Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer
et al., Viruses, doi:10.3390/v14102132, Sep 2022
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
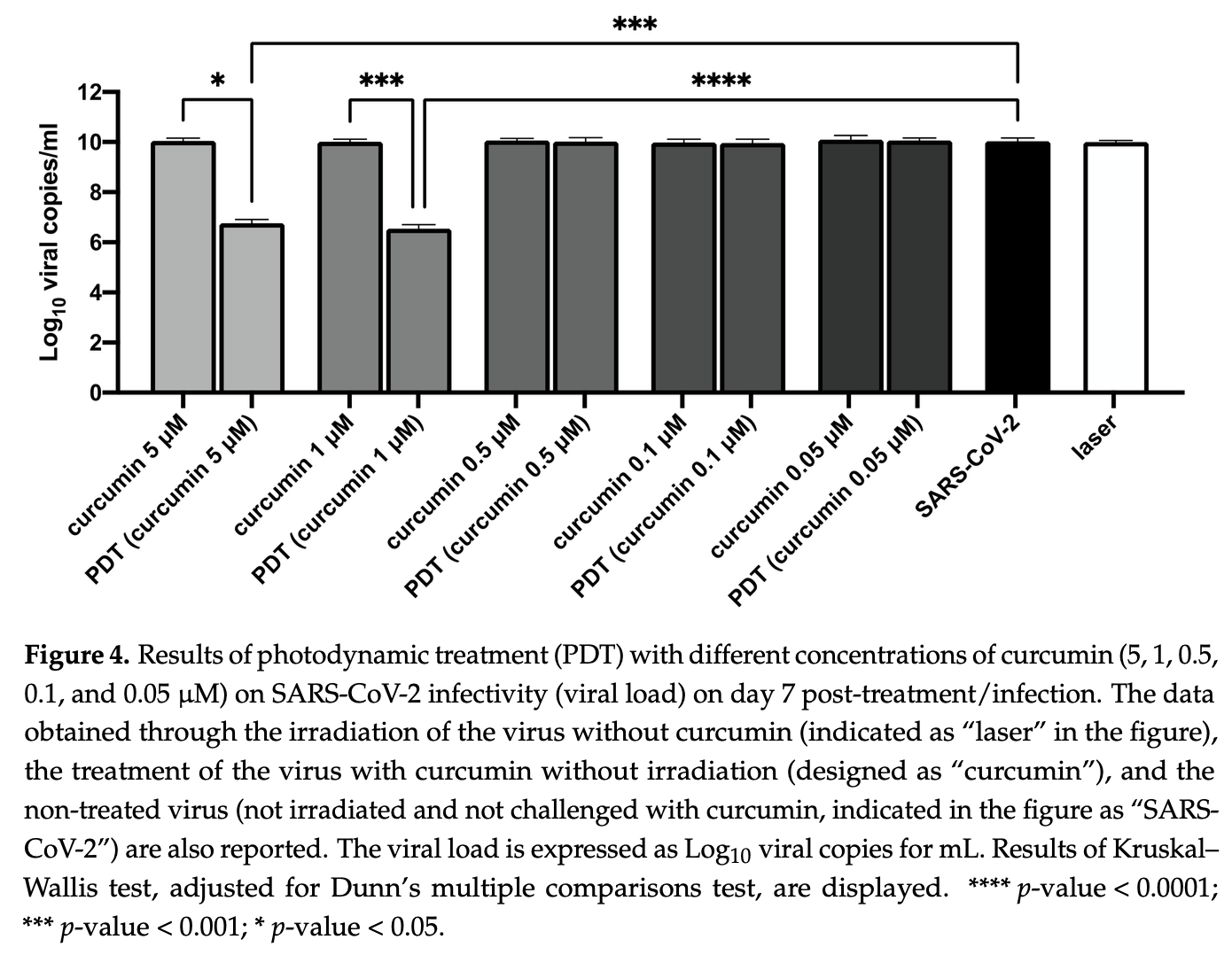
In vitro study showing that curcumin inhibits SARS-CoV-2 replication and can be used as a photosensitizer in photodynamic therapy. Authors found that 10 μM curcumin directly applied to the virus before cell inoculation inhibited SARS-CoV-2 replication by >99% in Vero E6 cells. The RNase protection assay suggested that curcumin may disrupt viral envelope integrity, allowing RNase to access viral RNA. When used at lower concentrations (5 μM and 1 μM) with photodynamic therapy using blue laser light (445 nm), curcumin also achieved >99% viral inhibition.
62 preclinical studies support the efficacy of curcumin for COVID-19:
In silico studies predict inhibition of SARS-CoV-2 with curcumin or metabolites via binding to the spikeA,1,5,6,11,16,18,24,27 (and specifically the receptor binding domainB,2,4,14,17,20 ), MproC,4-6,11,13,15-17,19,20,22,25,27,28,30,48 , RNA-dependent RNA polymeraseD,4-6,17,26 , PLproE,6, ACE2F,2,18,19,21 , nucleocapsidG,12,29 , nsp10H,29, and helicaseI,36 proteins, and inhibition of spike-ACE2 interactionJ,3.
In vitro studies demonstrate inhibition of the spikeA,41 (and specifically the receptor binding domainB,51), MproC,23,41,48,50 , ACE2F,51, and TMPRSS2K,51 proteins, and inhibition of spike-ACE2 interactionJ,3,34 .
In vitro studies demonstrate efficacy in Calu-3L,49, A549M,41, A549-ATN,31, 293TO,7, HEK293-hACE2P,23,39 , 293T/hACE2/TMPRSS2Q,40, Vero E6R,1,13,17,27,39,41,43,45,47,49 , and SH-SY5YS,38 cells.
Curcumin decreases pro-inflammatory cytokines induced by SARS-CoV-2 in peripheral blood mononuclear cells47, alleviates SARS-CoV-2 spike protein-induced mitochondrial membrane damage and oxidative stress7, may limit COVID-19 induced cardiac damage by inhibiting the NF-κB signaling pathway which mediates the profibrotic effects of the SARS-CoV-2 spike protein on cardiac fibroblasts35, is predicted to inhibit the interaction between the SARS-CoV-2 spike protein receptor binding domain and the human ACE2 receptor for the delta and omicron variants14, lowers ACE2 and STAT3, curbing lung inflammation and ARDS in preclinical COVID-19 models32, inhibits SARS-CoV-2 ORF3a ion channel activity, which contributes to viral pathogenicity and cytotoxicity42, has direct virucidal action by disrupting viral envelope integrity44, may inhibit viral replication and modulate inflammatory pathways like NF-κB via SIRT1 activation52, and can function as a photosensitizer in photodynamic therapy to generate reactive oxygen species that damage the virus44.
1.
Marzouk et al., Computational and Experimental Insights into the Antiviral Mechanism of Turmeric (Curcuma longa) against SARS-CoV-2 D614G, BIO Web of Conferences, doi:10.1051/bioconf/202519804002.
2.
Wu et al., Utilizing natural compounds as ligands to disrupt the binding of SARS-CoV-2 receptor-binding domain to angiotensin-converting enzyme 2, impeding viral infection, Phytochemistry Letters, doi:10.1016/j.phytol.2025.102999.
3.
Najimi et al., Phytochemical Inhibitors of SARS‐CoV‐2 Entry: Targeting the ACE2‐RBD Interaction with l‐Tartaric Acid, l‐Ascorbic Acid, and Curcuma longa Extract, ChemistrySelect, doi:10.1002/slct.202406035.
4.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
5.
Al balawi et al., Assessing multi-target antiviral and antioxidant activities of natural compounds against SARS-CoV-2: an integrated in vitro and in silico study, Bioresources and Bioprocessing, doi:10.1186/s40643-024-00822-z.
6.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
7.
Zhang et al., Computational Discovery of Mitochondrial Dysfunction Biomarkers in Severe SARS-CoV-2 Infection: Facilitating Pytomedicine Screening, Phytomedicine, doi:10.1016/j.phymed.2024.155784.
8.
Öztürkkan et al., In Silico investigation of the effects of curcuminoids on the spike protein of the omicron variant of SARS-CoV-2, Baku State University Journal of Chemistry and Material Sciences, 1:2, bsuj.bsu.edu.az/uploads/pdf/ec4204d62f7802de54e6092bf7860029.pdf.
9.
Yunze et al., Therapeutic effect and potential mechanism of curcumin, an active ingredient in Tongnao Decoction, on COVID-19 combined with stroke: a network pharmacology study and GEO database mining, Research Square, doi:10.21203/rs.3.rs-4329762/v1.
10.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
11.
Boseila et al., Throat spray formulated with virucidal Pharmaceutical excipients as an effective early prophylactic or treatment strategy against pharyngitis post-exposure to SARS CoV-2, European Journal of Pharmaceutics and Biopharmaceutics, doi:10.1016/j.ejpb.2024.114279.
12.
Hidayah et al., Bioinformatics study of curcumin, demethoxycurcumin, bisdemethoxycurcumin and cyclocurcumin compounds in Curcuma longa as an antiviral agent via nucleocapsid on SARS-CoV-2 inhibition, International Conference on Organic and Applied Chemistry, doi:10.1063/5.0197724.
13.
Singh et al., Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 M Pro protein - An in-silico and cell-based approach, Research Square, doi:10.21203/rs.3.rs-3888947/v1.
14.
Kant et al., Structure-based drug discovery to identify SARS-CoV2 spike protein–ACE2 interaction inhibitors, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2023.2300060.
15.
Naderi Beni et al., In silico studies of anti-oxidative and hot temperament-based phytochemicals as natural inhibitors of SARS-CoV-2 Mpro, PLOS ONE, doi:10.1371/journal.pone.0295014.
16.
Moschovou et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, International Journal of Molecular Sciences, doi:10.3390/ijms242115894.
17.
Eleraky et al., Curcumin Transferosome-Loaded Thermosensitive Intranasal in situ Gel as Prospective Antiviral Therapy for SARS-Cov-2, International Journal of Nanomedicine, doi:10.2147/IJN.S423251.
18.
Singh (B) et al., Computational studies to analyze effect of curcumin inhibition on coronavirus D614G mutated spike protein, The Seybold Report, doi:10.17605/OSF.IO/TKEXJ.
19.
Thapa et al., In-silico Approach for Predicting the Inhibitory Effect of Home Remedies on Severe Acute Respiratory Syndrome Coronavirus-2, Makara Journal of Science, doi:10.7454/mss.v27i3.1609.
20.
Srivastava et al., Paradigm of Well-Orchestrated Pharmacokinetic Properties of Curcuminoids Relative to Conventional Drugs for the Inactivation of SARS-CoV-2 Receptors: An In Silico Approach, Stresses, doi:10.3390/stresses3030043.
21.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
22.
Winih Kinasih et al., Analisis in silico interaksi senyawa kurkuminoid terhadap enzim main protease 6LU7 dari SARS-CoV-2, Duta Pharma Journal, doi:10.47701/djp.v3i1.2904.
23.
Wu (B) et al., Potential Mechanism of Curcumin and Resveratrol against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-2780614/v1.
24.
Nag et al., Curcumin inhibits spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in silico study, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2022.105552.
25.
Rampogu et al., Molecular Docking and Molecular Dynamics Simulations Discover Curcumin Analogue as a Plausible Dual Inhibitor for SARS-CoV-2, International Journal of Molecular Sciences, doi:10.3390/ijms23031771.
26.
Singh (C) et al., Potential of turmeric-derived compounds against RNA-dependent RNA polymerase of SARS-CoV-2: An in-silico approach, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2021.104965.
27.
Kandeil et al., Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2, Pathogens, doi:10.3390/pathogens10060758.
28.
Rajagopal et al., Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-020-00126-x.
29.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
30.
Sekiou et al., In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2, ChemRxiv, doi:10.26434/chemrxiv.12181404.v1.
31.
Grüneberg et al., Dose-dependent antiviral effects of glycyrrhizin, curcumin, and harmaline against clinical SARS-CoV-2 isolates, including D614G, Omicron BA.5, and Omicron XBB.1, BMC Complementary Medicine and Therapies, doi:10.1186/s12906-026-05253-1.
32.
Aktay et al., Oral Administration of Water-Soluble Curcumin Complex Prevents ARDS With the Potential for COVID-19 Treatment, Phytotherapy Research, doi:10.1002/ptr.70046.
33.
Olubiyi et al., Novel dietary herbal preparations with inhibitory activities against multiple SARS-CoV-2 targets: A multidisciplinary investigation into antiviral activities, Food Chemistry Advances, doi:10.1016/j.focha.2025.100969.
34.
Emam et al., Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors, AMB Express, doi:10.1186/s13568-024-01739-8.
35.
Van Tin et al., Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling, Cells, doi:10.3390/cells13161331.
36.
Li et al., Thermal shift assay (TSA)-based drug screening strategy for rapid discovery of inhibitors against the Nsp13 helicase of SARS-CoV-2, Animals and Zoonoses, doi:10.1016/j.azn.2024.06.001.
37.
Kamble et al., Nanoparticulate curcumin spray imparts prophylactic and therapeutic properties against SARS-CoV-2, Emergent Materials, doi:10.1007/s42247-024-00754-6.
38.
Nicoliche et al., Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2, Scientific Reports, doi:10.1038/s41598-024-61662-7.
39.
Nittayananta et al., A novel film spray containing curcumin inhibits SARS-CoV-2 and influenza virus infection and enhances mucosal immunity, Virology Journal, doi:10.1186/s12985-023-02282-x.
40.
Septisetyani et al., Curcumin and turmeric extract inhibited SARS-CoV-2 pseudovirus cell entry and Spike mediated cell fusion, bioRxiv, doi:10.1101/2023.09.28.560070.
41.
Mohd Abd Razak et al., In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds, Natural Product Communications, doi:10.1177/1934578X231188861.
42.
Fam et al., Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites, Scientific Reports, doi:10.1038/s41598-023-31764-9.
43.
Teshima et al., Antiviral activity of curcumin and its analogs selected by an artificial intelligence-supported activity prediction system in SARS-CoV-2-infected VeroE6 cells, Natural Product Research, doi:10.1080/14786419.2023.2194647.
44.
Zupin et al., Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer, Viruses, doi:10.3390/v14102132.
45.
Leka et al., In vitro antiviral activity against SARS-CoV-2 of common herbal medicinal extracts and their bioactive compounds, Phytotherapy Research, doi:10.1002/ptr.7463.
46.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
47.
Marín-Palma et al., Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms, Molecules, doi:10.3390/molecules26226900.
48.
Bahun et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594.
49.
Bormann et al., Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro, Viruses, doi:10.3390/v13101914.
50.
Guijarro-Real et al., Potential In Vitro Inhibition of Selected Plant Extracts against SARS-CoV-2 Chymotripsin-Like Protease (3CLPro) Activity, Foods, doi:10.3390/foods10071503.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The receptor binding domain is a specific region of the spike protein that binds ACE2 and is a major target of neutralizing antibodies. Focusing on the precise binding site allows highly specific disruption of viral attachment with reduced potential for off-target effects.
c.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
d.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
e.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
f.
The angiotensin converting enzyme 2 (ACE2) protein is a host cell transmembrane protein that serves as the cellular receptor for the SARS-CoV-2 spike protein. ACE2 is expressed on many cell types, including epithelial cells in the lungs, and allows the virus to enter and infect host cells. Inhibition may affect ACE2's physiological function in blood pressure control.
g.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
h.
Non-structural protein 10 (nsp10) serves as an RNA chaperone and stabilizes conformations of nsp12 and nsp14 in the replicase-transcriptase complex, which synthesizes new viral RNAs. Nsp10 disruption may destabilize replicase-transcriptase complex activity.
i.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
j.
The interaction between the SARS-CoV-2 spike protein and the human ACE2 receptor is a primary method of viral entry, inhibiting this interaction can prevent the virus from attaching to and entering host cells, halting infection at an early stage.
k.
Transmembrane protease serine 2 (TMPRSS2) is a host cell protease that primes the spike protein, facilitating cellular entry. TMPRSS2 activity helps enable cleavage of the spike protein required for membrane fusion and virus entry. Inhibition may especially protect respiratory epithelial cells, buy may have physiological effects.
l.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
m.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
n.
A549-AT is a human lung carcinoma cell line stably transfected with ACE2 and TMPRSS2 receptors. Unlike the parental line, this overexpression ensures stable infection and enhanced viral entry, allowing for the evaluation of antiviral efficacy against various SARS-CoV-2 variants.
o.
293T is a human embryonic kidney cell line that can be engineered for high ACE2 expression and SARS-CoV-2 susceptibility. 293T cells are easily transfected and support high protein expression.
p.
HEK293-hACE2 is a human embryonic kidney cell line with high ACE2 expression and SARS-CoV-2 susceptibility. Cells have been transfected with a plasmid to express the human ACE2 (hACE2) protein.
q.
293T/hACE2/TMPRSS2 is a human embryonic kidney cell line engineered for high ACE2 and TMPRSS2 expression, which mimics key aspects of human infection. 293T/hACE2/TMPRSS2 cells are very susceptible to SARS-CoV-2 infection.
r.
Vero E6 is an African green monkey kidney cell line with low/no ACE2 expression and high SARS-CoV-2 susceptibility. The cell line is easy to maintain and supports robust viral replication, however the monkey origin may not accurately represent human responses.
s.
SH-SY5Y is a human neuroblastoma cell line that exhibits neuronal phenotypes. It is commonly used as an in vitro model for studying neurotoxicity, neurodegenerative diseases, and neuronal differentiation.
Zupin et al., 27 Sep 2022, Italy, peer-reviewed, 7 authors.
Contact: luisa.zupin@burlo.trieste.it (corresponding author), sgrovella@qu.edu.qa.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer
Viruses, doi:10.3390/v14102132
Curcumin, the bioactive compound of the spice Curcuma longa, has already been reported as a potential COVID-19 adjuvant treatment due to its immunomodulatory and anti-inflammatory properties. In this study, SARS-CoV-2 was challenged with curcumin; moreover, curcumin was also coupled with laser light at 445 nm in a photodynamic therapy approach. Curcumin at a concentration of 10 µM, delivered to the virus prior to inoculation on cell culture, inhibited SARS-CoV-2 replication (reduction >99%) in Vero E6 cells, possibly due to disruption of the virion structure, as observed using the RNase protection assay. However, curcumin was not effective as a prophylactic treatment on already-infected Vero E6 cells. Notably, when curcumin was employed as a photosensitizer and blue laser light at 445 nm was delivered to a mix of curcumin/virus prior to the inoculation on the cells, virus inactivation was observed (>99%) using doses of curcumin that were not antiviral by themselves. Photodynamic therapy employing crude curcumin can be suggested as an antiviral option against SARS-CoV-2 infection.
Conflicts of Interest: The authors declare no conflict of interest.
References
Adamczak, Karpi Ński, Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity, Pharmaceuticals, doi:10.3390/ph13070153
Ahmadi, Salari, Sharifi, Reihani, Rostamiani et al., Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebo-controlled clinical trial, Food Sci. Nutr, doi:10.1002/fsn3.2226
Anggakusuma; Colpitts, Schang, Rachmawati, Frentzen, Pfaender et al., Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells, Gut, doi:10.1136/gutjnl-2012-304299
Araf, Akter, Tang, Fatemi, Alam Parvez et al., Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines, J. Med. Virol, doi:10.1002/jmv.27588
Asadirad, Nashibi, Khodadadi, Ghadiri, Sadeghi et al., Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebo-controlled clinical trial, Phytotherapy Res, doi:10.1002/ptr.7375
Bormann, Alt, Schipper, Van De Sand, Le-Trilling et al., Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro, Viruses, doi:10.3390/v13101914
Chen, Chen, Wen, Ou, Chiou et al., Inhibition of Enveloped Viruses Infectivity by Curcumin, PLoS ONE, doi:10.1371/journal.pone.0062482
Crovella, De Freitas, Zupin, Fontana, Ruscio et al., Surfactin Bacterial Antiviral Lipopeptide Blocks In Vitro Replication of SARS-CoV-2, Appl. Microbiol, doi:10.3390/applmicrobiol2030052
Elad, Meidan, Sellam, Simaan, Zeevi et al., Topical curcumin for the prevention of oral mucositis in pediatric patients: Case series, Altern. Ther. Health Med
Ellerkamp, Bortel, Schmid, Kirchner, Armeanu-Ebinger et al., Photodynamic Therapy Potentiates the Effects of Cur-cumin on Pediatric Epithelial Liver Tumor Cells, Anticancer Res
Goc, Rath, Niedzwiecki, Composition of naturally occurring compounds decreases activity of Omicron and SARS-CoV-2 RdRp complex, Eur. J. Microbiol. Immunol, doi:10.1556/1886.2022.00009
Goc, Sumera, Rath, Niedzwiecki, Phenolic compounds disrupt spike-mediated receptor-binding and entry of SARS-CoV-2 pseudo-virions, PLoS ONE, doi:10.1371/journal.pone.0253489
Gopi, Jacob, Varma, Jude, Amalraj et al., Comparative Oral Absorption of Curcumin in a Natural Turmeric Matrix with Two Other Curcumin Formulations: An Open-label Parallel-arm Study: Comparative Oral Absorption of Curcumin, Phytother. Res, doi:10.1002/ptr.5931
Hassaniazad, Eftekhar, Inchehsablagh, Kamali, Tousi et al., A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumincontaining nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients, Phytotherapy Res, doi:10.1002/ptr.7294
Henderson, Canna, Friedman, Gorelik, Lapidus et al., American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 2, Arthritis Rheumatol, doi:10.1002/art.41616
Hewlings, Kalman, Curcumin: A Review of Its Effects on Human Health, Foods, doi:10.3390/foods6100092
Jennings, Parks, Curcumin as an Antiviral Agent, Viruses, doi:10.3390/v12111242
Kaenkumchorn, Kesavan, Dietary Management of Pediatric Inflammatory Bowel Disease, J. Med. Food, doi:10.1089/jmf.2019.0063
Khan, Iqtadar, Mumtaz, Heinrich, Pascual-Figal et al., Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19-Results From a Pilot Open-Label, Randomized Controlled Trial, Front. Pharmacol, doi:10.3389/fphar.2022.898062
Kishimoto, Imaizumi, Wada, Yamakage, Satoh-Asahara et al., Newly Developed Highly Bioavailable Curcumin Formulation, curcuRougeTM, Reduces Neutrophil/Lymphocyte Ratio in the Elderly: A Double-Blind, Placebo-Controlled Clinical Trial, J. Nutr. Sci. Vitaminol, doi:10.3177/jnsv.67.249
Lee, Loo, Bebawy, Luk, Mason et al., Curcumin and its Derivatives: Their Application in Neuropharmacology and Neuroscience in the 21st Century, Curr. Neuropharmacol, doi:10.2174/1570159X11311040002
Marín-Palma, Tabares-Guevara, Zapata-Cardona, Flórez-Álvarez, Yepes et al., Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms, Molecules, doi:10.3390/molecules26226900
Miserocchi, Giuffrè, Cicinelli, Marchese, Gattinara et al., Oral phospholipidic curcumin in juvenile idiopathic arthritis-associated uveitis, Eur. J. Ophthalmol, doi:10.1177/1120672119892804
Moore, N-Acetyl Cysteine and Curcumin in Pediatric Acute-Onset Neuropsychiatric Syndrome, J. Child Adolesc. Psychopharmacol, doi:10.1089/cap.2017.0165
Moustapha, Pérétout, Rainey, Sureau, Geze et al., Curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization and mitochondrial events, Cell Death Discov, doi:10.1038/cddiscovery.2015.17
Pawar, Mastud, Pawar, Pawar, Bhoite et al., Oral Curcumin with Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial, Front. Pharmacol, doi:10.3389/fphar.2021.669362
Pourhajibagher, Azimi, Haddadi-Asl, Ahmadi, Gholamzad et al., Robust antimicrobial photodynamic therapy with curcumin-poly (lactic-co-glycolic acid) nanoparticles against COVID-19: A preliminary in vitro study in Vero cell line as a model, Photodiagnosis Photodyn. Ther, doi:10.1016/j.pdpdt.2021.102286
Rupel, Zupin, Brich, Mardirossian, Ottaviani et al., Antimicrobial activity of amphiphilic nanomicelles loaded with curcumin against Pseudomonas aeruginosa alone and activated by blue laser light, J. Biophotonics, doi:10.1002/jbio.202000350
Saber-Moghaddam, Salari, Hejazi, Amini, Taherzadeh et al., Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: An open label nonrandomized clinical trial, Phytotherapy Res, doi:10.1002/ptr.7004
Sadraeian, Junior, Miranda, Galinskas, Fernandes et al., Study of Viral Photoinactivation by UV-C Light and Photosensitizer Using a Pseudotyped Model, Pharmaceutics, doi:10.3390/pharmaceutics14030683
Santini, Tenore, Novellino, Nutraceuticals: A paradigm of proactive medicine, Eur. J. Pharm. Sci, doi:10.1016/j.ejps.2016.09.003
Shafie, Taheri, Alijani, Okhovvat, Goudarzi et al., Effect of nanocurcumin supplementation on the severity of symptoms and length of hospital stay in patients with COVID-19: A randomized double-blind placebo-controlled trial, Phytotherapy Res, doi:10.1002/ptr.7374
Sharma, Prateeksha, Singh, Singh, Rao et al., Nanocurcumin Potently Inhibits SARS-CoV-2 Spike Protein-Induced Cytokine Storm by Deactivation of MAPK/NF-κB Signaling in Epithelial Cells, ACS Appl. Bio. Mater, doi:10.1021/acsabm.1c00874
Sorg, Schmid, Bortel, Fuchs, Ellerkamp, Antitumor effects of curcumin in pediatric rhabdomyosarcoma in combination with chemotherapy and phototherapy in vitro, Int. J. Oncol, doi:10.3892/ijo.2020.5155
Tahmasebi, El-Esawi, Mahmoud, Timoshin, Valizadeh et al., Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients, J. Cell. Physiol, doi:10.1002/jcp.30233
Tahmasebi, Saeed, Temirgalieva, Yumashev, El-Esawi et al., Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV2, Life Sci, doi:10.1016/j.lfs.2021.119437
Tenero, Piazza, Zanoni, Bodini, Peroni et al., Antioxidant supplementation and exhaled nitric oxide in children with asthma, Allergy Asthma Proc, doi:10.2500/aap.2016.37.3920
Trigo-Gutierrez, Vega-Chacón, Soares, De Oliveira Mima, Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review, Int. J. Mol. Sci, doi:10.3390/ijms22137130
Valizadeh, Abdolmohammadi-Vahid, Danshina, Gencer, Ammari et al., Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients, Int. Immunopharmacol, doi:10.1016/j.intimp.2020.107088
Wiehe, O'brien, Senge, Trends and targets in antiviral phototherapy, Photochem. Photobiol. Sci, doi:10.1039/C9PP00211A
Zhai, Brockmüller, Kubatka, Shakibaei, Büsselberg, Curcumin's Beneficial Effects on Neuroblastoma: Mechanisms, Challenges, and Potential Solutions, Biomolecules, doi:10.3390/biom10111469
DOI record:
{
"DOI": "10.3390/v14102132",
"ISSN": [
"1999-4915"
],
"URL": "http://dx.doi.org/10.3390/v14102132",
"abstract": "<jats:p>Curcumin, the bioactive compound of the spice Curcuma longa, has already been reported as a potential COVID-19 adjuvant treatment due to its immunomodulatory and anti-inflammatory properties. In this study, SARS-CoV-2 was challenged with curcumin; moreover, curcumin was also coupled with laser light at 445 nm in a photodynamic therapy approach. Curcumin at a concentration of 10 μM, delivered to the virus prior to inoculation on cell culture, inhibited SARS-CoV-2 replication (reduction >99%) in Vero E6 cells, possibly due to disruption of the virion structure, as observed using the RNase protection assay. However, curcumin was not effective as a prophylactic treatment on already-infected Vero E6 cells. Notably, when curcumin was employed as a photosensitizer and blue laser light at 445 nm was delivered to a mix of curcumin/virus prior to the inoculation on the cells, virus inactivation was observed (>99%) using doses of curcumin that were not antiviral by themselves. Photodynamic therapy employing crude curcumin can be suggested as an antiviral option against SARS-CoV-2 infection.</jats:p>",
"alternative-id": [
"v14102132"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0001-5886-9129",
"affiliation": [
{
"name": "Institute for Maternal and Child Health IRCCS Burlo Garofolo, 34137 Trieste, Italy"
}
],
"authenticated-orcid": false,
"family": "Zupin",
"given": "Luisa",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Division of Laboratory Medicine, University Hospital Giuliano Isontina (ASUGI), 34129 Trieste, Italy"
}
],
"family": "Fontana",
"given": "Francesco",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-2264-6967",
"affiliation": [
{
"name": "Division of Laboratory Medicine, University Hospital Giuliano Isontina (ASUGI), 34129 Trieste, Italy"
}
],
"authenticated-orcid": false,
"family": "Clemente",
"given": "Libera",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Life Science, University of Trieste, 34127 Trieste, Italy"
}
],
"family": "Borelli",
"given": "Violetta",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-8031-1102",
"affiliation": [
{
"name": "Institute for Maternal and Child Health IRCCS Burlo Garofolo, 34137 Trieste, Italy"
},
{
"name": "Department of Medicine, Surgery and Health Sciences, University of Trieste, 34129 Trieste, Italy"
}
],
"authenticated-orcid": false,
"family": "Ricci",
"given": "Giuseppe",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-3446-6613",
"affiliation": [
{
"name": "Division of Laboratory Medicine, University Hospital Giuliano Isontina (ASUGI), 34129 Trieste, Italy"
}
],
"authenticated-orcid": false,
"family": "Ruscio",
"given": "Maurizio",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8493-1168",
"affiliation": [
{
"name": "Biological Science Program, Department of Biological and Environmental Sciences, College of Arts and Sciences, University of Qatar, Doha 2713, Qatar"
}
],
"authenticated-orcid": false,
"family": "Crovella",
"given": "Sergio",
"sequence": "additional"
}
],
"container-title": "Viruses",
"container-title-short": "Viruses",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
9,
29
]
],
"date-time": "2022-09-29T08:09:36Z",
"timestamp": 1664438976000
},
"deposited": {
"date-parts": [
[
2025,
1,
15
]
],
"date-time": "2025-01-15T02:41:17Z",
"timestamp": 1736908877000
},
"funder": [
{
"award": [
"RC 15/2017"
],
"name": "IRCCS Burlo Garofolo /Italian Ministry of Health"
},
{
"award": [
"RC 47/2020"
],
"name": "IRCCS Burlo Garofolo /Italian Ministry of Health"
},
{
"award": [
"QUCG-CAS-22/23-499"
],
"name": "Collaborative Grant from Qatar University"
}
],
"indexed": {
"date-parts": [
[
2025,
6,
20
]
],
"date-time": "2025-06-20T08:11:19Z",
"timestamp": 1750407079305,
"version": "3.37.3"
},
"is-referenced-by-count": 9,
"issue": "10",
"issued": {
"date-parts": [
[
2022,
9,
27
]
]
},
"journal-issue": {
"issue": "10",
"published-online": {
"date-parts": [
[
2022,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
27
]
],
"date-time": "2022-09-27T00:00:00Z",
"timestamp": 1664236800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4915/14/10/2132/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "2132",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
9,
27
]
]
},
"published-online": {
"date-parts": [
[
2022,
9,
27
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.3390/foods6100092",
"doi-asserted-by": "crossref",
"key": "ref_1",
"unstructured": "Hewlings, S.J., and Kalman, D.S. (2017). Curcumin: A Review of Its Effects on Human Health. Foods, 6."
},
{
"DOI": "10.1002/ptr.5931",
"article-title": "Comparative Oral Absorption of Curcumin in a Natural Turmeric Matrix with Two Other Curcumin Formulations: An Open-label Parallel-arm Study: Comparative Oral Absorption of Curcumin",
"author": "Gopi",
"doi-asserted-by": "crossref",
"first-page": "1883",
"journal-title": "Phytother. Res.",
"key": "ref_2",
"volume": "31",
"year": "2017"
},
{
"DOI": "10.1016/j.ejps.2016.09.003",
"article-title": "Nutraceuticals: A paradigm of proactive medicine",
"author": "Santini",
"doi-asserted-by": "crossref",
"first-page": "53",
"journal-title": "Eur. J. Pharm. Sci.",
"key": "ref_3",
"volume": "96",
"year": "2017"
},
{
"DOI": "10.1089/jmf.2019.0063",
"article-title": "Dietary Management of Pediatric Inflammatory Bowel Disease",
"author": "Kaenkumchorn",
"doi-asserted-by": "crossref",
"first-page": "1092",
"journal-title": "J. Med. Food",
"key": "ref_4",
"volume": "22",
"year": "2019"
},
{
"DOI": "10.3390/biom10111469",
"doi-asserted-by": "crossref",
"key": "ref_5",
"unstructured": "Zhai, K., Brockmüller, A., Kubatka, P., Shakibaei, M., and Büsselberg, D. (2020). Curcumin’s Beneficial Effects on Neuroblastoma: Mechanisms, Challenges, and Potential Solutions. Biomolecules, 10."
},
{
"DOI": "10.1089/cap.2017.0165",
"article-title": "N-Acetyl Cysteine and Curcumin in Pediatric Acute-Onset Neuropsychiatric Syndrome",
"author": "Moore",
"doi-asserted-by": "crossref",
"first-page": "293",
"journal-title": "J. Child Adolesc. Psychopharmacol.",
"key": "ref_6",
"volume": "28",
"year": "2018"
},
{
"article-title": "Topical curcumin for the prevention of oral mucositis in pediatric patients: Case series",
"author": "Elad",
"first-page": "21",
"journal-title": "Altern. Ther. Health Med.",
"key": "ref_7",
"volume": "19",
"year": "2013"
},
{
"DOI": "10.2500/aap.2016.37.3920",
"article-title": "Antioxidant supplementation and exhaled nitric oxide in children with asthma",
"author": "Tenero",
"doi-asserted-by": "crossref",
"first-page": "e8",
"journal-title": "Allergy Asthma Proc.",
"key": "ref_8",
"volume": "37",
"year": "2016"
},
{
"DOI": "10.1177/1120672119892804",
"article-title": "Oral phospholipidic curcumin in juvenile idiopathic arthritis-associated uveitis",
"author": "Miserocchi",
"doi-asserted-by": "crossref",
"first-page": "1390",
"journal-title": "Eur. J. Ophthalmol.",
"key": "ref_9",
"volume": "30",
"year": "2020"
},
{
"article-title": "Photodynamic Therapy Potentiates the Effects of Cur-cumin on Pediatric Epithelial Liver Tumor Cells",
"author": "Ellerkamp",
"first-page": "3363",
"journal-title": "Anticancer Res.",
"key": "ref_10",
"volume": "36",
"year": "2016"
},
{
"DOI": "10.3892/ijo.2020.5155",
"article-title": "Antitumor effects of curcumin in pediatric rhabdomyosarcoma in combination with chemotherapy and phototherapy in vitro",
"author": "Sorg",
"doi-asserted-by": "crossref",
"first-page": "266",
"journal-title": "Int. J. Oncol.",
"key": "ref_11",
"volume": "58",
"year": "2020"
},
{
"DOI": "10.1002/jbio.202000350",
"doi-asserted-by": "crossref",
"key": "ref_12",
"unstructured": "Rupel, K., Zupin, L., Brich, S., Mardirossian, M., Ottaviani, G., Gobbo, M., Di Lenarda, R., Pricl, S., Crovella, S., and Zacchigna, S. (2021). Antimicrobial activity of amphiphilic nanomicelles loaded with curcumin against Pseudomonas aeruginosa alone and activated by blue laser light. J. Biophotonics, 14."
},
{
"DOI": "10.3390/ph13070153",
"doi-asserted-by": "crossref",
"key": "ref_13",
"unstructured": "Adamczak, A., Ożarowski, M., and Karpiński, T.M. (2020). Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals, 13."
},
{
"DOI": "10.3390/v12111242",
"doi-asserted-by": "crossref",
"key": "ref_14",
"unstructured": "Jennings, M.R., and Parks, R.J. (2020). Curcumin as an Antiviral Agent. Viruses, 12."
},
{
"DOI": "10.1371/journal.pone.0253489",
"doi-asserted-by": "crossref",
"key": "ref_15",
"unstructured": "Goc, A., Sumera, W., Rath, M., and Niedzwiecki, A. (2021). Phenolic compounds disrupt spike-mediated receptor-binding and entry of SARS-CoV-2 pseudo-virions. PLoS ONE, 16."
},
{
"DOI": "10.3390/molecules26226900",
"doi-asserted-by": "crossref",
"key": "ref_16",
"unstructured": "Marín-Palma, D., Tabares-Guevara, J.H., Zapata-Cardona, M.I., Flórez-Álvarez, L., Yepes, L.M., Rugeles, M.T., Zapata-Builes, W., Hernandez, J.C., and Taborda, N.A. (2021). Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms. Molecules, 26."
},
{
"DOI": "10.3390/v13101914",
"doi-asserted-by": "crossref",
"key": "ref_17",
"unstructured": "Bormann, M., Alt, M., Schipper, L., van de Sand, L., Le-Trilling, V.T.K., Rink, L., Heinen, N., Madel, R.J., Otte, M., and Wuensch, K. (2021). Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro. Viruses, 13."
},
{
"DOI": "10.1556/1886.2022.00009",
"article-title": "Composition of naturally occurring compounds decreases activity of Omicron and SARS-CoV-2 RdRp complex",
"author": "Goc",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "Eur. J. Microbiol. Immunol.",
"key": "ref_18",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1021/acsabm.1c00874",
"article-title": "Nanocurcumin Potently Inhibits SARS-CoV-2 Spike Protein-Induced Cytokine Storm by Deactivation of MAPK/NF-κB Signaling in Epithelial Cells",
"author": "Sharma",
"doi-asserted-by": "crossref",
"first-page": "483",
"journal-title": "ACS Appl. Bio. Mater.",
"key": "ref_19",
"volume": "5",
"year": "2022"
},
{
"DOI": "10.1016/j.pdpdt.2021.102286",
"article-title": "Robust antimicrobial photodynamic therapy with curcumin-poly (lactic-co-glycolic acid) nanoparticles against COVID-19: A preliminary in vitro study in Vero cell line as a model",
"author": "Pourhajibagher",
"doi-asserted-by": "crossref",
"first-page": "102286",
"journal-title": "Photodiagnosis Photodyn. Ther.",
"key": "ref_20",
"volume": "34",
"year": "2021"
},
{
"DOI": "10.1002/fsn3.2226",
"article-title": "Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebo-controlled clinical trial",
"author": "Ahmadi",
"doi-asserted-by": "crossref",
"first-page": "4068",
"journal-title": "Food Sci. Nutr.",
"key": "ref_21",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7375",
"article-title": "Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebo-controlled clinical trial",
"author": "Asadirad",
"doi-asserted-by": "crossref",
"first-page": "1023",
"journal-title": "Phytotherapy Res.",
"key": "ref_22",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.1002/ptr.7294",
"article-title": "A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients",
"author": "Hassaniazad",
"doi-asserted-by": "crossref",
"first-page": "6417",
"journal-title": "Phytotherapy Res.",
"key": "ref_23",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7374",
"article-title": "Effect of nanocurcumin supplementation on the severity of symptoms and length of hospital stay in patients with COVID-19: A randomized double-blind placebo-controlled trial",
"author": "Shafie",
"doi-asserted-by": "crossref",
"first-page": "1013",
"journal-title": "Phytotherapy Res.",
"key": "ref_24",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.3389/fphar.2022.898062",
"doi-asserted-by": "crossref",
"key": "ref_25",
"unstructured": "Khan, A., Iqtadar, S., Mumtaz, S.U., Heinrich, M., Pascual-Figal, D.A., Livingstone, S., and Abaidullah, S. (2022). Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19—Results From a Pilot Open-Label, Randomized Controlled Trial. Front. Pharmacol., 13."
},
{
"DOI": "10.3177/jnsv.67.249",
"article-title": "Newly Developed Highly Bioavailable Curcumin Formulation, curcuRougeTM, Reduces Neutrophil/Lymphocyte Ratio in the Elderly: A Double-Blind, Placebo-Controlled Clinical Trial",
"author": "Kishimoto",
"doi-asserted-by": "crossref",
"first-page": "249",
"journal-title": "J. Nutr. Sci. Vitaminol.",
"key": "ref_26",
"volume": "67",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2021.669362",
"doi-asserted-by": "crossref",
"key": "ref_27",
"unstructured": "Pawar, K.S., Mastud, R.N., Pawar, S.K., Pawar, S.S., Bhoite, R.R., Bhoite, R.R., Kulkarni, M.V., and Deshpande, A.R. (2021). Oral Curcumin with Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial. Front. Pharmacol., 12."
},
{
"DOI": "10.1002/ptr.7004",
"article-title": "Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: An open label nonrandomized clinical trial",
"author": "Salari",
"doi-asserted-by": "crossref",
"first-page": "2616",
"journal-title": "Phytotherapy Res.",
"key": "ref_28",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1002/jcp.30233",
"article-title": "Immunomodulatory effects of nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients",
"author": "Tahmasebi",
"doi-asserted-by": "crossref",
"first-page": "5325",
"journal-title": "J. Cell. Physiol.",
"key": "ref_29",
"volume": "236",
"year": "2021"
},
{
"DOI": "10.1016/j.lfs.2021.119437",
"doi-asserted-by": "crossref",
"key": "ref_30",
"unstructured": "Tahmasebi, S., Saeed, B.Q., Temirgalieva, E., Yumashev, A.V., El-Esawi, M.A., Navashenaq, J.G., Valizadeh, H., Sadeghi, A., Aslani, S., and Yousefi, M. (2021). Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV2. Life Sci., 276."
},
{
"DOI": "10.1016/j.intimp.2020.107088",
"doi-asserted-by": "crossref",
"key": "ref_31",
"unstructured": "Valizadeh, H., Abdolmohammadi-Vahid, S., Danshina, S., Gencer, M.Z., Ammari, A., Sadeghi, A., Roshangar, L., Aslani, S., Esmaeilzadeh, A., and Ghaebi, M. (2020). Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol., 89."
},
{
"DOI": "10.3390/applmicrobiol2030052",
"article-title": "Surfactin Bacterial Antiviral Lipopeptide Blocks In Vitro Replication of SARS-CoV-2",
"author": "Crovella",
"doi-asserted-by": "crossref",
"first-page": "680",
"journal-title": "Appl. Microbiol.",
"key": "ref_32",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.1002/jmv.27588",
"article-title": "Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines",
"author": "Araf",
"doi-asserted-by": "crossref",
"first-page": "1825",
"journal-title": "J. Med. Virol.",
"key": "ref_33",
"volume": "94",
"year": "2022"
},
{
"key": "ref_34",
"unstructured": "CDC (2022, February 10). COVID Data Tracker, Available online: https://covid.cdc.gov/covid-data-tracker."
},
{
"DOI": "10.1002/art.41616",
"article-title": "American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS–CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 2",
"author": "Henderson",
"doi-asserted-by": "crossref",
"first-page": "e13",
"journal-title": "Arthritis Rheumatol.",
"key": "ref_35",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0062482",
"doi-asserted-by": "crossref",
"key": "ref_36",
"unstructured": "Chen, T.-Y., Chen, D.-Y., Wen, H.-W., Ou, J.-L., Chiou, S.-S., Chen, J.-M., Wong, M.-L., and Hsu, W.-L. (2013). Inhibition of Enveloped Viruses Infectivity by Curcumin. PLoS ONE, 8."
},
{
"DOI": "10.1136/gutjnl-2012-304299",
"article-title": "Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells",
"author": "Anggakusuma",
"doi-asserted-by": "crossref",
"first-page": "1137",
"journal-title": "Gut",
"key": "ref_37",
"volume": "63",
"year": "2013"
},
{
"DOI": "10.2174/1570159X11311040002",
"article-title": "Curcumin and its Derivatives: Their Application in Neuropharmacology and Neuroscience in the 21st Century",
"author": "Lee",
"doi-asserted-by": "crossref",
"first-page": "338",
"journal-title": "Curr. Neuropharmacol.",
"key": "ref_38",
"volume": "11",
"year": "2013"
},
{
"DOI": "10.1038/cddiscovery.2015.17",
"doi-asserted-by": "crossref",
"key": "ref_39",
"unstructured": "Moustapha, A., Pérétout, P.A., Rainey, N.E., Sureau, F., Geze, M., Petit, J.-M., Dewailly, E., Slomianny, C., and Petit, P.X. (2015). Curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization and mitochondrial events. Cell Death Discov., 1."
},
{
"DOI": "10.3390/ijms22137130",
"doi-asserted-by": "crossref",
"key": "ref_40",
"unstructured": "Trigo-Gutierrez, J.K., Vega-Chacón, Y., Soares, A.B., and de Oliveira Mima, E.G. (2021). Antimicrobial Activity of Curcumin in Nanoformulations: A Comprehensive Review. Int. J. Mol. Sci., 22."
},
{
"DOI": "10.1039/c9pp00211a",
"article-title": "Trends and targets in antiviral phototherapy",
"author": "Wiehe",
"doi-asserted-by": "crossref",
"first-page": "2565",
"journal-title": "Photochem. Photobiol. Sci.",
"key": "ref_41",
"volume": "18",
"year": "2019"
},
{
"DOI": "10.3390/pharmaceutics14030683",
"doi-asserted-by": "crossref",
"key": "ref_42",
"unstructured": "Sadraeian, M., Junior, F.F.P., Miranda, M., Galinskas, J., Fernandes, R.S., da Cruz, E.F., Fu, L., Zhang, L., Diaz, R.S., and Cabral-Miranda, G. (2022). Study of Viral Photoinactivation by UV-C Light and Photosensitizer Using a Pseudotyped Model. Pharmaceutics, 14."
}
],
"reference-count": 42,
"references-count": 42,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4915/14/10/2132"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer",
"type": "journal-article",
"volume": "14"
}
