR59",
"unstructured": "Zhong B, Robinson NA, Warner RD, Barrow CJ, Dunshea FR, Suleria HAR (2020) LC-ESI-QTOF-MS/MS characterization of seaweed phenolics and their antioxidant potential. Mar Drugs 18:331",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1016/j.foodres.2011.04.003",
"author": "C Engels",
"doi-asserted-by": "publisher",
"first-page": "557",
"journal-title": "Food Res Int",
"key": "907_CR60",
"unstructured": "Engels C, Gräter D, Esquivel P, Jiménez VM, Gänzle MG, Schieber A (2012) Characterization of phenolic compounds in jocote (Spondias purpurea L.) peels by ultra high-performance liquid chromatography/electrospray ionization mass spectrometry. Food Res Int 46:557–562",
"volume": "46",
"year": "2012"
},
{
"DOI": "10.1016/j.jchromb.2014.05.001",
"author": "H-P Song",
"doi-asserted-by": "publisher",
"first-page": "56",
"journal-title": "J Chromatogr B",
"key": "907_CR61",
"unstructured": "Song H-P, Zhang H, Fu Y, Mo H-Y, Zhang M, Chen J et al (2014) Screening for selective inhibitors of xanthine oxidase from Flos Chrysanthemum using ultrafiltration LC–MS combined with enzyme channel blocking. J Chromatogr B 961:56–61",
"volume": "961",
"year": "2014"
},
{
"DOI": "10.1016/j.jchromb.2013.08.024",
"author": "L Wang",
"doi-asserted-by": "publisher",
"first-page": "91",
"journal-title": "J Chromatogr B",
"key": "907_CR62",
"unstructured": "Wang L, Halquist MS, Sweet DH (2013) Simultaneous determination of gallic acid and gentisic acid in organic anion transporter expressing cells by liquid chromatography–tandem mass spectrometry. J Chromatogr B 937:91–96",
"volume": "937",
"year": "2013"
},
{
"DOI": "10.1111/j.1755-0238.2012.00190.x",
"author": "MB Sánchez-Ilárduya",
"doi-asserted-by": "publisher",
"first-page": "203",
"journal-title": "Aust J Grape Wine Res",
"key": "907_CR63",
"unstructured": "Sánchez-Ilárduya MB, Sánchez-Fernández C, Viloria-Bernal M, López-Márquez DM, Berrueta LA, Gallo B et al (2012) Mass spectrometry fragmentation pattern of coloured flavanol-anthocyanin and anthocyanin-flavanol derivatives in aged red wines of Rioja. Aust J Grape Wine Res 18:203–214",
"volume": "18",
"year": "2012"
},
{
"DOI": "10.3390/molecules15128543",
"author": "D Lopes-Lutz",
"doi-asserted-by": "publisher",
"first-page": "8543",
"journal-title": "Molecules",
"key": "907_CR64",
"unstructured": "Lopes-Lutz D, Dettmann J, Nimalaratne C, Schieber A (2010) Characterization and quantification of polyphenols in Amazon grape (Pourouma cecropiifolia Martius). Molecules 15:8543–8552",
"volume": "15",
"year": "2010"
},
{
"DOI": "10.1039/C9FO01721F",
"author": "L Mazzoni",
"doi-asserted-by": "publisher",
"first-page": "7103",
"journal-title": "Food Funct",
"key": "907_CR65",
"unstructured": "Mazzoni L, Giampieri F, Suarez JMA, Gasparrini M, Mezzetti B, Hernandez TYF et al (2019) Isolation of strawberry anthocyanin-rich fractions and their mechanisms of action against murine breast cancer cell lines. Food Funct 10:7103–7120",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.3389/fphar.2018.00756",
"author": "DN Olennikov",
"doi-asserted-by": "publisher",
"journal-title": "Front Pharmacol",
"key": "907_CR66",
"unstructured": "Olennikov DN, Chirikova NK, Kashchenko NI, Nikolaev VM, Kim S-W, Vennos C (2018) Bioactive phenolics of the genus Artemisia (Asteraceae): HPLC-DAD-ESI-TQ-MS/MS profile of the Siberian species and their inhibitory potential against α-amylase and α-glucosidase. Front Pharmacol 9:756",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1016/j.sjbs.2016.08.001",
"author": "GH Jang",
"doi-asserted-by": "publisher",
"first-page": "1622",
"journal-title": "Saudi J Biol Sci",
"key": "907_CR67",
"unstructured": "Jang GH, Kim HW, Lee MK, Jeong SY, Bak AR, Lee DJ et al (2018) Characterization and quantification of flavonoid glycosides in the Prunus genus by UPLC-DAD-QTOF/MS. Saudi J Biol Sci 25:1622–1631",
"volume": "25",
"year": "2018"
},
{
"author": "RO Ferreira",
"first-page": "617",
"journal-title": "Rev Bras",
"key": "907_CR68",
"unstructured": "Ferreira RO, de Carvalho Junior AR, da Silva TMG, Castro RN, da Silva TMS, de Carvalho MG (2014) Distribution of metabolites in galled and non-galled leaves of Clusia lanceolata and its antioxidant activity. Rev Bras 6:617–625",
"volume": "6",
"year": "2014"
},
{
"DOI": "10.1002/rcm.1370",
"author": "F Sánchez-Rabaneda",
"doi-asserted-by": "publisher",
"first-page": "553",
"journal-title": "Rapid Commun Mass Spectrom",
"key": "907_CR69",
"unstructured": "Sánchez-Rabaneda F, Jáuregui O, Lamuela-Raventós RM, Viladomat F, Bastida J, Codina C (2004) Qualitative analysis of phenolic compounds in apple pomace using liquid chromatography coupled to mass spectrometry in tandem mode. Rapid Commun Mass Spectrom 18:553–563",
"volume": "18",
"year": "2004"
},
{
"DOI": "10.1002/pca.1018",
"author": "NM Islam",
"doi-asserted-by": "publisher",
"first-page": "71",
"journal-title": "Phytochem Anal",
"key": "907_CR70",
"unstructured": "Islam NM, Yoo HH, Lee MW, Dong Ms, Park YI, Jeong HS et al (2008) Simultaneous quantitation of five flavonoid glycosides in herba Epimedii by high-performance liquid chromatography–tandem mass spectrometry. Phytochem Anal 19:71–77",
"volume": "19",
"year": "2008"
},
{
"DOI": "10.1021/jf048152r",
"author": "P Bonaccorsi",
"doi-asserted-by": "publisher",
"first-page": "2733",
"journal-title": "J Agric Food Chem",
"key": "907_CR71",
"unstructured": "Bonaccorsi P, Caristi C, Gargiulli C, Leuzzi U (2005) Flavonol glucoside profile of southern Italian red onion (Allium cepa L.). J Agric Food Chem 53:2733–2740",
"volume": "53",
"year": "2005"
},
{
"DOI": "10.1002/jssc.200700246",
"author": "L Giordano",
"doi-asserted-by": "publisher",
"first-page": "3127",
"journal-title": "J Sep Sci",
"key": "907_CR72",
"unstructured": "Giordano L, Coletta W, Rapisarda P, Donati MB, Rotilio D (2007) Development and validation of an LC-MS/MS analysis for simultaneous determination of delphinidin-3-glucoside, cyanidin-3-glucoside and cyanidin-3-(6-malonylglucoside) in human plasma and urine after blood orange juice administration. J Sep Sci 30:3127–3136",
"volume": "30",
"year": "2007"
},
{
"DOI": "10.1021/jf0204102",
"author": "E Cantos",
"doi-asserted-by": "publisher",
"first-page": "5691",
"journal-title": "J Agric Food Chem",
"key": "907_CR73",
"unstructured": "Cantos E, Espin JC, Tomás-Barberán FA (2002) Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC− DAD− MS− MS. J Agric Food Chem 50:5691–5696",
"volume": "50",
"year": "2002"
},
{
"DOI": "10.3390/antiox11071249",
"author": "EE Eltamany",
"doi-asserted-by": "publisher",
"journal-title": "Antioxidants",
"key": "907_CR74",
"unstructured": "Eltamany EE, Goda MS, Nafie MS, Abu-Elsaoud AM, Hareeri RH, Aldurdunji MM et al (2022) Comparative assessment of the antioxidant and anticancer activities of Plicosepalus acacia and Plicosepalus curviflorus: metabolomic profiling and in silico studies. Antioxidants. https://doi.org/10.3390/antiox11071249",
"year": "2022"
},
{
"DOI": "10.3390/antiox11010017",
"author": "MS Goda",
"doi-asserted-by": "publisher",
"journal-title": "Antioxidants",
"key": "907_CR75",
"unstructured": "Goda MS, Nafie MS, Awad BM, Abdel-Kader MS, Ibrahim AK, Badr JM et al (2021) In vitro and in vivo studies of anti-lung cancer activity of Artemesia judaica L. crude extract combined with LC-MS/MS metabolic profiling, docking simulation and HPLC-DAD quantification. Antioxidants 11:17",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.3390/12030593",
"author": "D Tsimogiannis",
"doi-asserted-by": "publisher",
"first-page": "593",
"journal-title": "Molecules",
"key": "907_CR76",
"unstructured": "Tsimogiannis D, Samiotaki M, Panayotou G, Oreopoulou V (2007) Characterization of flavonoid subgroups and hydroxy substitution by HPLC-MS/MS. Molecules 12:593–606",
"volume": "12",
"year": "2007"
},
{
"author": "VC Devaraj",
"first-page": "1022",
"journal-title": "Zhong xi yi jie he xue bao J Chin Integr Med",
"key": "907_CR77",
"unstructured": "Devaraj VC, Krishna BG, Viswanatha GL (2011) Simultaneous determination of quercetin, rutin and kaempferol in the leaf extracts of Moringa oleifera Lam. and Raphinus sativus Linn. by liquid chromatography-tandem mass spectrometry. Zhong xi yi jie he xue bao J Chin Integr Med 9:1022–1030",
"volume": "9",
"year": "2011"
},
{
"author": "SC Shin",
"first-page": "1470",
"journal-title": "Food Sci Biotechnol",
"key": "907_CR78",
"unstructured": "Shin SC, Lee S-J, Lee SJ, Chung JI, Bae DW, Kim ST et al (2009) Comparison of anthocyanin content in seed coats of black soybean [Glycine max (L.) Merr.] cultivars using liquid chromatography coupled to tandem mass spectrometry. Food Sci Biotechnol 18:1470–1475",
"volume": "18",
"year": "2009"
},
{
"DOI": "10.1080/14786419.2020.1779716",
"author": "D Zhang",
"doi-asserted-by": "publisher",
"first-page": "312",
"journal-title": "Nat Prod Res",
"key": "907_CR79",
"unstructured": "Zhang D, Sun Y, Shi Y, Wu X, Jia Q, Chen K et al (2021) Four new indole alkaloids from the roots of Isatis tinctoria. Nat Prod Res 36:312–318",
"volume": "36",
"year": "2021"
},
{
"DOI": "10.3390/molecules24142615",
"author": "P-K Revelou",
"doi-asserted-by": "publisher",
"first-page": "2615",
"journal-title": "Molecules",
"key": "907_CR80",
"unstructured": "Revelou P-K, Kokotou MG, Constantinou-Kokotou V (2019) Identification of auxin metabolites in Brassicaceae by ultra-performance liquid chromatography coupled with high-resolution mass spectrometry. Molecules 24:2615",
"volume": "24",
"year": "2019"
},
{
"DOI": "10.1007/s11306-014-0676-4",
"author": "F Allen",
"doi-asserted-by": "publisher",
"first-page": "98",
"journal-title": "Metabolomics",
"key": "907_CR81",
"unstructured": "Allen F, Greiner R, Wishart D (2015) Competitive fragmentation modeling of ESI-MS/MS spectra for putative metabolite identification. Metabolomics 11:98–110",
"volume": "11",
"year": "2015"
},
{
"DOI": "10.1556/1326.2019.00546",
"author": "F Šibul",
"doi-asserted-by": "publisher",
"first-page": "86",
"journal-title": "Acta Chromatogr",
"key": "907_CR82",
"unstructured": "Šibul F, Orčić D, Berežni S, Anačkov G, Mimica-Dukić N (2020) HPLC–MS/MS profiling of wild-growing scentless chamomile. Acta Chromatogr 32:86–94",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.5935/0100-4042.20140069",
"author": "LdS Farias",
"doi-asserted-by": "publisher",
"first-page": "483",
"journal-title": "Quim Nova",
"key": "907_CR83",
"unstructured": "Farias LdS, Mendez ASL (2014) LC/ESI-MS method applied to characterization of flavonoids glycosides in B. forficata subsp. pruinosa. Quim Nova 37:483–486",
"volume": "37",
"year": "2014"
},
{
"DOI": "10.1007/s00216-013-7237-y",
"author": "L Silvestro",
"doi-asserted-by": "publisher",
"first-page": "8295",
"journal-title": "Anal Bioanal Chem",
"key": "907_CR84",
"unstructured": "Silvestro L, Tarcomnicu I, Dulea C, Attili NRBN, Ciuca V, Peru D et al (2013) Confirmation of diosmetin 3-O-glucuronide as major metabolite of diosmin in humans, using micro-liquid-chromatography–mass spectrometry and ion mobility mass spectrometry. Anal Bioanal Chem 405:8295–8310",
"volume": "405",
"year": "2013"
},
{
"DOI": "10.1016/j.heliyon.2024.e30080",
"doi-asserted-by": "crossref",
"key": "907_CR85",
"unstructured": "Ho W-Y, Shen Z-h, Chen Y, Chen T-H, Lu X, Fu Y-S (2024) Therapeutic implications of quercetin and its derived-products in COVID-19 protection and prophylactic Heliyon 10,"
},
{
"author": "PK Agrawal",
"journal-title": "Nat Prod Commun",
"key": "907_CR86",
"unstructured": "Agrawal PK, Agrawal C, Blunden G (2020) Quercetin: antiviral significance and possible COVID-19 integrative considerations. Nat Prod Commun 15:1934578X20976293",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.3390/nu11102288",
"author": "WM Dabeek",
"doi-asserted-by": "publisher",
"first-page": "2288",
"journal-title": "Nutrients",
"key": "907_CR87",
"unstructured": "Dabeek WM, Marra MV (2019) Dietary quercetin and kaempferol: bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 11:2288",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.3390/ijms22137048",
"author": "F Mangiavacchi",
"doi-asserted-by": "publisher",
"journal-title": "Int J Mol Sci",
"key": "907_CR88",
"unstructured": "Mangiavacchi F, Botwina P, Menichetti E, Bagnoli L, Rosati O, Marini F et al (2021) Seleno-functionalization of quercetin improves the non-covalent inhibition of Mpro and its antiviral activity in cells against SARS-CoV-2. Int J Mol Sci 22:7048",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.3390/v14071458",
"author": "OA Chaves",
"doi-asserted-by": "publisher",
"journal-title": "Viruses",
"key": "907_CR89",
"unstructured": "Chaves OA, Fintelman-Rodrigues N, Wang X, Sacramento CQ, Temerozo JR, Ferreira AC et al (2022) Commercially available flavonols are better SARS-CoV-2 inhibitors than isoflavone and flavones. Viruses 14:1458",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1073/pnas.1835675100",
"author": "H Yang",
"doi-asserted-by": "publisher",
"first-page": "13190",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "907_CR90",
"unstructured": "Yang H, Yang M, Ding Y, Liu Y, Lou Z, Zhou Z et al (2003) The crystal structures of severe acute respiratory syndrome virus main protease and its complex with an inhibitor. Proc Natl Acad Sci U S A 100:13190–13195",
"volume": "100",
"year": "2003"
},
{
"DOI": "10.1002/mco2.151",
"author": "Q Hu",
"doi-asserted-by": "publisher",
"journal-title": "MedComm",
"key": "907_CR91",
"unstructured": "Hu Q, Xiong Y, Zhu GH, Zhang YN, Zhang YW, Huang P et al (2022) The SARS-CoV-2 main protease (Mpro): structure, function, and emerging therapies for COVID-19. MedComm 3:e151",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1074/jbc.AC120.016154",
"author": "DW Kneller",
"doi-asserted-by": "publisher",
"first-page": "17365",
"journal-title": "J Biol Chem",
"key": "907_CR92",
"unstructured": "Kneller DW, Phillips G, Weiss KL, Pant S, Zhang Q, O’Neill HM et al (2020) Unusual zwitterionic catalytic site of SARS–CoV-2 main protease revealed by neutron crystallography. J Biol Chem 295:17365–17373",
"volume": "295",
"year": "2020"
},
{
"DOI": "10.1021/acs.jmedchem.2c01716",
"author": "S Gao",
"doi-asserted-by": "publisher",
"first-page": "16902",
"journal-title": "J Med Chem",
"key": "907_CR93",
"unstructured": "Gao S, Song L, Claff T, Woodson M, Sylvester K, Jing L et al (2022) Discovery and crystallographic studies of nonpeptidic piperazine derivatives as covalent SARS-CoV-2 main protease inhibitors. J Med Chem 65:16902–16917",
"volume": "65",
"year": "2022"
},
{
"DOI": "10.1021/jm0302997",
"author": "M Kontoyianni",
"doi-asserted-by": "publisher",
"first-page": "558",
"journal-title": "J Med Chem",
"key": "907_CR94",
"unstructured": "Kontoyianni M, McClellan LM, Sokol GS (2004) Evaluation of docking performance: comparative data on docking algorithms. J Med Chem 47:558–565",
"volume": "47",
"year": "2004"
},
{
"DOI": "10.1016/j.bioorg.2022.105770",
"author": "AM El-Naggar",
"doi-asserted-by": "publisher",
"journal-title": "Bioorg Chem",
"key": "907_CR95",
"unstructured": "El-Naggar AM, Hassan AMA, Elkaeed EB, Alesawy MS, Al-Karmalawy AA (2022) Design, synthesis, and SAR studies of novel 4-methoxyphenyl pyrazole and pyrimidine derivatives as potential dual tyrosine kinase inhibitors targeting both EGFR and VEGFR-2. Bioorg Chem 123:105770",
"volume": "123",
"year": "2022"
},
{
"DOI": "10.1016/j.drudis.2023.103579",
"author": "T Kronenberger",
"doi-asserted-by": "publisher",
"journal-title": "Drug Discov Today",
"key": "907_CR96",
"unstructured": "Kronenberger T, Laufer SA, Pillaiyar T (2023) COVID-19 therapeutics: small-molecule drug development targeting SARS-CoV-2 main protease. Drug Discov Today 28:103579",
"volume": "28",
"year": "2023"
},
{
"DOI": "10.3390/metabo12111109",
"author": "HI Abd-Alla",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "Metabolites",
"key": "907_CR97",
"unstructured": "Abd-Alla HI, Kutkat O, Sweelam H-T, Eldehna WM, Mostafa MA, Ibrahim MT, Moatasim Y, GabAllah M, Al-Karmalawy AA (2022) Investigating the potential anti-SARS-CoV-2 and anti-MERS-CoV activities of Yellow necklacepod among three selected medicinal plants: extraction, isolation, identification, in vitro, modes of action, and molecular docking studies. Metabolites 12(11):1109",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1081/DMR-120015690",
"author": "M Bajpai",
"doi-asserted-by": "publisher",
"first-page": "679",
"issue": "4",
"journal-title": "Drug Metab Rev",
"key": "907_CR98",
"unstructured": "Bajpai M, Esmay JD (2002) In vitro studies in drug discovery and development: an analysis of study objectives and application of good laboratory practices (GLP). Drug Metab Rev 34(4):679–689",
"volume": "34",
"year": "2002"
}
],
"reference-count": 98,
"references-count": 98,
"relation": {},
"resource": {
"primary": {
"URL": "https://fjps.springeropen.com/articles/10.1186/s43094-025-00907-2"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Phytochemical and antiviral investigation of Cynanchum acutum L. extract and derived semi-synthetic analogs targeting SARS-CoV-2 main protease",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "11"
}
