Curcumin Transferosome-Loaded Thermosensitive Intranasal in situ Gel as Prospective Antiviral Therapy for SARS-Cov-2
et al., International Journal of Nanomedicine, doi:10.2147/IJN.S423251, Oct 2023
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
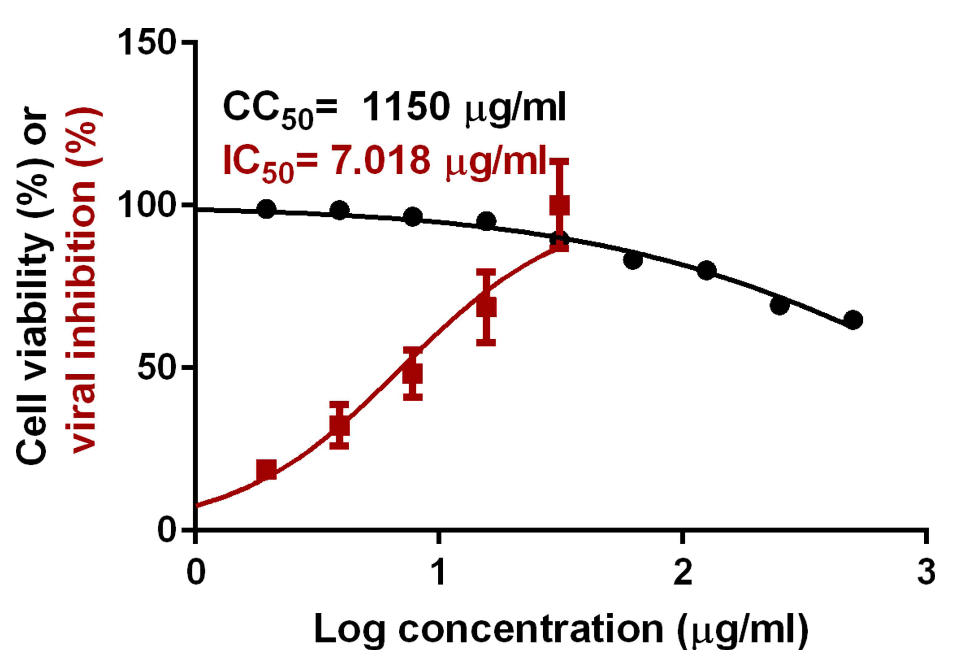
In silico, in vitro, and in vivo study showing antiviral efficacy of a curcumin nanoformulation against SARS-CoV-2. Molecular docking revealed potential binding and inhibition of curcumin with viral proteins involved in entry and replication, including the main protease, RNA-dependent RNA polymerase (RdRp), and the receptor binding domain (RBD) of the spike protein. An optimized intranasal curcumin transferosome-loaded thermosensitive gel was formulated. This nanoformulation displayed strong anti-SARS-CoV-2 activity at low concentrations in Vero E6 cells with minimal cytotoxicity. After intranasal administration in rabbits, the nanoformulation showed 226% higher relative bioavailability and 2.5 times longer mean residence time compared to free curcumin gel, indicating improved lung delivery. Authors conclude the nanoformulation is a promising antiviral treatment for COVID-19.
62 preclinical studies support the efficacy of curcumin for COVID-19:
In silico studies predict inhibition of SARS-CoV-2 with curcumin or metabolites via binding to the spikeA,1,5,6,11,16,18,24,27 (and specifically the receptor binding domainB,2,4,14,17,20 ), MproC,4-6,11,13,15-17,19,20,22,25,27,28,30,48 , RNA-dependent RNA polymeraseD,4-6,17,26 , PLproE,6, ACE2F,2,18,19,21 , nucleocapsidG,12,29 , nsp10H,29, and helicaseI,36 proteins, and inhibition of spike-ACE2 interactionJ,3.
In vitro studies demonstrate inhibition of the spikeA,41 (and specifically the receptor binding domainB,51), MproC,23,41,48,50 , ACE2F,51, and TMPRSS2K,51 proteins, and inhibition of spike-ACE2 interactionJ,3,34 .
In vitro studies demonstrate efficacy in Calu-3L,49, A549M,41, A549-ATN,31, 293TO,7, HEK293-hACE2P,23,39 , 293T/hACE2/TMPRSS2Q,40, Vero E6R,1,13,17,27,39,41,43,45,47,49 , and SH-SY5YS,38 cells.
Curcumin decreases pro-inflammatory cytokines induced by SARS-CoV-2 in peripheral blood mononuclear cells47, alleviates SARS-CoV-2 spike protein-induced mitochondrial membrane damage and oxidative stress7, may limit COVID-19 induced cardiac damage by inhibiting the NF-κB signaling pathway which mediates the profibrotic effects of the SARS-CoV-2 spike protein on cardiac fibroblasts35, is predicted to inhibit the interaction between the SARS-CoV-2 spike protein receptor binding domain and the human ACE2 receptor for the delta and omicron variants14, lowers ACE2 and STAT3, curbing lung inflammation and ARDS in preclinical COVID-19 models32, inhibits SARS-CoV-2 ORF3a ion channel activity, which contributes to viral pathogenicity and cytotoxicity42, has direct virucidal action by disrupting viral envelope integrity44, may inhibit viral replication and modulate inflammatory pathways like NF-κB via SIRT1 activation52, and can function as a photosensitizer in photodynamic therapy to generate reactive oxygen species that damage the virus44.
1.
Marzouk et al., Computational and Experimental Insights into the Antiviral Mechanism of Turmeric (Curcuma longa) against SARS-CoV-2 D614G, BIO Web of Conferences, doi:10.1051/bioconf/202519804002.
2.
Wu et al., Utilizing natural compounds as ligands to disrupt the binding of SARS-CoV-2 receptor-binding domain to angiotensin-converting enzyme 2, impeding viral infection, Phytochemistry Letters, doi:10.1016/j.phytol.2025.102999.
3.
Najimi et al., Phytochemical Inhibitors of SARS‐CoV‐2 Entry: Targeting the ACE2‐RBD Interaction with l‐Tartaric Acid, l‐Ascorbic Acid, and Curcuma longa Extract, ChemistrySelect, doi:10.1002/slct.202406035.
4.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
5.
Al balawi et al., Assessing multi-target antiviral and antioxidant activities of natural compounds against SARS-CoV-2: an integrated in vitro and in silico study, Bioresources and Bioprocessing, doi:10.1186/s40643-024-00822-z.
6.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
7.
Zhang et al., Computational Discovery of Mitochondrial Dysfunction Biomarkers in Severe SARS-CoV-2 Infection: Facilitating Pytomedicine Screening, Phytomedicine, doi:10.1016/j.phymed.2024.155784.
8.
Öztürkkan et al., In Silico investigation of the effects of curcuminoids on the spike protein of the omicron variant of SARS-CoV-2, Baku State University Journal of Chemistry and Material Sciences, 1:2, bsuj.bsu.edu.az/uploads/pdf/ec4204d62f7802de54e6092bf7860029.pdf.
9.
Yunze et al., Therapeutic effect and potential mechanism of curcumin, an active ingredient in Tongnao Decoction, on COVID-19 combined with stroke: a network pharmacology study and GEO database mining, Research Square, doi:10.21203/rs.3.rs-4329762/v1.
10.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
11.
Boseila et al., Throat spray formulated with virucidal Pharmaceutical excipients as an effective early prophylactic or treatment strategy against pharyngitis post-exposure to SARS CoV-2, European Journal of Pharmaceutics and Biopharmaceutics, doi:10.1016/j.ejpb.2024.114279.
12.
Hidayah et al., Bioinformatics study of curcumin, demethoxycurcumin, bisdemethoxycurcumin and cyclocurcumin compounds in Curcuma longa as an antiviral agent via nucleocapsid on SARS-CoV-2 inhibition, International Conference on Organic and Applied Chemistry, doi:10.1063/5.0197724.
13.
Singh et al., Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 M Pro protein - An in-silico and cell-based approach, Research Square, doi:10.21203/rs.3.rs-3888947/v1.
14.
Kant et al., Structure-based drug discovery to identify SARS-CoV2 spike protein–ACE2 interaction inhibitors, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2023.2300060.
15.
Naderi Beni et al., In silico studies of anti-oxidative and hot temperament-based phytochemicals as natural inhibitors of SARS-CoV-2 Mpro, PLOS ONE, doi:10.1371/journal.pone.0295014.
16.
Moschovou et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, International Journal of Molecular Sciences, doi:10.3390/ijms242115894.
17.
Eleraky et al., Curcumin Transferosome-Loaded Thermosensitive Intranasal in situ Gel as Prospective Antiviral Therapy for SARS-Cov-2, International Journal of Nanomedicine, doi:10.2147/IJN.S423251.
18.
Singh (B) et al., Computational studies to analyze effect of curcumin inhibition on coronavirus D614G mutated spike protein, The Seybold Report, doi:10.17605/OSF.IO/TKEXJ.
19.
Thapa et al., In-silico Approach for Predicting the Inhibitory Effect of Home Remedies on Severe Acute Respiratory Syndrome Coronavirus-2, Makara Journal of Science, doi:10.7454/mss.v27i3.1609.
20.
Srivastava et al., Paradigm of Well-Orchestrated Pharmacokinetic Properties of Curcuminoids Relative to Conventional Drugs for the Inactivation of SARS-CoV-2 Receptors: An In Silico Approach, Stresses, doi:10.3390/stresses3030043.
21.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
22.
Winih Kinasih et al., Analisis in silico interaksi senyawa kurkuminoid terhadap enzim main protease 6LU7 dari SARS-CoV-2, Duta Pharma Journal, doi:10.47701/djp.v3i1.2904.
23.
Wu (B) et al., Potential Mechanism of Curcumin and Resveratrol against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-2780614/v1.
24.
Nag et al., Curcumin inhibits spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in silico study, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2022.105552.
25.
Rampogu et al., Molecular Docking and Molecular Dynamics Simulations Discover Curcumin Analogue as a Plausible Dual Inhibitor for SARS-CoV-2, International Journal of Molecular Sciences, doi:10.3390/ijms23031771.
26.
Singh (C) et al., Potential of turmeric-derived compounds against RNA-dependent RNA polymerase of SARS-CoV-2: An in-silico approach, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2021.104965.
27.
Kandeil et al., Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2, Pathogens, doi:10.3390/pathogens10060758.
28.
Rajagopal et al., Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-020-00126-x.
29.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
30.
Sekiou et al., In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2, ChemRxiv, doi:10.26434/chemrxiv.12181404.v1.
31.
Grüneberg et al., Dose-dependent antiviral effects of glycyrrhizin, curcumin, and harmaline against clinical SARS-CoV-2 isolates, including D614G, Omicron BA.5, and Omicron XBB.1, BMC Complementary Medicine and Therapies, doi:10.1186/s12906-026-05253-1.
32.
Aktay et al., Oral Administration of Water-Soluble Curcumin Complex Prevents ARDS With the Potential for COVID-19 Treatment, Phytotherapy Research, doi:10.1002/ptr.70046.
33.
Olubiyi et al., Novel dietary herbal preparations with inhibitory activities against multiple SARS-CoV-2 targets: A multidisciplinary investigation into antiviral activities, Food Chemistry Advances, doi:10.1016/j.focha.2025.100969.
34.
Emam et al., Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors, AMB Express, doi:10.1186/s13568-024-01739-8.
35.
Van Tin et al., Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling, Cells, doi:10.3390/cells13161331.
36.
Li et al., Thermal shift assay (TSA)-based drug screening strategy for rapid discovery of inhibitors against the Nsp13 helicase of SARS-CoV-2, Animals and Zoonoses, doi:10.1016/j.azn.2024.06.001.
37.
Kamble et al., Nanoparticulate curcumin spray imparts prophylactic and therapeutic properties against SARS-CoV-2, Emergent Materials, doi:10.1007/s42247-024-00754-6.
38.
Nicoliche et al., Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2, Scientific Reports, doi:10.1038/s41598-024-61662-7.
39.
Nittayananta et al., A novel film spray containing curcumin inhibits SARS-CoV-2 and influenza virus infection and enhances mucosal immunity, Virology Journal, doi:10.1186/s12985-023-02282-x.
40.
Septisetyani et al., Curcumin and turmeric extract inhibited SARS-CoV-2 pseudovirus cell entry and Spike mediated cell fusion, bioRxiv, doi:10.1101/2023.09.28.560070.
41.
Mohd Abd Razak et al., In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds, Natural Product Communications, doi:10.1177/1934578X231188861.
42.
Fam et al., Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites, Scientific Reports, doi:10.1038/s41598-023-31764-9.
43.
Teshima et al., Antiviral activity of curcumin and its analogs selected by an artificial intelligence-supported activity prediction system in SARS-CoV-2-infected VeroE6 cells, Natural Product Research, doi:10.1080/14786419.2023.2194647.
44.
Zupin et al., Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer, Viruses, doi:10.3390/v14102132.
45.
Leka et al., In vitro antiviral activity against SARS-CoV-2 of common herbal medicinal extracts and their bioactive compounds, Phytotherapy Research, doi:10.1002/ptr.7463.
46.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
47.
Marín-Palma et al., Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms, Molecules, doi:10.3390/molecules26226900.
48.
Bahun et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594.
49.
Bormann et al., Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro, Viruses, doi:10.3390/v13101914.
50.
Guijarro-Real et al., Potential In Vitro Inhibition of Selected Plant Extracts against SARS-CoV-2 Chymotripsin-Like Protease (3CLPro) Activity, Foods, doi:10.3390/foods10071503.
51.
Goc (B) et al., Phenolic compounds disrupt spike-mediated receptor-binding and entry of SARS-CoV-2 pseudo-virions, PLOS ONE, doi:10.1371/journal.pone.0253489.
52.
Shokri-Afra et al., Targeting SIRT1: A Potential Strategy for Combating Severe COVID‐19, BioMed Research International, doi:10.1155/bmri/9507417.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The receptor binding domain is a specific region of the spike protein that binds ACE2 and is a major target of neutralizing antibodies. Focusing on the precise binding site allows highly specific disruption of viral attachment with reduced potential for off-target effects.
c.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
d.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
e.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
f.
The angiotensin converting enzyme 2 (ACE2) protein is a host cell transmembrane protein that serves as the cellular receptor for the SARS-CoV-2 spike protein. ACE2 is expressed on many cell types, including epithelial cells in the lungs, and allows the virus to enter and infect host cells. Inhibition may affect ACE2's physiological function in blood pressure control.
g.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
h.
Non-structural protein 10 (nsp10) serves as an RNA chaperone and stabilizes conformations of nsp12 and nsp14 in the replicase-transcriptase complex, which synthesizes new viral RNAs. Nsp10 disruption may destabilize replicase-transcriptase complex activity.
i.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
j.
The interaction between the SARS-CoV-2 spike protein and the human ACE2 receptor is a primary method of viral entry, inhibiting this interaction can prevent the virus from attaching to and entering host cells, halting infection at an early stage.
k.
Transmembrane protease serine 2 (TMPRSS2) is a host cell protease that primes the spike protein, facilitating cellular entry. TMPRSS2 activity helps enable cleavage of the spike protein required for membrane fusion and virus entry. Inhibition may especially protect respiratory epithelial cells, buy may have physiological effects.
l.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
m.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
n.
A549-AT is a human lung carcinoma cell line stably transfected with ACE2 and TMPRSS2 receptors. Unlike the parental line, this overexpression ensures stable infection and enhanced viral entry, allowing for the evaluation of antiviral efficacy against various SARS-CoV-2 variants.
o.
293T is a human embryonic kidney cell line that can be engineered for high ACE2 expression and SARS-CoV-2 susceptibility. 293T cells are easily transfected and support high protein expression.
p.
HEK293-hACE2 is a human embryonic kidney cell line with high ACE2 expression and SARS-CoV-2 susceptibility. Cells have been transfected with a plasmid to express the human ACE2 (hACE2) protein.
q.
293T/hACE2/TMPRSS2 is a human embryonic kidney cell line engineered for high ACE2 and TMPRSS2 expression, which mimics key aspects of human infection. 293T/hACE2/TMPRSS2 cells are very susceptible to SARS-CoV-2 infection.
r.
Vero E6 is an African green monkey kidney cell line with low/no ACE2 expression and high SARS-CoV-2 susceptibility. The cell line is easy to maintain and supports robust viral replication, however the monkey origin may not accurately represent human responses.
s.
SH-SY5Y is a human neuroblastoma cell line that exhibits neuronal phenotypes. It is commonly used as an in vitro model for studying neurotoxicity, neurodegenerative diseases, and neuronal differentiation.
Eleraky et al., 16 Oct 2023, peer-reviewed, 7 authors.
Contact: nermineleraky@pharm.aun.edu.eg.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Curcumin Transferosome-Loaded Thermosensitive Intranasal in situ Gel as Prospective Antiviral Therapy for SARS-Cov-2
International Journal of Nanomedicine, doi:10.2147/ijn.s423251
Purpose: Immunomodulatory and broad-spectrum antiviral activities have motivated the evaluation of curcumin for Coronavirus infection 2019 (COVID-19) management. Inadequate bioavailability is the main impediment to the therapeutic effects of oral Cur. This study aimed to develop an optimal curcumin transferosome-loaded thermosensitive in situ gel to improve its delivery to the lungs. Methods: Transferosomes were developed by using 3 3 screening layouts. The phospholipid concentration as well as the concentration and type of surfactant were considered independent variables. The entrapment efficiency (EE%), size, surface charge, and polydispersity index (PDI) were regarded as dependent factors. A cold technique was employed to develop thermosensitive in-situ gels. Optimized transferosomes were loaded onto the selected gels. The produced gel was assessed based on shape attributes, ex vivo permeability enhancement, and the safety of the nasal mucosa. The in vitro cytotoxicity, antiviral cytopathic effect, and plaque assay (CV/CPE/Plaque activity), and in vivo performance were evaluated after intranasal administration in experimental rabbits.
Results: The optimized preparation displayed a particle size of 664.3 ± 69.3 nm, EE% of 82.8 ± 0.02%, ZP of -11.23 ± 2.5 mV, and PDI of 0.6 ± 0.03. The in vitro curcumin release from the optimized transferosomal gel was markedly improved compared with that of the free drug-loaded gel. An ex vivo permeation study revealed a significant improvement (2.58-fold) in drug permeability across nasal tissues of sheep. Histopathological screening confirmed the safety of these preparations. This formulation showed high antiviral activity against SARS-CoV-2 at reduced concentrations. High relative bioavailability (226.45%) was attained after the formula intranasally administered to rabbits compared to the free drug in-situ gel. The curcumin transferosome gel displayed a relatively high lung accumulation after intranasal administration.
Conclusion: This study provides a promising formulation for the antiviral treatment of COVID-19 patients, which can be evaluated further in preclinical and clinical studies.
Author Contributions All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this study.
References
-Q, -H L, Curcumin regulates the differentiation of naïve CD4+ T cells and activates IL-10 immune modulation against acute lung injury in mice, Biomed Pharmacother, doi:10.1016/j.biopha.2020.109946
Abdelbary, Aburahma, Oro-dental mucoadhesive proniosomal gel formulation loaded with lornoxicam for management of dental pain, J Liposome Res, doi:10.3109/08982104.2014.941861
Abdellatif, Aldosari, Subaiyel, Transethosomal Gel for the Topical Delivery of Celecoxib: formulation and Estimation of Skin Cancer Progression, Pharmaceutics, doi:10.3390/pharmaceutics15010022
Abdellatif, Tawfeek, Transfersomal nanoparticles for enhanced transdermal delivery of clindamycin, Aaps Pharmscitech, doi:10.1208/s12249-015-0441-7
Abdulbaqi, Darwis, Assi, Khan, Ahmed et al., In situ misemgel as a multifunctional dual-absorption platform for nasal delivery of raloxifene hydrochloride: formulation, characterization, and in vivo performance, International Journal of Nanomedicine, doi:10.2147/IJN.S181587
Aboud, Ali, El-Menshawe, Elbary, Nanotransfersomes of carvedilol for intranasal delivery: formulation, characterization and in vivo evaluation, Drug Deliv, doi:10.3109/10717544.2015.1013587
Ahad, Al-Saleh, Formulation and characterization of Phospholipon 90 G and tween 80 based transfersomes for transdermal delivery of eprosartan mesylate, Pharm Dev Technol, doi:10.1080/10837450.2017.1330345
Ahad, Al-Saleh, Formulation and characterization of novel soft nanovesicles for enhanced transdermal delivery of eprosartan mesylate, Saudi Pharmaceutical j, doi:10.1016/j.jsps.2017.01.006
Ahad, Aqil, Kohli, Sultana, Mujeeb et al., Formulation and optimization of nanotransfersomes using experimental design technique for accentuated transdermal delivery of valsartan, Nanomedicine, doi:10.1016/j.nano.2011.06.004
Ahmed, Badr-Eldin, Ahmed, Aldawsari, Intranasal optimized solid lipid nanoparticles loaded in situ gel for enhancing trans-mucosal delivery of simvastatin, J Drug Deliv Sci Technol, doi:10.1016/j.jddst.2018.10.027
Ahmed, Fahmy, Badr-Eldin, Application of nanopharmaceutics for flibanserin brain delivery augmentation via the nasal route, Nanomaterials, doi:10.3390/nano10071270
Ahmed, Preparation of transfersomes encapsulating sildenafil aimed for transdermal drug delivery: plackett-Burman design and characterization, J Liposome Res, doi:10.3109/08982104.2014.950276
Alhakamy, Ahmed, Repurposing of sitagliptin-melittin optimized nanoformula against sars-cov-2; antiviral screening and molecular docking studies, Pharmaceutics, doi:10.3390/pharmaceutics13030307
Allam, Eleraky, Diab, Development of Sedative Dexmedetomidine Sublingual In Situ Gels: In vitro and In Vivo Evaluations, Pharmaceutics, doi:10.3390/pharmaceutics14020220
Allam, Elsabahy, Badry, Eleraky, Betaxolol-loaded niosomes integrated within pH-sensitive in situ forming gel for management of glaucoma, Int J Pharm, doi:10.1016/j.ijpharm.2021.120380
Almeida, Amaral, Lobão, Lobo, Pluronic ® F-127 and Pluronic Lecithin Organogel (PLO): main features and their applications in topical and transdermal administration of drugs, J Pharm Pharmaceutical Sci, doi:10.18433/J3HW2B
Altuntaş, Yener, Formulation and evaluation of thermoreversible in situ nasal gels containing mometasone furoate for allergic rhinitis, AAPS PharmSciTech, doi:10.1208/s12249-017-0747-8
Arkin, Elham, Anwar, Kalimanjiang, Iminjan, Optimization and Evaluation of the Quercus infectoria Galls Thermosensitive In Situ Gel for Rectal Delivery, Evidence Based Complementary Alternative Med, doi:10.1155/2022/8451055
Arunothayanun, Bernard, Craig, Uchegbu, Florence, The effect of processing variables on the physical characteristics of non-ionic surfactant vesicles (niosomes) formed from a hexadecyl diglycerol ether, Int J Pharm, doi:10.1016/S0378-5173(00)00362-8
Asmari, Ullah, Tariq, Fatani, Preparation, characterization, and in vivo evaluation of intranasally administered liposomal formulation of donepezil, Drug Des Devel Ther
Avasarala, Zhang, Liu, Wang, London et al., Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome, PLoS One, doi:10.1371/journal.pone.0057285
Aziz, Abdelbary, Elassasy, Fabrication of novel elastosomes for boosting the transdermal delivery of diacerein: statistical optimization, ex-vivo permeation, in-vivo skin deposition and pharmacokinetic assessment compared to oral formulation, Drug Deliv, doi:10.1080/10717544.2018.1451572
Badr-Eldin, Ahmed, Optimized nano-transfersomal films for enhanced sildenafil citrate transdermal delivery: ex vivo and in vivo evaluation, Drug Des Devel Ther, doi:10.2147/DDDT.S103122
Badria, Abdelaziz, Hassan, Elgazar, Mazyed, Development of provesicular nanodelivery system of curcumin as a safe and effective antiviral agent: statistical optimization, in vitro characterization, and antiviral effectiveness, Molecules, doi:10.3390/molecules25235668
Bary, Preparation and Characterization of Thermosensitive Mucoadhesive In_Situ Gels for Nasal Delivery of Ondansetron Hydrochloride, Al-Azhar J Pharm Sci, doi:10.21608/ajps.2014.6953
Basha, El-Alim, Shamma, Awad, Design and optimization of surfactant-based nanovesicles for ocular delivery of Clotrimazole, J Liposome Res, doi:10.3109/08982104.2013.788025
Benvenuto, Giovanetti, Ciccozzi, Spoto, Angeletti et al., The 2019-new coronavirus epidemic: evidence for virus evolution, J Med Virol, doi:10.1002/jmv.25688
Bnyan, Khan, Ehtezazi, Formulation and optimisation of novel transfersomes for sustained release of local anaesthetic, J Pharm Pharmacol, doi:10.1111/jphp.13149
Bnyan, Khan, Ehtezazi, Surfactant effects on lipid-based vesicles properties, J Pharm Sci, doi:10.1016/j.xphs.2018.01.005
Bonaccorso, Gigliobianco, Pellitteri, Optimization of curcumin nanocrystals as promising strategy for nose-to-brain delivery application, Pharmaceutics, doi:10.3390/pharmaceutics12050476
Bonfim, Monteleoni, Calmon, Antiviral activity of curcumin-nanoemulsion associated with photodynamic therapy in vulvar cell lines transducing different variants of HPV-16, Artif Cells, Nanomed Biotechnol, doi:10.1080/21691401.2020.1725023
Boonlai, Tantishaiyakul, Hirun, Sangfai, Suknuntha, Thermosensitive poloxamer 407/poly (acrylic acid) hydrogels with potential application as injectable drug delivery system, AAPS PharmSciTech, doi:10.1208/s12249-018-1010-7
Brambilla, Locarno, Gallo, Poloxamer-based hydrogel as drug delivery system: how polymeric excipients influence the chemical-physical properties, Polymers, doi:10.3390/polym14173624
Brezáni, Leláková, Hassan, Anti-infectivity against herpes simplex virus and selected microbes and anti-inflammatory activities of compounds isolated from Eucalyptus globulus labill, Viruses, doi:10.3390/v10070360
Cas, Ghidoni, Dietary curcumin: correlation between bioavailability and health potential, Nutrients, doi:10.3390/nu11092147
Chan, Yuan, Kok, A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster, Lancet, doi:10.1016/S0140-6736(20)30154-9
Chang, Oh, -G, Kim, Kim, Rheological evaluation of thermosensitive and mucoadhesive vaginal gels in physiological conditions, Int J Pharm, doi:10.1016/S0378-5173(02)00232-6
Chaudhary, Kohli, Kumar, Nano-transfersomes as a novel carrier for transdermal delivery, Int J Pharm, doi:10.1016/j.ijpharm.2013.07.031
Chauhan, Kumari, Kumar, Intranasal curcumin and its evaluation in murine model of asthma, Int Immunopharmacol, doi:10.1016/j.intimp.2013.08.008
Chauhan, Madou, Kalra, Chopra, Ghosh et al., Nanotechnology for COVID-19: therapeutics and vaccine research, ACS nano, doi:10.1021/acsnano.0c04006
Chen, Lee, Meng, An overview on thermosensitive oral gel based on poloxamer 407, Materials, doi:10.3390/ma14164522
Chen, Liu, Liu, A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19, J Zhejiang Univ
Chen, Zou, Niu, Liu, Peng et al., The stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes, Molecules, doi:10.3390/molecules200814293
Choi, Jung, Ryu, Yoon, Kim, Development of in situ-gelling and mucoadhesive Acetaminophen liquid suppository, Int J Pharm, doi:10.1016/S0378-5173(97)00386-4
Chu, Cheng, Hung, Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings, Thorax, doi:10.1136/thorax.2003.012658
Clogston, Patri, Zeta potential measurement, Characterization Nanoparticles Intended Drug Delivery
Das, Sen, Maji, Nayak, Sen, Transferosomal gel for transdermal delivery of risperidone: formulation optimization and ex vivo permeation, J Drug Deliv Sci Technol, doi:10.1016/j.jddst.2017.01.006
De Barros, Portugal, Batain, Formulation, design and strategies for efficient nanotechnology-based nasal delivery systems, RPS Pharm Pharmacol Rep, doi:10.1093/rpsppr/rqac003
Dourado, Freire, Pereira, Will curcumin nanosystems be the next promising antiviral alternatives in COVID-19 treatment trials?, Biomed Pharmacother, doi:10.1016/j.biopha.2021.111578
Dubey, Chaudhry, Singh, Gupta, Kaushik, Perspectives on nano-nutraceuticals to manage pre and post COVID-19 infections, Biotechnol Rep, doi:10.1016/j.btre.2022.e00712
Durgun, Mesut, Hacıoğlu, Güngör, Özsoy, Optimization of the Micellar-Based In Situ Gelling Systems Posaconazole with Quality by Design (QbD) Approach and Characterization by In Vitro Studies, International Journal of Nanomedicine, doi:10.3390/pharmaceutics14030526
El Zaafarany, Awad, Holayel, Mortada, Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery, Int J Pharm, doi:10.1016/j.ijpharm.2010.06.034
El-Feky, Fares, Zayed, El-Telbany, Ahmed et al., Repurposing of nifedipine loaded in situ ophthalmic gel as a novel approach for glaucoma treatment, Biomed Pharmacother, doi:10.1016/j.biopha.2021.112008
El-Gizawy, Nouh, Saber, Kira, Deferoxamine-loaded transfersomes accelerates healing of pressure ulcers in streptozotocin-induced diabetic rats, J Drug Deliv Sci Technol, doi:10.1016/j.jddst.2020.101732
El-Halim, Mamdouh, El-Haddad, Soliman, Fabrication of anti-HSV-1 curcumin stabilized nanostructured proniosomal gel: molecular docking studies on thymidine kinase proteins, Sci Pharm, doi:10.3390/scipharm88010009
El-Sayed, Hussein, Sarhan, Mansour, Flurbiprofen-loaded niosomes-in-gel system improves the ocular bioavailability of flurbiprofen in the aqueous humor, Drug Dev Ind Pharm, doi:10.1080/03639045.2016.1272120
Eleraky, Omar, Mahmoud, Abou-Taleb, Nanostructured lipid carriers to mediate brain delivery of temazepam: design and in vivo study, Pharmaceutics, doi:10.3390/pharmaceutics12050451
Elkomy, Menshawe, Abou-Taleb, Elkarmalawy, Loratadine bioavailability via buccal transferosomal gel: formulation, statistical optimization, in vitro/in vivo characterization, and pharmacokinetics in human volunteers, Drug Deliv, doi:10.1080/10717544.2017.1321061
Elsenosy, Abdelbary, Elshafeey, Elsayed, Fares et al., Brain targeting of duloxetine HCL via intranasal delivery of loaded cubosomal gel: in vitro characterization, ex vivo permeation, and in vivo biodistribution studies, Int J Nanomedicine, doi:10.1016/S0378-5173(00)00493-2
Elshagea, Makar, Salama, Elkasabgy, Basalious, Investigating the Targeting Power to Brain Tissues of Intranasal Rasagiline Mesylate-Loaded Transferosomal In Situ Gel for Efficient Treatment of Parkinson's Disease, Pharmaceutics, doi:10.3390/pharmaceutics15020533
Elsheikh, Elnaggar, Hamdy, Abdallah, Novel cremochylomicrons for improved oral bioavailability of the antineoplastic phytomedicine berberine chloride: optimization and pharmacokinetics, Int J Pharm, doi:10.1016/j.ijpharm.2017.11.023
Fathalla, Mustafa, Abdelkader, Moharram, Sabry et al., Hybrid thermosensitive-mucoadhesive in situ forming gels for enhanced corneal wound healing effect of L-carnosine, Drug Deliv, doi:10.1080/10717544.2021.2023236
Fathalla, Vangala, Longman, Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: design, characterisation, toxicity and transcorneal permeation studies, Eur J Pharmaceutics Biopharmaceutics, doi:10.1016/j.ejpb.2017.01.008
Fu, Wang, Yuan, Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis, J Infection, doi:10.1016/j.jinf.2020.03.041
Fu, Ye, Feng, Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease, Nat Commun, doi:10.1038/s41467-020-18233-x
Galgatte, Kumbhar, Chaudhari, Development of in situ gel for nasal delivery: design, optimization, in vitro and in vivo evaluation, Drug Deliv, doi:10.3109/10717544.2013.849778
Garg, Garg, Beg, Singh, Katare, Nanosized ethosomes-based hydrogel formulations of methoxsalen for enhanced topical delivery against vitiligo: formulation optimization, in vitro evaluation and preclinical assessment, J Drug Target, doi:10.3109/1061186X.2015.1070855
Guo, Fan, Chen, Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19), JAMA cardiol, doi:10.1001/jamacardio.2020.1017
Hirun, Kraisit, Tantishaiyakul, Thermosensitive Polymer Blend Composed of Poloxamer 407, Poloxamer 188 and Polycarbophil for the Use as Mucoadhesive In Situ Gel, Polymers, doi:10.3390/polym14091836
Hosny, Rizg, Khallaf, Preparation and optimization of in situ gel loaded with rosuvastatin-ellagic acid nanotransfersomes to enhance the anti-proliferative activity, Pharmaceutics, doi:10.3390/pharmaceutics12030263
Jain, Jain, Umamaheshwari, Jain, Transfersomes-a novel vesicular carrier for enhanced transdermal delivery: development, characterization, and performance evaluation, Drug Dev Ind Pharm, doi:10.1081/DDC-120025458
Jiang, Yu, Liu, Ding, Ma et al., Biodistribution of curcumin and its derivatives new aspects for curcumin administration, IEEE
Joshi, Kaur, Kulkarni, Chaudhari, In-vitro and ex-vivo evaluation of raloxifene hydrochloride delivery using nano-transfersome based formulations, J Drug Deliv Sci Technol, doi:10.1016/j.jddst.2018.02.006
Jyoti, Anandhakrishnan, Singh, A three-pronged formulation approach to improve oral bioavailability and therapeutic efficacy of two lipophilic drugs with gastric lability, Drug Deliv Transl Res, doi:10.1007/s13346-019-00635-0
Karimi, Mashreghi, Saremi, Jaafari, Spectrofluorometric method development and validation for the determination of curcumin in nanoliposomes and plasma, J Fluoresc, doi:10.1007/s10895-020-02574-3
Kaushik, Manipulative magnetic nanomedicine: the future of COVID-19 pandemic/endemic therapy, Expert Opin Drug Deliv, doi:10.1080/17425247.2021.1860938
Kazmi, Al-Abbasi, Nadeem, Altayb, Alshehri et al., Formulation, Optimization and Evaluation of Luteolin-Loaded Topical Nanoparticulate Delivery System for the Skin Cancer, Pharmaceutics, doi:10.3390/pharmaceutics13111749
Khan, Aqil, Imam, Ursolic acid loaded intra nasal nano lipid vesicles for brain tumour: formulation, optimization, in-vivo brain/ plasma distribution study and histopathological assessment, Biomed Pharmacother, doi:10.1016/j.biopha.2018.07.127
Khowessah, Shoukri, Nano-transfersomal ciprofloxacin loaded vesicles for non-invasive trans-tympanic ototopical delivery: in-vitro optimization, ex-vivo permeation studies, and in-vivo assessment, Int J Pharm, doi:10.1016/j.ijpharm.2014.06.041
Kolbina, Rachmawati, Budiputra, Mauludin, Curcumin nanoemulsion for transdermal application: formulation and evaluation, Drug Dev Ind Pharm, doi:10.3109/03639045.2014.884127
Kuo, Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus, J Med Chem, doi:10.1021/jm070295s
Lelli, Sahebkar, Johnston, Pedone, Curcumin use in pulmonary diseases: state of the art and future perspectives, Pharmacol Res, doi:10.1016/j.phrs.2016.11.017
Li, Moore, Vasilieva, Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus, Nature, doi:10.1038/nature02145
Li, Structural analysis of major species barriers between humans and palm civets for severe acute respiratory syndrome coronavirus infections, J Virol, doi:10.1128/jvi.00442-08
Liu, Zhai, Heng, Oral bioavailability of curcumin: problems and advancements, J Drug Target, doi:10.3109/1061186X.2016.1157883
Ma, Almahri, Micellar sensitized Resonance Rayleigh Scattering and spectrofluorometric methods based on isoindole formation for determination of Eflornithine in cream and biological samples, Spectrochim Acta A Mol Biomol Spectrosc, doi:10.1016/j.saa.2021.119806
Ma, Wang, He, Tang, Pharmaceutical strategies of improving oral systemic bioavailability of curcumin for clinical application, J Controlled Release, doi:10.1016/j.jconrel.2019.10.053
Madane, Mahajan, Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: design, characterization, and in vivo study, Drug Deliv, doi:10.3109/10717544.2014.975382
Mahajan, Gattani, In situ gels of metoclopramide hydrochloride for intranasal delivery: in vitro evaluation and in vivo pharmacokinetic study in rabbits, Drug Deliv, doi:10.3109/10717540903447194
Mahmoud, Bakr, Al-Karmalawy, Moatasim, Taweel et al., Scrutinizing the feasibility of nonionic surfactants to form isotropic bicelles of curcumin: a potential antiviral candidate against COVID-19, AAPS PharmSciTech, doi:10.1208/s12249-021-02197-2
Malakar, Sen, Nayak, Sen, Formulation, optimization and evaluation of transferosomal gel for transdermal insulin delivery, Saudi Pharmaceutical j, doi:10.1016/j.jsps.2012.02.001
Manca, Peris, Melis, Lattuada, Nanoincorporation of Curcumin in Polymer-Glycerosomes and Evaluation of their In Vitro-In Vivo Suitability as Pulmonary Delivery Systems, RSC Adv, doi:10.1039/C5RA24032H
Manconi, Caddeo, Sinico, Ex vivo skin delivery of diclofenac by transcutol containing liposomes and suggested mechanism of vesicle-skin interaction, Eur J Pharmaceutics Biopharmaceutics, doi:10.1016/j.ejpb.2010.12.010
Manconi, Sinico, Valenti, Loy, Fadda, Niosomes as carriers for tretinoin. I. Preparation and properties, Int J Pharm, doi:10.1016/S0378-5173(01)00971-1
Marasini, Kaminskas, Subunit-based mucosal vaccine delivery systems for pulmonary delivery-Are they feasible?, Drug Dev Ind Pharm, doi:10.1080/03639045.2019.1583758
Mathew, Hsu, Antiviral potential of curcumin, J Funct Foods, doi:10.1016/j.jff.2017.12.017
Mazyed, Abdelaziz, Fabrication of transgelosomes for enhancing the ocular delivery of Acetazolamide: statistical optimization, in vitro characterization, and in vivo study, Pharmaceutics, doi:10.3390/pharmaceutics12050465
Mazzone, Simons, Van Geelen, In Vitro Biological Activity of Natural Products from the Endophytic Fungus Paraboeremia selaginellae against Toxoplasma gondii, Antibiotics, doi:10.3390/antibiotics11091176
Mehanny, Hathout, Geneidi, Mansour, Exploring the use of nanocarrier systems to deliver the magical molecule; curcumin and its derivatives, J Controlled Release, doi:10.1016/j.jconrel.2016.01.018
Mekkawy, Eleraky, Soliman, Elnaggar, Elnaggar, Combinatorial Therapy of Letrozole-and Quercetin-Loaded Spanlastics for Enhanced Cytotoxicity against MCF-7 Breast Cancer Cells, Pharmaceutics, doi:10.3390/pharmaceutics14081727
Mircioiu, Voicu, Anuta, Mathematical modeling of release kinetics from supramolecular drug delivery systems, Pharmaceutics, doi:10.3390/pharmaceutics11030140
Moawad, Ali, Salem, Nanotransfersomes-loaded thermosensitive in situ gel as a rectal delivery system of tizanidine HCl: preparation, in vitro and in vivo performance, Drug Deliv, doi:10.1080/10717544.2016.1245369
Monavari, Bolouri, Ebrahimi, Ataei-Pirkooh, Ataei-Pirkooh, The inhibitory effect of Acyclovir loaded nano-niosomes against herpes simplex virus type-1 in cell culture, Med J Islam Repub Iran
Moore, June, Cytokine release syndrome in severe COVID-19, Science, doi:10.1126/science.abb8925
Mostafa, Kandeil, Elshaier, FDA-approved drugs with potent in vitro antiviral activity against severe acute respiratory syndrome coronavirus 2, Pharmaceuticals, doi:10.3390/ph13120443
Mura, Mennini, Nativi, Richichi, In situ mucoadhesive-thermosensitive liposomal gel as a novel vehicle for nasal extended delivery of opiorphin, Eur J Pharmaceutics Biopharmaceutics, doi:10.1016/j.ejpb.2017.10.008
Nair, Chaudhary, Shah, Intranasal Delivery of Darunavir-Loaded Mucoadhesive In Situ Gel: experimental Design, In Vitro Evaluation, and Pharmacokinetic Studies, Gels, doi:10.3390/gels8060342
Nemmar, Subramaniyan, Ali, Protective effect of curcumin on pulmonary and cardiovascular effects induced by repeated exposure to diesel exhaust particles in mice, PLoS One, doi:10.1371/journal.pone.0039554
Ng, Selvarajah, Hussein, Yeap, Omar, In Vitro Evaluation of Curcumin-Encapsulated Chitosan Nanoparticles against Feline Infectious Peritonitis Virus and Pharmacokinetics Study in Cats, Biomed Res Int, doi:10.1155/2020/3012198
Ochani, Jatoi, Epidemic amidst the coronavirus disease-19 pandemic, J Glob Health, doi:10.7189/jogh.11.03056
Oh, Kim, Shin, Skin permeation of retinol in Tween 20-based deformable liposomes: in-vitro evaluation in human skin and keratinocyte models, J Pharm Pharmacol, doi:10.1211/jpp.58.2.0002
Omar, Hasan, Zaki, Eleraky, Externally triggered novel rapid-release sonosensitive folate-modified liposomes for gemcitabine: development and characteristics, Int J Nanomedicine, doi:10.2147/IJN.S266676
Opatha, Titapiwatanakun, Chutoprapat, Transfersomes: a promising nanoencapsulation technique for transdermal drug delivery, Pharmaceutics, doi:10.3390/pharmaceutics12090855
Paliwal, Sargolzaei, Bhardwaj, Bhardwaj, Dixit et al., Grand challenges in bio-nanotechnology to manage the COVID-19 pandemic, Front Nanotechnol, doi:10.3389/fnano.2020.571284
Pan, Huang, Lin, Biotransformation of curcumin through reduction and glucuronidation in mice, International Journal of Nanomedicine, doi:10.2147/IJN.S423251DovePress
Patel, Koradia, Parikh, Design and development of intranasal in situ gelling system of Midazolam hydrochloride using 32 full factorial design, J Drug Deliv Sci Technol, doi:10.1016/j.jddst.2015.10.010
Patel, Kumar, Thakkar, Formulation of niosomal gel for enhanced transdermal lopinavir delivery and its comparative evaluation with ethosomal gel, AAPS pharmscitech, doi:10.1208/s12249-012-9871-7
Petchsomrit, Sermkaew, Wiwattanapatapee, Effect of alginate and surfactant on physical properties of oil entrapped alginate bead formulation of curcumin, Int J Pharmacol Pharmaceutical Sci
Pitta, Dudhipala, Narala, Veerabrahma, Development of zolmitriptan transfersomes by Box-Behnken design for nasal delivery: in vitro and in vivo evaluation, Drug Dev Ind Pharm, doi:10.1080/03639045.2017.1402918
Prasad, Tyagi, Aggarwal, Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice, Cancer Res Treatment, doi:10.4143/crt.2014.46.1.2
Qi, Chen, Huang, Development of a poloxamer analogs/carbopol-based in situ gelling and mucoadhesive ophthalmic delivery system for puerarin, Int J Pharm, doi:10.1016/j.ijpharm.2006.12.038
Qushawy, Nasr, Abd-Alhaseeb, Swidan, Design, optimization and characterization of a transfersomal gel using miconazole nitrate for the treatment of candida skin infections, Pharmaceutics, doi:10.3390/pharmaceutics10010026
Rabia, Khaleeq, Batool, Rifampicin-loaded nanotransferosomal gel for treatment of cutaneous leishmaniasis: passive targeting via topical route, Nanomedicine, doi:10.2217/nnm-2019-0320
Raj, Murali, Formulation and Evaluation of Curcumin Loaded Transferosomal Nasal In-Situ Gel for Alzheimer's Disease, Res Rev AJ Drug Formul Dev Prod
Ravindranath, Chandrasekhara, Absorption and tissue distribution of curcumin in rats, Toxicology, doi:10.1016/0300-483X(80)90122-5
Rubin, Chan-Tack, Farley, Sherwat, FDA Approval of Remdesivir -a Step in the Right Direction, N Eng J Med, doi:10.1056/NEJMp2032369
Ryu, Choe, Lee, Choi, Kim, Curcumin and dehydrozingerone derivatives: synthesis, radiolabeling, and evaluation for βamyloid plaque imaging, J Med Chem, doi:10.1021/jm0607193
Saber-Moghaddam, Salari, Hejazi, Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: an open label nonrandomized clinical trial, CAS, SciSearch ® , Current Contents ® /Clinical Medicine, Journal Citation Reports/Science Edition, doi:10.1002/ptr.7004
Sahoo, Chakraborti, Mishra, Qualitative analysis of controlled release ciprofloxacin/carbopol 934 mucoadhesive suspension, J Adv Pharm Technol Res, doi:10.4103/2231-4040.85541
Salama, Badran, Elmowafy, Soliman, Spironolactone-loaded leciplexes as potential topical delivery systems for female acne: in vitro appraisal and ex vivo skin permeability studies, Pharmaceutics, doi:10.3390/pharmaceutics12010025
Salatin, Barar, Barzegar-Jalali, Adibkia, Milani et al., Hydrogel nanoparticles and nanocomposites for nasal drug/vaccine delivery, Arch Pharm Res, doi:10.1007/s12272-016-0782-0
Salem, Kharshoum, Abou-Taleb, Naguib, Nanosized transferosome-based intranasal in situ gel for brain targeting of resveratrol: formulation, optimization, in vitro evaluation, and in vivo pharmacokinetic study, Aaps pharmscitech, doi:10.1208/s12249-019-1353-8
Sanidad, Sukamtoh, Xiao, Mcclements, Zhang, Curcumin: recent advances in the development of strategies to improve oral bioavailability, Annu Rev Food Sci Technol, doi:10.1146/annurev-food-032818-121738
Shah, Nair, Shah, Jacob, Shehata et al., Enhancement in antinociceptive and anti-inflammatory effects of tramadol by transdermal proniosome gel, Asian J Pharm Sci, doi:10.1016/j.ajps.2019.05.001
Shang, Ye, Shi, Structural basis of receptor recognition by SARS-CoV-2, Nature, doi:10.1038/s41586-020-2179-y
Singh, Wani, Kaul-Ghanekar, Prabhune, Ogale, From micron to nano-curcumin by sophorolipid co-processing: highly enhanced bioavailability, fluorescence, and anti-cancer efficacy, RSC Adv, doi:10.1039/C4RA07300B
Sinico, Manconi, Peppi, Lai, Valenti et al., Liposomes as carriers for dermal delivery of tretinoin: in vitro evaluation of drug permeation and vesicle-skin interaction, J Controlled Release, doi:10.1016/j.jconrel.2004.11.020
Sintov, AmyloLipid Nanovesicles: a self-assembled lipid-modified starch hybrid system constructed for direct nose-to-brain delivery of curcumin, Int J Pharm, doi:10.1016/j.ijpharm.2020.119725
Soliman, Fetih, Abbas, Thermosensitive bioadhesive gels for the vaginal delivery of sildenafil citrate: in vitro characterization and clinical evaluation in women using clomiphene citrate for induction of ovulation, Drug Dev Ind Pharm, doi:10.1080/03639045.2016.1254239
Sood, Jain, Gowthamarajan, Optimization of curcumin nanoemulsion for intranasal delivery using design of experiment and its toxicity assessment, Colloids Surf B Biointerfaces, doi:10.1016/j.colsurfb.2013.09.030
Stadio, Ishai, Gambacorta, Nutraceuticals as immune-stimulating therapy to fight COVID-19. Combination of elements to improve the efficacy, Eur Rev Med Pharmacol Sci
Studart, Amstad, Gauckler, Colloidal stabilization of nanoparticles in concentrated suspensions, Langmuir, doi:10.1021/la062042s
Sung, Pulliam, Edwards, Nanoparticles for drug delivery to the lungs, Trends Biotechnol, doi:10.1016/j.tibtech.2007.09.005
Suresh, Wagner, Rosania, Pulmonary administration of a water-soluble curcumin complex reduces severity of acute lung injury, Am J Respir Cell Mol Biol, doi:10.1165/rcmb.2011-0175OC
Sylvester, Optimization of the tetrazolium dye (MTT) colorimetric assay for cellular growth and viability, Drug Des Discov
Tahmasebi, El-Esawi, Mahmoud, Immunomodulatory effects of Nanocurcumin on Th17 cell responses in mild and severe COVID-19 patients, J Cell Physiol, doi:10.1002/jcp.30233
Tamilarasan, Yasmin, Anitha, Box-Behnken Design: optimization of Proanthocyanidin-Loaded Transferosomes as an Effective Therapeutic Approach for Osteoarthritis, Nanomaterials, doi:10.3390/nano12172954
Tanaka, Narazaki, Kishimoto, Immunotherapeutic implications of IL-6 blockade for cytokine storm, International Journal of Nanomedicine, doi:10.2217/imt-2016-0020
Teaima, Helal, Alsofany, El-Nabarawi, Yasser, Ion-Triggered In Situ Gelling Intranasal Spray of Dronedarone Hydrochloride Nanocarriers: In vitro Optimization and In Vivo Pharmacokinetic Appraisal, Pharmaceutics, doi:10.3390/pharmaceutics14112405
Teaima, Mohamady, El-Nabarawi, Mohamed, Formulation and evaluation of niosomal vesicles containing ondansetron HCL for trans-mucosal nasal drug delivery, Drug Dev Ind Pharm, doi:10.1080/03639045.2020.1753061
Thimmulappa, Mudnakudu-Nagaraju, Shivamallu, Antiviral and immunomodulatory activity of curcumin: a case for prophylactic therapy for COVID-19, Heliyon, doi:10.1016/j.heliyon.2021.e06350
Thorne, Pronk, Padmanabhan, Ii, Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration, Neuroscience, doi:10.1016/j.neuroscience.2004.05.029
Uwaezuoke, Toit, Kumar, Ally, Choonara, Linoleic Acid-Based Transferosomes for Topical Ocular Delivery of Cyclosporine A, Pharmaceutics, doi:10.3390/pharmaceutics14081695
Valizadeh, Abdolmohammadi-Vahid, Danshina, Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients, Int Immunopharmacol, doi:10.1016/j.intimp.2020.107088
Varahachalam, Lahooti, Chamaneh, Nanomedicine for the SARS-CoV-2: state-of-The-art and future prospects, Int J Nanomedicine, doi:10.2147/IJN.S283686
Varia, Joshi, Jadeja, Katariya, Detholia et al., Development and evaluation of ultradeformable vesicles loaded transdermal film of boswellic acid, Future J Pharm Sci, doi:10.1186/s43094-022-00428-2
Varia, Joshi, Jadeja, Katariya, Detholia et al., Development and evaluation of ultradeformable vesicles loaded transdermal film of boswellic acid, International Journal of Nanomedicine, doi:10.1186/s43094-022-00428-2
Varthya, Thangaraju, Venkatesan, Curcumin and fungal infection-commonly available herbs for common female infection, J Family Med Primary Care, doi:10.4103/jfmpc.jfmpc_1218_19
Verekar, Gurav, Bolmal, Thermosensitive mucoadhesive in situ gel for intranasal delivery of Almotriptan malate: formulation, characterization, and evaluation, J Drug Deliv Sci Technol, doi:10.1016/j.jddst.2020.101778
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Wang, Chen, Zhang, Yang, Zhai, Formulation and evaluation of microemulsion-based in situ ion-sensitive gelling systems for intranasal administration of curcumin, J Drug Target, doi:10.3109/1061186X.2012.719230
Wang, He, Dynamics of vesicle formation from lipid droplets: mechanism and controllability, J Chem Phys, doi:10.1063/1.3079097
Wang, Pan, Cheng, Stability of curcumin in buffer solutions and characterization of its degradation products, J Pharm Biomed Anal, doi:10.1016/S0731-7085(96)02024-9
Wang, Wong, Chan, Lee, Zuo, Statistical Design of Experiment (DoE) based development and optimization of DB213 in situ thermosensitive gel for intranasal delivery, Int J Pharm, doi:10.1016/j.ijpharm.2018.01.032
Wei, Xu, Ding, Zheng, Zheng, Thermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studies, J Controlled Release, doi:10.1016/S0168-3659(02)00175-X
Wu, Peng, Wilken, Geraghty, Li, Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus, J Biol Chem, doi:10.1074/jbc.M111.325803
Yeo, Olusanya, Chaw, Elkordy, Brief effect of a small hydrophobic drug (cinnarizine) on the physicochemical characterisation of niosomes produced by thin-film hydration and microfluidic methods, Pharmaceutics, doi:10.3390/pharmaceutics10040185
Yin, Mao, Luan, Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir, Science, doi:10.1126/science.abc1560
Young, Johnston, Pace, Mishra, Phospholipid-stabilized nanoparticles of cyclosporine A by rapid expansion from supercritical to aqueous solution, Aaps Pharmscitech, doi:10.1208/pt050111
Yu, Wang, Wang, Formulation optimization and bioavailability after oral and nasal administration in rabbits of puerarin-loaded microemulsion, J Pharm Sci, doi:10.1002/jps.22333
Yuki, Fujiogi, Koutsogiannaki, COVID-19 pathophysiology: a review, Clin immunol, doi:10.1016/j.clim.2020.108427
Zahedipour, Hosseini, Sathyapalan, Potential effects of curcumin in the treatment of COVID-19 infection, Phytother Res, doi:10.1002/ptr.6738
Zakaria, Fayad, Althobaiti, Zaki, Almaaty, Statistical optimization of bile salt deployed nanovesicles as a potential platform for oral delivery of piperine: accentuated antiviral and anti-inflammatory activity in MERS-CoV challenged mice, Drug Deliv, doi:10.1080/10717544.2021.1934190
Zhai, Ding, Wu, Long, Zhong et al., The epidemiology, diagnosis and treatment of COVID-19, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2020.105955
Zhang, Swamy, Balijepalli, Direct pulmonary delivery of solubilized curcumin reduces severity of lethal pneumonia, FASEB J, doi:10.1096/fj.201901047RR
Zhang, Yang, Wong, Xie, Ho, In vitro and in vivo comparison of curcumin-encapsulated chitosan-coated poly (lactic-coglycolic acid) nanoparticles and curcumin/hydroxypropyl-β-Cyclodextrin inclusion complexes administered intranasally as therapeutic strategies for Alzheimer's Disease, Mol Pharm, doi:10.1021/acs.molpharmaceut.0c00675
Zheng, Shao, Chen, Zhang, Wang et al., Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis, Int J Infectious Dis, doi:10.1016/j.ijid.2021.11.009
Zhou, Zhong, Wei, Dou, Chou et al., Baicalein and hydroxypropyl-γ-cyclodextrin complex in poloxamer thermal sensitive hydrogel for vaginal administration, Int J Pharm, doi:10.1016/j.ijpharm.2013.07.006
Zueva, Makarova, Zvereva, Industrial block copolymer surfactants: diversity of associative forms and interaction with carbon nanomaterial, J Mol Liq, doi:10.1016/j.molliq.2022.119267
DOI record:
{
"DOI": "10.2147/ijn.s423251",
"ISSN": [
"1178-2013"
],
"URL": "http://dx.doi.org/10.2147/IJN.S423251",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-5218-4575",
"affiliation": [],
"authenticated-orcid": true,
"family": "Eleraky",
"given": "Nermin E",
"sequence": "first"
},
{
"affiliation": [],
"family": "El-Badry",
"given": "Mahmoud",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Omar",
"given": "Mahmoud",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7778-6238",
"affiliation": [],
"authenticated-orcid": true,
"family": "El-Koussi",
"given": "Wesam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mohamed",
"given": "Noha",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3020-4966",
"affiliation": [],
"authenticated-orcid": true,
"family": "Abdel-Lateef",
"given": "Mohamed",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8984-2957",
"affiliation": [],
"authenticated-orcid": true,
"family": "Hassan",
"given": "Abeer",
"sequence": "additional"
}
],
"container-title": "International Journal of Nanomedicine",
"container-title-short": "IJN",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
10,
17
]
],
"date-time": "2023-10-17T04:10:15Z",
"timestamp": 1697515815000
},
"deposited": {
"date-parts": [
[
2023,
10,
17
]
],
"date-time": "2023-10-17T04:10:28Z",
"timestamp": 1697515828000
},
"indexed": {
"date-parts": [
[
2023,
10,
18
]
],
"date-time": "2023-10-18T04:52:09Z",
"timestamp": 1697604729191
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/3.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
1
]
],
"date-time": "2023-10-01T00:00:00Z",
"timestamp": 1696118400000
}
}
],
"link": [
{
"URL": "https://www.dovepress.com/getfile.php?fileID=93508",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.dovepress.com/getfile.php?fileID=93508",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "5831-5869",
"prefix": "10.2147",
"published": {
"date-parts": [
[
2023,
10
]
]
},
"published-online": {
"date-parts": [
[
2023,
10
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.2147/IJN.S283686",
"author": "Varahachalam",
"doi-asserted-by": "publisher",
"first-page": "539",
"journal-title": "Int J Nanomedicine",
"key": "ref1",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.7189/jogh.11.03056",
"author": "Ochani",
"doi-asserted-by": "publisher",
"journal-title": "J Glob Health",
"key": "ref2",
"volume": "11",
"year": "2021"
},
{
"key": "ref3",
"unstructured": "World Health Organization. Coronavirus disease (COVID-19); 2021; 2021."
},
{
"DOI": "10.1016/j.ijid.2021.11.009",
"author": "Zheng",
"doi-asserted-by": "publisher",
"first-page": "252",
"journal-title": "Int J Infectious Dis",
"key": "ref4",
"volume": "114",
"year": "2022"
},
{
"DOI": "10.3389/fnano.2020.571284",
"author": "Paliwal",
"doi-asserted-by": "publisher",
"first-page": "571284",
"journal-title": "Front Nanotechnol",
"key": "ref5",
"volume": "2",
"year": "2020"
},
{
"DOI": "10.1016/j.heliyon.2021.e06350",
"author": "Thimmulappa",
"doi-asserted-by": "publisher",
"first-page": "e06350",
"journal-title": "Heliyon",
"key": "ref6",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1016/j.clim.2020.108427",
"author": "Yuki",
"doi-asserted-by": "publisher",
"first-page": "108427",
"journal-title": "Clin immunol",
"key": "ref7",
"volume": "215",
"year": "2020"
},
{
"DOI": "10.1080/17425247.2021.1860938",
"author": "Kaushik",
"doi-asserted-by": "publisher",
"first-page": "531",
"journal-title": "Expert Opin Drug Deliv",
"key": "ref8",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2020.03.041",
"author": "Fu",
"doi-asserted-by": "publisher",
"first-page": "656",
"journal-title": "J Infection",
"key": "ref9",
"volume": "80",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30154-9",
"author": "Chan",
"doi-asserted-by": "publisher",
"first-page": "514",
"journal-title": "Lancet",
"key": "ref10",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jamacardio.2020.1017",
"author": "Guo",
"doi-asserted-by": "publisher",
"first-page": "811",
"journal-title": "JAMA cardiol",
"key": "ref11",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "269",
"journal-title": "Cell Res",
"key": "ref12",
"volume": "30",
"year": "2020"
},
{
"author": "Chen",
"first-page": "215",
"journal-title": "J Zhejiang Univ",
"key": "ref13",
"volume": "49",
"year": "2020"
},
{
"DOI": "10.1136/thorax.2003.012658",
"author": "Chu",
"doi-asserted-by": "publisher",
"first-page": "252",
"journal-title": "Thorax",
"key": "ref14",
"volume": "59",
"year": "2004"
},
{
"DOI": "10.2217/imt-2016-0020",
"author": "Tanaka",
"doi-asserted-by": "publisher",
"first-page": "959",
"journal-title": "Immunotherapy",
"key": "ref15",
"volume": "8",
"year": "2016"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105955",
"author": "Zhai",
"doi-asserted-by": "publisher",
"first-page": "105955",
"journal-title": "Int J Antimicrob Agents",
"key": "ref16",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25688",
"author": "Benvenuto",
"doi-asserted-by": "publisher",
"first-page": "455",
"journal-title": "J Med Virol",
"key": "ref17",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1126/science.abb8925",
"author": "Moore",
"doi-asserted-by": "publisher",
"first-page": "473",
"journal-title": "Science",
"key": "ref18",
"volume": "368",
"year": "2020"
},
{
"author": "Di Stadio",
"first-page": "56",
"journal-title": "Eur Rev Med Pharmacol Sci",
"key": "ref19",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1016/j.btre.2022.e00712",
"author": "Dubey",
"doi-asserted-by": "publisher",
"first-page": "e00712",
"journal-title": "Biotechnol Rep",
"key": "ref20",
"volume": "33",
"year": "2022"
},
{
"DOI": "10.1016/j.phrs.2016.11.017",
"author": "Lelli",
"doi-asserted-by": "publisher",
"first-page": "133",
"journal-title": "Pharmacol Res",
"key": "ref21",
"volume": "115",
"year": "2017"
},
{
"DOI": "10.4103/jfmpc.jfmpc_1218_19",
"author": "Varthya",
"doi-asserted-by": "publisher",
"first-page": "1272",
"journal-title": "J Family Med Primary Care",
"key": "ref22",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1021/jm070295s",
"author": "Wen",
"doi-asserted-by": "publisher",
"first-page": "4087",
"journal-title": "J Med Chem",
"key": "ref23",
"volume": "50",
"year": "2007"
},
{
"DOI": "10.1016/j.intimp.2020.107088",
"author": "Valizadeh",
"doi-asserted-by": "publisher",
"first-page": "107088",
"journal-title": "Int Immunopharmacol",
"key": "ref24",
"volume": "89",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0039554",
"author": "Nemmar",
"doi-asserted-by": "publisher",
"first-page": "e39554",
"journal-title": "PLoS One",
"key": "ref25",
"volume": "7",
"year": "2012"
},
{
"DOI": "10.1016/j.biopha.2020.109946",
"author": "Y-s",
"doi-asserted-by": "publisher",
"first-page": "109946",
"journal-title": "Biomed Pharmacother",
"key": "ref26",
"volume": "125",
"year": "2020"
},
{
"DOI": "10.1002/ptr.6738",
"author": "Zahedipour",
"doi-asserted-by": "publisher",
"first-page": "2911",
"journal-title": "Phytother Res",
"key": "ref27",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1016/j.biopha.2021.111578",
"author": "Dourado",
"doi-asserted-by": "publisher",
"first-page": "111578",
"journal-title": "Biomed Pharmacother",
"key": "ref28",
"volume": "139",
"year": "2021"
},
{
"DOI": "10.3109/1061186X.2016.1157883",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "694",
"journal-title": "J Drug Target",
"key": "ref29",
"volume": "24",
"year": "2016"
},
{
"DOI": "10.1007/s13346-019-00635-0",
"author": "Jyoti",
"doi-asserted-by": "publisher",
"first-page": "848",
"journal-title": "Drug Deliv Transl Res",
"key": "ref30",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1021/acsnano.0c04006",
"author": "Chauhan",
"doi-asserted-by": "publisher",
"first-page": "7760",
"journal-title": "ACS nano",
"key": "ref31",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.colsurfb.2013.09.030",
"author": "Sood",
"doi-asserted-by": "publisher",
"first-page": "330",
"journal-title": "Colloids Surf B Biointerfaces",
"key": "ref32",
"volume": "113",
"year": "2014"
},
{
"DOI": "10.1093/rpsppr/rqac003",
"author": "de Barros",
"doi-asserted-by": "publisher",
"first-page": "rqac003",
"journal-title": "RPS Pharm Pharmacol Rep",
"key": "ref33",
"volume": "1",
"year": "2022"
},
{
"DOI": "10.1016/j.biopha.2018.07.127",
"author": "Khan",
"doi-asserted-by": "publisher",
"first-page": "1578",
"journal-title": "Biomed Pharmacother",
"key": "ref34",
"volume": "106",
"year": "2018"
},
{
"DOI": "10.1080/03639045.2019.1583758",
"author": "Marasini",
"doi-asserted-by": "publisher",
"first-page": "882",
"journal-title": "Drug Dev Ind Pharm",
"key": "ref35",
"volume": "45",
"year": "2019"
},
{
"DOI": "10.1016/j.tibtech.2007.09.005",
"author": "Sung",
"doi-asserted-by": "publisher",
"first-page": "563",
"journal-title": "Trends Biotechnol",
"key": "ref36",
"volume": "25",
"year": "2007"
},
{
"DOI": "10.3390/pharmaceutics12030263",
"author": "Hosny",
"doi-asserted-by": "publisher",
"first-page": "263",
"journal-title": "Pharmaceutics",
"key": "ref37",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.3390/pharmaceutics12090855",
"author": "Opatha",
"doi-asserted-by": "publisher",
"first-page": "855",
"journal-title": "Pharmaceutics",
"key": "ref38",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1080/03639045.2017.1402918",
"author": "Pitta",
"doi-asserted-by": "publisher",
"first-page": "484",
"journal-title": "Drug Dev Ind Pharm",
"key": "ref39",
"volume": "44",
"year": "2018"
},
{
"DOI": "10.3390/pharmaceutics15020533",
"author": "ElShagea",
"doi-asserted-by": "publisher",
"first-page": "533",
"journal-title": "Pharmaceutics",
"key": "ref40",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1016/j.ijpharm.2021.120380",
"author": "Allam",
"doi-asserted-by": "publisher",
"first-page": "120380",
"journal-title": "Int J Pharm",
"key": "ref41",
"volume": "598",
"year": "2021"
},
{
"DOI": "10.3390/ma14164522",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "4522",
"journal-title": "Materials",
"key": "ref42",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1016/j.jddst.2018.02.006",
"author": "Joshi",
"doi-asserted-by": "publisher",
"first-page": "151",
"journal-title": "J Drug Deliv Sci Technol",
"key": "ref43",
"volume": "45",
"year": "2018"
},
{
"DOI": "10.1007/978-1-60327-198-1_6",
"author": "Clogston",
"doi-asserted-by": "crossref",
"first-page": "63",
"journal-title": "Characterization Nanoparticles Intended Drug Delivery",
"key": "ref44",
"year": "2011"
},
{
"DOI": "10.2147/DDDT.S103122",
"author": "Badr-Eldin",
"doi-asserted-by": "publisher",
"first-page": "1323",
"journal-title": "Drug Des Devel Ther",
"key": "ref45",
"volume": "10",
"year": "2016"
},
{
"DOI": "10.1016/S0168-3659(02)00175-X",
"author": "Wei",
"doi-asserted-by": "publisher",
"first-page": "65",
"journal-title": "J Controlled Release",
"key": "ref46",
"volume": "83",
"year": "2002"
},
{
"DOI": "10.1016/j.ijpharm.2006.12.038",
"author": "Qi",
"doi-asserted-by": "publisher",
"first-page": "178",
"journal-title": "Int J Pharm",
"key": "ref47",
"volume": "337",
"year": "2007"
},
{
"DOI": "10.21608/ajps.2014.6953",
"author": "Abdel Bary",
"doi-asserted-by": "publisher",
"first-page": "191",
"journal-title": "Al-Azhar J Pharm Sci",
"key": "ref48",
"volume": "50",
"year": "2014"
},
{
"DOI": "10.3390/polym14173624",
"author": "Brambilla",
"doi-asserted-by": "publisher",
"first-page": "3624",
"journal-title": "Polymers",
"key": "ref49",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/S0378-5173(97)00386-4",
"author": "Choi",
"doi-asserted-by": "publisher",
"first-page": "33",
"journal-title": "Int J Pharm",
"key": "ref50",
"volume": "165",
"year": "1998"
},
{
"DOI": "10.3390/polym14091836",
"author": "Hirun",
"doi-asserted-by": "publisher",
"first-page": "1836",
"journal-title": "Polymers",
"key": "ref51",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1080/03639045.2016.1254239",
"author": "Soliman",
"doi-asserted-by": "publisher",
"first-page": "399",
"journal-title": "Drug Dev Ind Pharm",
"key": "ref52",
"volume": "43",
"year": "2017"
},
{
"DOI": "10.3390/pharmaceutics14081727",
"author": "Mekkawy",
"doi-asserted-by": "publisher",
"first-page": "1727",
"journal-title": "Pharmaceutics",
"key": "ref53",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1080/10717544.2021.2023236",
"author": "Fathalla",
"doi-asserted-by": "publisher",
"first-page": "374",
"journal-title": "Drug Deliv",
"key": "ref54",
"volume": "29",
"year": "2022"
},
{
"DOI": "10.3390/pharmaceutics14020220",
"author": "Allam",
"doi-asserted-by": "publisher",
"first-page": "220",
"journal-title": "Pharmaceutics",
"key": "ref55",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3390/pharmaceutics11030140",
"author": "Mircioiu",
"doi-asserted-by": "publisher",
"first-page": "140",
"journal-title": "Pharmaceutics",
"key": "ref56",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1016/j.jddst.2020.101778",
"author": "Verekar",
"doi-asserted-by": "publisher",
"first-page": "101778",
"journal-title": "J Drug Deliv Sci Technol",
"key": "ref57",
"volume": "58",
"year": "2020"
},
{
"DOI": "10.1016/j.jddst.2015.10.010",
"author": "Patel",
"doi-asserted-by": "publisher",
"first-page": "154",
"journal-title": "J Drug Deliv Sci Technol",
"key": "ref58",
"volume": "30",
"year": "2015"
},
{
"DOI": "10.3109/10717544.2013.849778",
"author": "Galgatte",
"doi-asserted-by": "publisher",
"first-page": "62",
"journal-title": "Drug Deliv",
"key": "ref59",
"volume": "21",
"year": "2014"
},
{
"author": "Sylvester",
"first-page": "157",
"journal-title": "Drug Des Discov",
"key": "ref60",
"volume": "2011",
"year": "2011"
},
{
"DOI": "10.3390/ph13120443",
"author": "Mostafa",
"doi-asserted-by": "publisher",
"first-page": "443",
"journal-title": "Pharmaceuticals",
"key": "ref61",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1007/s10895-020-02574-3",
"author": "Karimi",
"doi-asserted-by": "publisher",
"first-page": "1113",
"journal-title": "J Fluoresc",
"key": "ref62",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1016/j.saa.2021.119806",
"author": "Abdel-Lateef",
"doi-asserted-by": "publisher",
"first-page": "119806",
"journal-title": "Spectrochim Acta A Mol Biomol Spectrosc",
"key": "ref63",
"volume": "258",
"year": "2021"
},
{
"DOI": "10.1016/j.intimp.2013.08.008",
"author": "Chauhan",
"doi-asserted-by": "publisher",
"first-page": "733",
"journal-title": "Int Immunopharmacol",
"key": "ref64",
"volume": "17",
"year": "2013"
},
{
"DOI": "10.3390/pharmaceutics12050451",
"author": "E. Eleraky",
"doi-asserted-by": "publisher",
"first-page": "451",
"journal-title": "Pharmaceutics",
"key": "ref65",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.2147/IJN.S266676",
"author": "Omar",
"doi-asserted-by": "publisher",
"first-page": "683",
"journal-title": "Int J Nanomedicine",
"key": "ref66",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1016/j.jddst.2018.10.027",
"author": "Ahmed",
"doi-asserted-by": "publisher",
"first-page": "499",
"journal-title": "J Drug Deliv Sci Technol",
"key": "ref67",
"volume": "48",
"year": "2018"
},
{
"DOI": "10.1038/s41467-020-18233-x",
"author": "Fu",
"doi-asserted-by": "publisher",
"first-page": "4417",
"journal-title": "Nat Commun",
"key": "ref68",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1056/NEJMp2032369",
"author": "Rubin",
"doi-asserted-by": "publisher",
"first-page": "2598",
"journal-title": "N Eng J Med",
"key": "ref69",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1126/science.abc1560",
"author": "Yin",
"doi-asserted-by": "publisher",
"first-page": "1499",
"journal-title": "Science",
"key": "ref70",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1038/nature02145",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "450",
"journal-title": "Nature",
"key": "ref71",
"volume": "426",
"year": "2003"
},
{
"DOI": "10.1128/jvi.00442-08",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "6984",
"journal-title": "J Virol",
"key": "ref72",
"volume": "82",
"year": "2008"
},
{
"DOI": "10.1074/jbc.M111.325803",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "8904",
"journal-title": "J Biol Chem",
"key": "ref73",
"volume": "287",
"year": "2012"
},
{
"DOI": "10.1038/s41586-020-2179-y",
"author": "Shang",
"doi-asserted-by": "publisher",
"first-page": "221",
"journal-title": "Nature",
"key": "ref74",
"volume": "581",
"year": "2020"
},
{
"DOI": "10.3390/nano12172954",
"author": "Tamilarasan",
"doi-asserted-by": "publisher",
"first-page": "2954",
"journal-title": "Nanomaterials",
"key": "ref75",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.3390/pharmaceutics14081695",
"author": "Uwaezuoke",
"doi-asserted-by": "publisher",
"first-page": "1695",
"journal-title": "Pharmaceutics",
"key": "ref76",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3390/pharmaceutics12050465",
"author": "Mazyed",
"doi-asserted-by": "publisher",
"first-page": "465",
"journal-title": "Pharmaceutics",
"key": "ref77",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1186/s43094-022-00428-2",
"author": "Varia",
"doi-asserted-by": "publisher",
"first-page": "39",
"journal-title": "Future J Pharm Sci",
"key": "ref78",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1016/j.xphs.2018.01.005",
"author": "Bnyan",
"doi-asserted-by": "publisher",
"first-page": "1237",
"journal-title": "J Pharm Sci",
"key": "ref79",
"volume": "107",
"year": "2018"
},
{
"DOI": "10.1186/s43094-022-00428-2",
"author": "Varia",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Future J Pharm Sci",
"key": "ref80",
"volume": "8",
"year": "2022"
},
{
"author": "Petchsomrit",
"first-page": "864",
"journal-title": "Int J Pharmacol Pharmaceutical Sci",
"key": "ref81",
"volume": "7",
"year": "2013"
},
{
"DOI": "10.1016/j.ijpharm.2017.11.023",
"author": "Elsheikh",
"doi-asserted-by": "publisher",
"first-page": "316",
"journal-title": "Int J Pharm",
"key": "ref82",
"volume": "535",
"year": "2018"
},
{
"DOI": "10.3109/08982104.2014.950276",
"author": "Ahmed",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "J Liposome Res",
"key": "ref83",
"volume": "25",
"year": "2015"
},
{
"DOI": "10.1016/j.ijpharm.2010.06.034",
"author": "El Zaafarany",
"doi-asserted-by": "publisher",
"first-page": "164",
"journal-title": "Int J Pharm",
"key": "ref84",
"volume": "397",
"year": "2010"
},
{
"DOI": "10.1016/j.jddst.2020.101732",
"author": "El-Gizawy",
"doi-asserted-by": "publisher",
"first-page": "101732",
"journal-title": "J Drug Deliv Sci Technol",
"key": "ref85",
"volume": "58",
"year": "2020"
},
{
"DOI": "10.1016/j.ijpharm.2013.07.031",
"author": "Chaudhary",
"doi-asserted-by": "publisher",
"first-page": "367",
"journal-title": "Int J Pharm",
"key": "ref86",
"volume": "454",
"year": "2013"
},
{
"DOI": "10.1016/S0378-5173(01)00971-1",
"author": "Manconi",
"doi-asserted-by": "publisher",
"first-page": "237",
"journal-title": "Int J Pharm",
"key": "ref87",
"volume": "234",
"year": "2002"
},
{
"DOI": "10.1063/1.3079097",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "094905",
"journal-title": "J Chem Phys",
"key": "ref88",
"volume": "130",
"year": "2009"
},
{
"DOI": "10.1081/DDC-120025458",
"author": "Jain",
"doi-asserted-by": "publisher",
"first-page": "1013",
"journal-title": "Drug Dev Ind Pharm",
"key": "ref89",
"volume": "29",
"year": "2003"
},
{
"DOI": "10.3109/10717544.2015.1013587",
"author": "Aboud",
"doi-asserted-by": "publisher",
"first-page": "2471",
"journal-title": "Drug Deliv",
"key": "ref90",
"volume": "23",
"year": "2016"
},
{
"DOI": "10.3109/08982104.2014.941861",
"author": "Abdelbary",
"doi-asserted-by": "publisher",
"first-page": "107",
"journal-title": "J Liposome Res",
"key": "ref91",
"volume": "25",
"year": "2015"
},
{
"DOI": "10.1111/jphp.13149",
"author": "Bnyan",
"doi-asserted-by": "publisher",
"first-page": "1508",
"journal-title": "J Pharm Pharmacol",
"key": "ref92",
"volume": "71",
"year": "2019"
},
{
"DOI": "10.1208/pt050111",
"author": "Young",
"doi-asserted-by": "publisher",
"first-page": "70",
"journal-title": "Aaps Pharmscitech",
"key": "ref93",
"volume": "5",
"year": "2004"
},
{
"DOI": "10.3390/pharmaceutics10010026",
"author": "Qushawy",
"doi-asserted-by": "publisher",
"first-page": "26",
"journal-title": "Pharmaceutics",
"key": "ref94",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.3390/pharmaceutics10040185",
"author": "Yeo",
"doi-asserted-by": "publisher",
"first-page": "185",
"journal-title": "Pharmaceutics",
"key": "ref95",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.2217/nnm-2019-0320",
"author": "Rabia",
"doi-asserted-by": "publisher",
"first-page": "183",
"journal-title": "Nanomedicine",
"key": "ref96",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1021/la062042s",
"author": "Studart",
"doi-asserted-by": "publisher",
"first-page": "1081",
"journal-title": "Langmuir",
"key": "ref97",
"volume": "23",
"year": "2007"
},
{
"DOI": "10.1016/j.jsps.2017.01.006",
"author": "Ahad",
"doi-asserted-by": "publisher",
"first-page": "1040",
"journal-title": "Saudi Pharmaceutical j",
"key": "ref98",
"volume": "25",
"year": "2017"
},
{
"key": "ref99",
"volume-title": "Phospholipids as Functional Excipients in Solid Oral Dosage Forms",
"year": "2018"
},
{
"DOI": "10.3109/03639045.2014.884127",
"author": "Rachmawati",
"doi-asserted-by": "publisher",
"first-page": "560",
"journal-title": "Drug Dev Ind Pharm",
"key": "ref100",
"volume": "41",
"year": "2015"
},
{
"DOI": "10.1080/10837450.2017.1330345",
"author": "Ahad",
"doi-asserted-by": "publisher",
"first-page": "787",
"journal-title": "Pharm Dev Technol",
"key": "ref101",
"volume": "23",
"year": "2018"
},
{
"DOI": "10.1016/j.jconrel.2004.11.020",
"author": "Sinico",
"doi-asserted-by": "publisher",
"first-page": "123",
"journal-title": "J Controlled Release",
"key": "ref102",
"volume": "103",
"year": "2005"
},
{
"DOI": "10.3109/08982104.2013.788025",
"author": "Basha",
"doi-asserted-by": "publisher",
"first-page": "203",
"journal-title": "J Liposome Res",
"key": "ref103",
"volume": "23",
"year": "2013"
},
{
"DOI": "10.1080/10717544.2018.1451572",
"author": "Aziz",
"doi-asserted-by": "publisher",
"first-page": "815",
"journal-title": "Drug Deliv",
"key": "ref104",
"volume": "25",
"year": "2018"
},
{
"DOI": "10.1016/S0378-5173(00)00362-8",
"author": "Arunothayanun",
"doi-asserted-by": "publisher",
"first-page": "7",
"journal-title": "Int J Pharm",
"key": "ref105",
"volume": "201",
"year": "2000"
},
{
"DOI": "10.1016/j.jsps.2012.02.001",
"author": "Malakar",
"doi-asserted-by": "publisher",
"first-page": "355",
"journal-title": "Saudi Pharmaceutical j",
"key": "ref106",
"volume": "20",
"year": "2012"
},
{
"DOI": "10.3390/pharmaceutics13111749",
"author": "Kazmi",
"doi-asserted-by": "publisher",
"first-page": "1749",
"journal-title": "Pharmaceutics",
"key": "ref107",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1208/s12249-019-1353-8",
"author": "Salem",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Aaps pharmscitech",
"key": "ref108",
"volume": "20",
"year": "2019"
},
{
"DOI": "10.1016/j.ejpb.2017.10.008",
"author": "Mura",
"doi-asserted-by": "publisher",
"first-page": "54",
"journal-title": "Eur J Pharmaceutics Biopharmaceutics",
"key": "ref109",
"volume": "122",
"year": "2018"
},
{
"DOI": "10.1208/s12249-017-0747-8",
"author": "Altuntaş",
"doi-asserted-by": "publisher",
"first-page": "2673",
"journal-title": "AAPS PharmSciTech",
"key": "ref110",
"volume": "18",
"year": "2017"
},
{
"DOI": "10.3390/gels8060342",
"author": "Nair",
"doi-asserted-by": "publisher",
"first-page": "342",
"journal-title": "Gels",
"key": "ref111",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.3390/pharmaceutics14030526",
"author": "Durgun",
"doi-asserted-by": "publisher",
"first-page": "526",
"journal-title": "Pharmaceutics",
"key": "ref112",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1016/j.ejpb.2017.01.008",
"author": "Fathalla",
"doi-asserted-by": "publisher",
"first-page": "119",
"journal-title": "Eur J Pharmaceutics Biopharmaceutics",
"key": "ref113",
"volume": "114",
"year": "2017"
},
{
"DOI": "10.1208/s12249-018-1010-7",
"author": "Boonlai",
"doi-asserted-by": "publisher",
"first-page": "2103",
"journal-title": "AAPS PharmSciTech",
"key": "ref114",
"volume": "19",
"year": "2018"
},
{
"DOI": "10.1016/j.ijpharm.2018.01.032",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "50",
"journal-title": "Int J Pharm",
"key": "ref115",
"volume": "539",
"year": "2018"
},
{
"DOI": "10.1016/j.molliq.2022.119267",
"author": "Zueva",
"doi-asserted-by": "publisher",
"first-page": "119267",
"journal-title": "J Mol Liq",
"key": "ref116",
"volume": "359",
"year": "2022"
},
{
"DOI": "10.1155/2022/8451055",
"author": "Arkin",
"doi-asserted-by": "publisher",
"journal-title": "Evidence Based Complementary Alternative Med",
"key": "ref117",
"volume": "2022",
"year": "2022"
},
{
"DOI": "10.1016/j.ijpharm.2013.07.006",
"author": "Zhou",
"doi-asserted-by": "publisher",
"first-page": "125",
"journal-title": "Int J Pharm",
"key": "ref118",
"volume": "454",
"year": "2013"
},
{
"DOI": "10.1080/03639045.2020.1753061",
"author": "Teaima",
"doi-asserted-by": "publisher",
"first-page": "751",
"journal-title": "Drug Dev Ind Pharm",
"key": "ref119",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1016/S0378-5173(02)00232-6",
"author": "Chang",
"doi-asserted-by": "publisher",
"first-page": "155",
"journal-title": "Int J Pharm",
"key": "ref120",
"volume": "241",
"year": "2002"
},
{
"DOI": "10.1016/j.biopha.2021.112008",
"author": "El-Feky",
"doi-asserted-by": "publisher",
"first-page": "112008",
"journal-title": "Biomed Pharmacother",
"key": "ref121",
"volume": "142",
"year": "2021"
},
{
"DOI": "10.3390/pharmaceutics14112405",
"author": "Teaima",
"doi-asserted-by": "publisher",
"first-page": "2405",
"journal-title": "Pharmaceutics",
"key": "ref122",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.3390/molecules200814293",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "14293",
"journal-title": "Molecules",
"key": "ref123",
"volume": "20",
"year": "2015"
},
{
"DOI": "10.1039/C4RA07300B",
"author": "Singh",
"doi-asserted-by": "publisher",
"first-page": "60334",
"journal-title": "RSC Adv",
"key": "ref124",
"volume": "4",
"year": "2014"
},
{
"DOI": "10.3390/pharmaceutics12010025",
"author": "Salama",
"doi-asserted-by": "publisher",
"first-page": "25",
"journal-title": "Pharmaceutics",
"key": "ref125",
"volume": "12",
"year": "2019"
},
{
"DOI": "10.4103/2231-4040.85541",
"author": "Sahoo",
"doi-asserted-by": "publisher",
"first-page": "195",
"journal-title": "J Adv Pharm Technol Res",
"key": "ref126",
"volume": "2",
"year": "2011"
},
{
"DOI": "10.1002/jcp.30233",
"author": "Tahmasebi",
"doi-asserted-by": "publisher",
"first-page": "5325",
"journal-title": "J Cell Physiol",
"key": "ref127",
"volume": "236",
"year": "2021"
},
{
"author": "Arun Raj",
"first-page": "19",
"journal-title": "Res Rev AJ Drug Formul Dev Prod",
"key": "ref128",
"volume": "6",
"year": "2019"
},
{
"DOI": "10.3390/pharmaceutics12050476",
"author": "Bonaccorso",
"doi-asserted-by": "publisher",
"first-page": "476",
"journal-title": "Pharmaceutics",
"key": "ref129",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1080/03639045.2016.1272120",
"author": "El-Sayed",
"doi-asserted-by": "publisher",
"first-page": "902",
"journal-title": "Drug Dev Ind Pharm",
"key": "ref130",
"volume": "43",
"year": "2017"
},
{
"DOI": "10.1146/annurev-food-032818-121738",
"author": "Sanidad",
"doi-asserted-by": "publisher",
"first-page": "597",
"journal-title": "Annu Rev Food Sci Technol",
"key": "ref131",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1016/j.ejpb.2010.12.010",
"author": "Manconi",
"doi-asserted-by": "publisher",
"first-page": "27",
"journal-title": "Eur J Pharmaceutics Biopharmaceutics",
"key": "ref132",
"volume": "78",
"year": "2011"
},
{
"DOI": "10.3109/10717540903447194",
"author": "Mahajan",
"doi-asserted-by": "publisher",
"first-page": "19",
"journal-title": "Drug Deliv",
"key": "ref133",
"volume": "17",
"year": "2010"
},
{
"DOI": "10.1208/s12249-015-0441-7",
"author": "Abdellatif",
"doi-asserted-by": "publisher",
"first-page": "1067",
"journal-title": "Aaps Pharmscitech",
"key": "ref134",
"volume": "17",
"year": "2016"
},
{
"DOI": "10.1016/j.ijpharm.2014.06.041",
"author": "Al-Mahallawi",
"doi-asserted-by": "publisher",
"first-page": "304",
"journal-title": "Int J Pharm",
"key": "ref135",
"volume": "472",
"year": "2014"
},
{
"DOI": "10.1080/10717544.2017.1321061",
"author": "Elkomy",
"doi-asserted-by": "publisher",
"first-page": "781",
"journal-title": "Drug Deliv",
"key": "ref136",
"volume": "24",
"year": "2017"
},
{
"DOI": "10.2147/IJN.S277352",
"author": "Elsenosy",
"doi-asserted-by": "publisher",
"first-page": "9517",
"journal-title": "Int J Nanomedicine",
"key": "ref137",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1016/S0378-5173(00)00493-2",
"author": "El Maghraby",
"doi-asserted-by": "publisher",
"first-page": "159",
"journal-title": "Int J Pharm",
"key": "ref138",
"volume": "204",
"year": "2000"
},
{
"DOI": "10.1211/jpp.58.2.0002",
"author": "Oh",
"doi-asserted-by": "publisher",
"first-page": "161",
"journal-title": "J Pharm Pharmacol",
"key": "ref139",
"volume": "58",
"year": "2006"
},
{
"DOI": "10.1016/j.nano.2011.06.004",
"author": "Ahad",
"doi-asserted-by": "publisher",
"first-page": "237",
"journal-title": "Nanomedicine",
"key": "ref140",
"volume": "8",
"year": "2012"
},
{
"DOI": "10.1080/10717544.2016.1245369",
"author": "Moawad",
"doi-asserted-by": "publisher",
"first-page": "252",
"journal-title": "Drug Deliv",
"key": "ref141",
"volume": "24",
"year": "2017"
},
{
"DOI": "10.2147/DDDT.S158018",
"author": "Abdulbaqi",
"doi-asserted-by": "publisher",
"first-page": "795",
"journal-title": "Drug Des Devel Ther",
"key": "ref142",
"volume": "12",
"year": "2018"
},
{
"DOI": "10.2147/IJN.S181587",
"author": "Ahmed",
"doi-asserted-by": "publisher",
"first-page": "6325",
"journal-title": "Int J Nanomedicine",
"key": "ref143",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.18433/J3HW2B",
"author": "Almeida",
"doi-asserted-by": "publisher",
"first-page": "592",
"journal-title": "J Pharm Pharmaceutical Sci",
"key": "ref144",
"volume": "15",
"year": "2012"
},
{
"DOI": "10.1208/s12249-012-9871-7",
"author": "Patel",
"doi-asserted-by": "publisher",
"first-page": "1502",
"journal-title": "AAPS pharmscitech",
"key": "ref145",
"volume": "13",
"year": "2012"
},
{
"DOI": "10.1007/s12272-016-0782-0",
"author": "Salatin",
"doi-asserted-by": "publisher",
"first-page": "1181",
"journal-title": "Arch Pharm Res",
"key": "ref146",
"volume": "39",
"year": "2016"
},
{
"DOI": "10.3390/nano10071270",
"author": "Ahmed",
"doi-asserted-by": "publisher",
"first-page": "1270",
"journal-title": "Nanomaterials",
"key": "ref147",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1016/j.jddst.2017.01.006",
"author": "Das",
"doi-asserted-by": "publisher",
"first-page": "59",
"journal-title": "J Drug Deliv Sci Technol",
"key": "ref148",
"volume": "38",
"year": "2017"
},
{
"DOI": "10.3109/1061186X.2015.1070855",
"author": "Garg",
"doi-asserted-by": "publisher",
"first-page": "233",
"journal-title": "J Drug Target",
"key": "ref149",
"volume": "24",
"year": "2016"
},
{
"DOI": "10.3390/antibiotics11091176",
"author": "Mazzone",
"doi-asserted-by": "publisher",
"first-page": "1176",
"journal-title": "Antibiotics",
"key": "ref150",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.3390/pharmaceutics15010022",
"author": "Abdellatif",
"doi-asserted-by": "publisher",
"first-page": "22",
"journal-title": "Pharmaceutics",
"key": "ref151",
"volume": "15",
"year": "2022"
},
{
"DOI": "10.3390/pharmaceutics13030307",
"author": "Al-Rabia",
"doi-asserted-by": "publisher",
"first-page": "307",
"journal-title": "Pharmaceutics",
"key": "ref152",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1155/2020/3012198",
"author": "Ng",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Biomed Res Int",
"key": "ref153",
"volume": "2020",
"year": "2020"
},
{
"author": "Monavari",
"first-page": "99",
"journal-title": "Med J Islam Repub Iran",
"key": "ref154",
"volume": "28",
"year": "2014"
},
{
"DOI": "10.1016/j.ajps.2019.05.001",
"author": "Shah",
"doi-asserted-by": "publisher",
"first-page": "786",
"journal-title": "Asian J Pharm Sci",
"key": "ref155",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1080/10717544.2021.1934190",
"author": "Zakaria",
"doi-asserted-by": "publisher",
"first-page": "1150",
"journal-title": "Drug Deliv",
"key": "ref156",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.3390/molecules25235668",
"author": "Badria",
"doi-asserted-by": "publisher",
"first-page": "5668",
"journal-title": "Molecules",
"key": "ref157",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.3390/scipharm88010009",
"author": "El-Halim",
"doi-asserted-by": "publisher",
"first-page": "9",
"journal-title": "Sci Pharm",
"key": "ref158",
"volume": "88",
"year": "2020"
},
{
"DOI": "10.3390/v10070360",
"author": "Brezáni",
"doi-asserted-by": "publisher",
"first-page": "360",
"journal-title": "Viruses",
"key": "ref159",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.1208/s12249-021-02197-2",
"author": "Mahmoud",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "AAPS PharmSciTech",
"key": "ref160",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1016/j.jff.2017.12.017",
"author": "Mathew",
"doi-asserted-by": "publisher",
"first-page": "692",
"journal-title": "J Funct Foods",
"key": "ref161",
"volume": "40",
"year": "2018"
},
{
"DOI": "10.1080/21691401.2020.1725023",
"author": "Bonfim",
"doi-asserted-by": "publisher",
"first-page": "515",
"journal-title": "Artif Cells, Nanomed Biotechnol",
"key": "ref162",
"volume": "48",
"year": "2020"
},
{
"DOI": "10.1016/j.jconrel.2019.10.053",
"author": "Ma",
"doi-asserted-by": "publisher",
"first-page": "359",
"journal-title": "J Controlled Release",
"key": "ref163",
"volume": "316",
"year": "2019"
},
{
"DOI": "10.1016/j.jconrel.2016.01.018",
"author": "Mehanny",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "J Controlled Release",
"key": "ref164",
"volume": "225",
"year": "2016"
},
{
"DOI": "10.1021/acs.molpharmaceut.0c00675",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "4256",
"journal-title": "Mol Pharm",
"key": "ref165",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.4143/crt.2014.46.1.2",
"author": "Prasad",
"doi-asserted-by": "publisher",
"first-page": "2",
"journal-title": "Cancer Res Treatment",
"key": "ref166",
"volume": "46",
"year": "2014"
},
{
"DOI": "10.1016/S0731-7085(96)02024-9",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "1867",
"journal-title": "J Pharm Biomed Anal",
"key": "ref167",
"volume": "15",
"year": "1997"
},
{
"DOI": "10.3109/1061186X.2012.719230",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "831",
"journal-title": "J Drug Target",
"key": "ref168",
"volume": "20",
"year": "2012"
},
{
"DOI": "10.1002/jps.22333",
"author": "Yu",
"doi-asserted-by": "publisher",
"first-page": "933",
"journal-title": "J Pharm Sci",
"key": "ref169",
"volume": "100",
"year": "2011"
},
{
"DOI": "10.2147/DDDT.S113171",
"author": "Al Asmari",
"doi-asserted-by": "crossref",
"first-page": "205",
"journal-title": "Drug Des Devel Ther",
"key": "ref170",
"volume": "10",
"year": "2016"
},
{
"DOI": "10.1021/jm0607193",
"author": "Ryu",
"doi-asserted-by": "publisher",
"first-page": "6111",
"journal-title": "J Med Chem",
"key": "ref171",
"volume": "49",
"year": "2006"
},
{
"DOI": "10.1016/0300-483X(80)90122-5",
"author": "Ravindranath",
"doi-asserted-by": "publisher",
"first-page": "259",
"journal-title": "Toxicology",
"key": "ref172",
"volume": "16",
"year": "1980"
},
{
"author": "Pan",
"first-page": "486",
"journal-title": "Drug Metabolism Disposition",
"key": "ref173",
"volume": "27",
"year": "1999"
},
{
"author": "Jiang",
"first-page": "3878",
"journal-title": "IEEE",
"key": "ref174",
"year": "2011"
},
{
"DOI": "10.1039/C5RA24032H",
"author": "Manca",
"doi-asserted-by": "publisher",
"first-page": "105149",
"journal-title": "RSC Adv",
"key": "ref175",
"volume": "5",
"year": "2015"
},
{
"DOI": "10.1371/journal.pone.0057285",
"author": "Avasarala",
"doi-asserted-by": "publisher",
"first-page": "e57285",
"journal-title": "PLoS One",
"key": "ref176",
"volume": "8",
"year": "2013"
},
{
"DOI": "10.1096/fj.201901047RR",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "13294",
"journal-title": "FASEB J",
"key": "ref177",
"volume": "33",
"year": "2019"
},
{
"DOI": "10.1165/rcmb.2011-0175OC",
"author": "Suresh",
"doi-asserted-by": "publisher",
"first-page": "280",
"journal-title": "Am J Respir Cell Mol Biol",
"key": "ref178",
"volume": "47",
"year": "2012"
},
{
"DOI": "10.3390/nu11092147",
"author": "Dei Cas",
"doi-asserted-by": "publisher",
"first-page": "2147",
"journal-title": "Nutrients",
"key": "ref179",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1016/j.neuroscience.2004.05.029",
"author": "Thorne",
"doi-asserted-by": "publisher",
"first-page": "481",
"journal-title": "Neuroscience",
"key": "ref180",
"volume": "127",
"year": "2004"
},
{
"DOI": "10.3109/10717544.2014.975382",
"author": "Madane",
"doi-asserted-by": "publisher",
"first-page": "1326",
"journal-title": "Drug Deliv",
"key": "ref181",
"volume": "23",
"year": "2016"
},
{
"DOI": "10.1016/j.ijpharm.2020.119725",
"author": "Sintov",
"doi-asserted-by": "publisher",
"first-page": "119725",
"journal-title": "Int J Pharm",
"key": "ref182",
"volume": "588",
"year": "2020"
},
{
"DOI": "10.1002/ptr.7004",
"author": "Saber‐Moghaddam",
"doi-asserted-by": "publisher",
"first-page": "2616",
"journal-title": "Phytotherapy Res",
"key": "ref183",
"volume": "35",
"year": "2021"
}
],
"reference-count": 183,
"references-count": 183,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.dovepress.com/curcumin-transferosome-loaded-thermosensitive-intranasal-in-situ-gel-a-peer-reviewed-fulltext-article-IJN"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Organic Chemistry",
"Drug Discovery",
"General Medicine",
"Biomaterials",
"Bioengineering",
"Biophysics",
"Pharmaceutical Science"
],
"subtitle": [],
"title": "Curcumin Transferosome-Loaded Thermosensitive Intranasal in situ Gel as Prospective Antiviral Therapy for SARS-Cov-2",
"type": "journal-article",
"volume": "Volume 18"
}
