In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds
et al., Natural Product Communications, doi:10.1177/1934578X231188861, Sep 2023
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
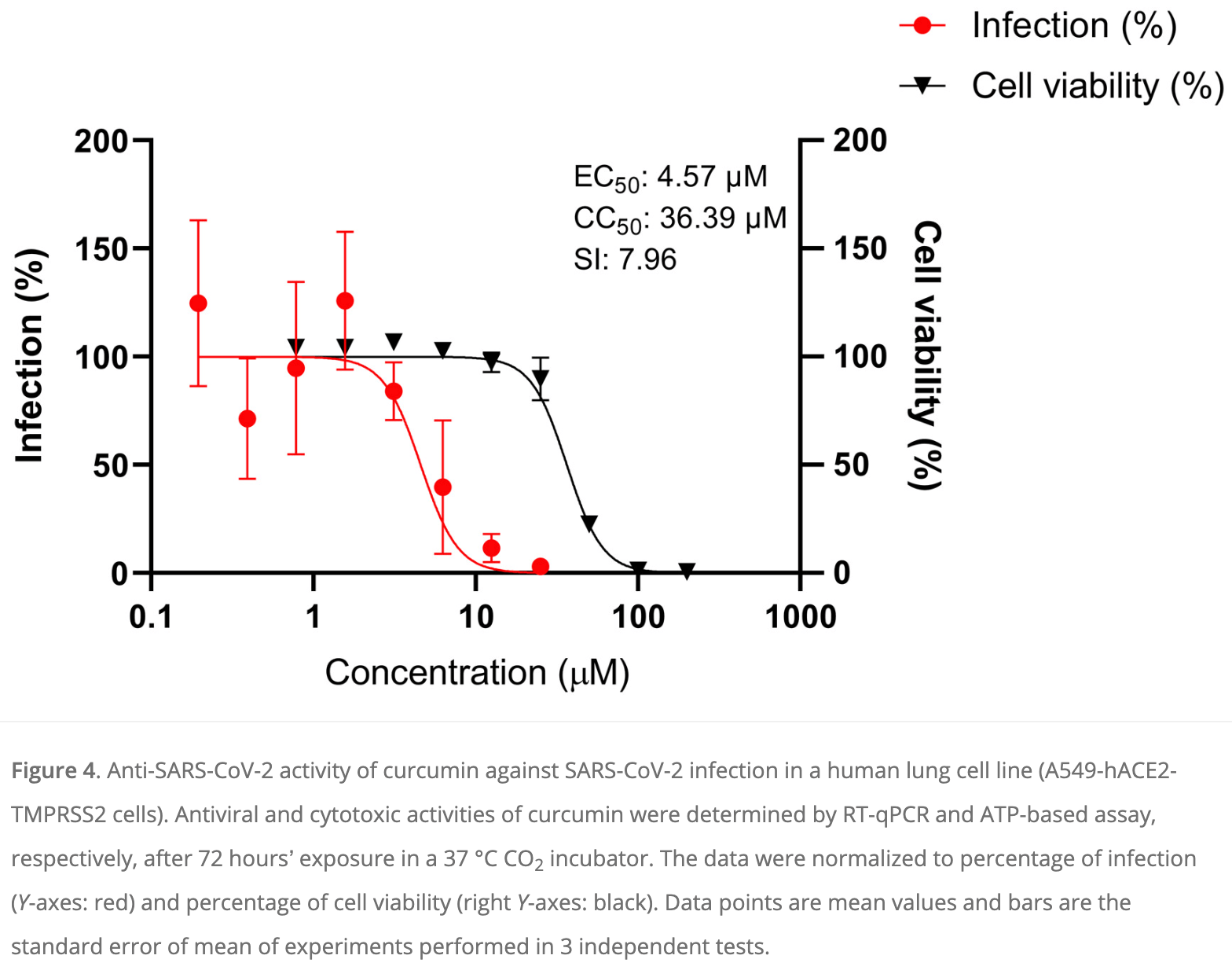
In vitro study showing that of 9 phenolic compounds tested, only curcumin inhibited SARS-CoV-2 cytopathic effects in infected monkey kidney Vero E6 cells. Curcumin showed antiviral activity against wildtype, alpha, delta, and omicron variants, with EC50 values around 25µM for the variants and 13.63µM for the wildtype, but with a low selectivity index (SI < 5). Curcumin was more effective against SARS-CoV-2 infection in human lung A549 cells expressing ACE2 and TMPRSS2 receptors, with an EC50 of 4.57μM and a higher selectivity index of 7.96. Curcumin also inhibited SARS-CoV-2 spike protein-ACE2 interaction and 3CL protease activity at 10-20μM concentrations. The results suggest curcumin has moderate antiviral activity against SARS-CoV-2 through multiple targets, although bioavailability may limit efficacy, requiring formulations for improved bioavailability.
Positive controls andrographolide, chloroquine, and remdesivir inhibited the viral-induced CPE with EC50 values of less than 10μM with a high selectivity index (SI ≥ 10).
62 preclinical studies support the efficacy of curcumin for COVID-19:
In silico studies predict inhibition of SARS-CoV-2 with curcumin or metabolites via binding to the spikeA,1,5,6,11,16,18,24,27 (and specifically the receptor binding domainB,2,4,14,17,20 ), MproC,4-6,11,13,15-17,19,20,22,25,27,28,30,48 , RNA-dependent RNA polymeraseD,4-6,17,26 , PLproE,6, ACE2F,2,18,19,21 , nucleocapsidG,12,29 , nsp10H,29, and helicaseI,36 proteins, and inhibition of spike-ACE2 interactionJ,3.
In vitro studies demonstrate inhibition of the spikeA,41 (and specifically the receptor binding domainB,51), MproC,23,41,48,50 , ACE2F,51, and TMPRSS2K,51 proteins, and inhibition of spike-ACE2 interactionJ,3,34 .
In vitro studies demonstrate efficacy in Calu-3L,49, A549M,41, A549-ATN,31, 293TO,7, HEK293-hACE2P,23,39 , 293T/hACE2/TMPRSS2Q,40, Vero E6R,1,13,17,27,39,41,43,45,47,49 , and SH-SY5YS,38 cells.
Curcumin decreases pro-inflammatory cytokines induced by SARS-CoV-2 in peripheral blood mononuclear cells47, alleviates SARS-CoV-2 spike protein-induced mitochondrial membrane damage and oxidative stress7, may limit COVID-19 induced cardiac damage by inhibiting the NF-κB signaling pathway which mediates the profibrotic effects of the SARS-CoV-2 spike protein on cardiac fibroblasts35, is predicted to inhibit the interaction between the SARS-CoV-2 spike protein receptor binding domain and the human ACE2 receptor for the delta and omicron variants14, lowers ACE2 and STAT3, curbing lung inflammation and ARDS in preclinical COVID-19 models32, inhibits SARS-CoV-2 ORF3a ion channel activity, which contributes to viral pathogenicity and cytotoxicity42, has direct virucidal action by disrupting viral envelope integrity44, may inhibit viral replication and modulate inflammatory pathways like NF-κB via SIRT1 activation52, and can function as a photosensitizer in photodynamic therapy to generate reactive oxygen species that damage the virus44.
1.
Marzouk et al., Computational and Experimental Insights into the Antiviral Mechanism of Turmeric (Curcuma longa) against SARS-CoV-2 D614G, BIO Web of Conferences, doi:10.1051/bioconf/202519804002.
2.
Wu et al., Utilizing natural compounds as ligands to disrupt the binding of SARS-CoV-2 receptor-binding domain to angiotensin-converting enzyme 2, impeding viral infection, Phytochemistry Letters, doi:10.1016/j.phytol.2025.102999.
3.
Najimi et al., Phytochemical Inhibitors of SARS‐CoV‐2 Entry: Targeting the ACE2‐RBD Interaction with l‐Tartaric Acid, l‐Ascorbic Acid, and Curcuma longa Extract, ChemistrySelect, doi:10.1002/slct.202406035.
4.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
5.
Al balawi et al., Assessing multi-target antiviral and antioxidant activities of natural compounds against SARS-CoV-2: an integrated in vitro and in silico study, Bioresources and Bioprocessing, doi:10.1186/s40643-024-00822-z.
6.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
7.
Zhang et al., Computational Discovery of Mitochondrial Dysfunction Biomarkers in Severe SARS-CoV-2 Infection: Facilitating Pytomedicine Screening, Phytomedicine, doi:10.1016/j.phymed.2024.155784.
8.
Öztürkkan et al., In Silico investigation of the effects of curcuminoids on the spike protein of the omicron variant of SARS-CoV-2, Baku State University Journal of Chemistry and Material Sciences, 1:2, bsuj.bsu.edu.az/uploads/pdf/ec4204d62f7802de54e6092bf7860029.pdf.
9.
Yunze et al., Therapeutic effect and potential mechanism of curcumin, an active ingredient in Tongnao Decoction, on COVID-19 combined with stroke: a network pharmacology study and GEO database mining, Research Square, doi:10.21203/rs.3.rs-4329762/v1.
10.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
11.
Boseila et al., Throat spray formulated with virucidal Pharmaceutical excipients as an effective early prophylactic or treatment strategy against pharyngitis post-exposure to SARS CoV-2, European Journal of Pharmaceutics and Biopharmaceutics, doi:10.1016/j.ejpb.2024.114279.
12.
Hidayah et al., Bioinformatics study of curcumin, demethoxycurcumin, bisdemethoxycurcumin and cyclocurcumin compounds in Curcuma longa as an antiviral agent via nucleocapsid on SARS-CoV-2 inhibition, International Conference on Organic and Applied Chemistry, doi:10.1063/5.0197724.
13.
Singh et al., Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 M Pro protein - An in-silico and cell-based approach, Research Square, doi:10.21203/rs.3.rs-3888947/v1.
14.
Kant et al., Structure-based drug discovery to identify SARS-CoV2 spike protein–ACE2 interaction inhibitors, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2023.2300060.
15.
Naderi Beni et al., In silico studies of anti-oxidative and hot temperament-based phytochemicals as natural inhibitors of SARS-CoV-2 Mpro, PLOS ONE, doi:10.1371/journal.pone.0295014.
16.
Moschovou et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, International Journal of Molecular Sciences, doi:10.3390/ijms242115894.
17.
Eleraky et al., Curcumin Transferosome-Loaded Thermosensitive Intranasal in situ Gel as Prospective Antiviral Therapy for SARS-Cov-2, International Journal of Nanomedicine, doi:10.2147/IJN.S423251.
18.
Singh (B) et al., Computational studies to analyze effect of curcumin inhibition on coronavirus D614G mutated spike protein, The Seybold Report, doi:10.17605/OSF.IO/TKEXJ.
19.
Thapa et al., In-silico Approach for Predicting the Inhibitory Effect of Home Remedies on Severe Acute Respiratory Syndrome Coronavirus-2, Makara Journal of Science, doi:10.7454/mss.v27i3.1609.
20.
Srivastava et al., Paradigm of Well-Orchestrated Pharmacokinetic Properties of Curcuminoids Relative to Conventional Drugs for the Inactivation of SARS-CoV-2 Receptors: An In Silico Approach, Stresses, doi:10.3390/stresses3030043.
21.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
22.
Winih Kinasih et al., Analisis in silico interaksi senyawa kurkuminoid terhadap enzim main protease 6LU7 dari SARS-CoV-2, Duta Pharma Journal, doi:10.47701/djp.v3i1.2904.
23.
Wu (B) et al., Potential Mechanism of Curcumin and Resveratrol against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-2780614/v1.
24.
Nag et al., Curcumin inhibits spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in silico study, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2022.105552.
25.
Rampogu et al., Molecular Docking and Molecular Dynamics Simulations Discover Curcumin Analogue as a Plausible Dual Inhibitor for SARS-CoV-2, International Journal of Molecular Sciences, doi:10.3390/ijms23031771.
26.
Singh (C) et al., Potential of turmeric-derived compounds against RNA-dependent RNA polymerase of SARS-CoV-2: An in-silico approach, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2021.104965.
27.
Kandeil et al., Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2, Pathogens, doi:10.3390/pathogens10060758.
28.
Rajagopal et al., Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-020-00126-x.
29.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
30.
Sekiou et al., In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2, ChemRxiv, doi:10.26434/chemrxiv.12181404.v1.
31.
Grüneberg et al., Dose-dependent antiviral effects of glycyrrhizin, curcumin, and harmaline against clinical SARS-CoV-2 isolates, including D614G, Omicron BA.5, and Omicron XBB.1, BMC Complementary Medicine and Therapies, doi:10.1186/s12906-026-05253-1.
32.
Aktay et al., Oral Administration of Water-Soluble Curcumin Complex Prevents ARDS With the Potential for COVID-19 Treatment, Phytotherapy Research, doi:10.1002/ptr.70046.
33.
Olubiyi et al., Novel dietary herbal preparations with inhibitory activities against multiple SARS-CoV-2 targets: A multidisciplinary investigation into antiviral activities, Food Chemistry Advances, doi:10.1016/j.focha.2025.100969.
34.
Emam et al., Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors, AMB Express, doi:10.1186/s13568-024-01739-8.
35.
Van Tin et al., Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling, Cells, doi:10.3390/cells13161331.
36.
Li et al., Thermal shift assay (TSA)-based drug screening strategy for rapid discovery of inhibitors against the Nsp13 helicase of SARS-CoV-2, Animals and Zoonoses, doi:10.1016/j.azn.2024.06.001.
37.
Kamble et al., Nanoparticulate curcumin spray imparts prophylactic and therapeutic properties against SARS-CoV-2, Emergent Materials, doi:10.1007/s42247-024-00754-6.
38.
Nicoliche et al., Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2, Scientific Reports, doi:10.1038/s41598-024-61662-7.
39.
Nittayananta et al., A novel film spray containing curcumin inhibits SARS-CoV-2 and influenza virus infection and enhances mucosal immunity, Virology Journal, doi:10.1186/s12985-023-02282-x.
40.
Septisetyani et al., Curcumin and turmeric extract inhibited SARS-CoV-2 pseudovirus cell entry and Spike mediated cell fusion, bioRxiv, doi:10.1101/2023.09.28.560070.
41.
Mohd Abd Razak et al., In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds, Natural Product Communications, doi:10.1177/1934578X231188861.
42.
Fam et al., Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites, Scientific Reports, doi:10.1038/s41598-023-31764-9.
43.
Teshima et al., Antiviral activity of curcumin and its analogs selected by an artificial intelligence-supported activity prediction system in SARS-CoV-2-infected VeroE6 cells, Natural Product Research, doi:10.1080/14786419.2023.2194647.
44.
Zupin et al., Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer, Viruses, doi:10.3390/v14102132.
45.
Leka et al., In vitro antiviral activity against SARS-CoV-2 of common herbal medicinal extracts and their bioactive compounds, Phytotherapy Research, doi:10.1002/ptr.7463.
46.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
47.
Marín-Palma et al., Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms, Molecules, doi:10.3390/molecules26226900.
48.
Bahun et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594.
49.
Bormann et al., Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro, Viruses, doi:10.3390/v13101914.
50.
Guijarro-Real et al., Potential In Vitro Inhibition of Selected Plant Extracts against SARS-CoV-2 Chymotripsin-Like Protease (3CLPro) Activity, Foods, doi:10.3390/foods10071503.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The receptor binding domain is a specific region of the spike protein that binds ACE2 and is a major target of neutralizing antibodies. Focusing on the precise binding site allows highly specific disruption of viral attachment with reduced potential for off-target effects.
c.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
d.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
e.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
f.
The angiotensin converting enzyme 2 (ACE2) protein is a host cell transmembrane protein that serves as the cellular receptor for the SARS-CoV-2 spike protein. ACE2 is expressed on many cell types, including epithelial cells in the lungs, and allows the virus to enter and infect host cells. Inhibition may affect ACE2's physiological function in blood pressure control.
g.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
h.
Non-structural protein 10 (nsp10) serves as an RNA chaperone and stabilizes conformations of nsp12 and nsp14 in the replicase-transcriptase complex, which synthesizes new viral RNAs. Nsp10 disruption may destabilize replicase-transcriptase complex activity.
i.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
j.
The interaction between the SARS-CoV-2 spike protein and the human ACE2 receptor is a primary method of viral entry, inhibiting this interaction can prevent the virus from attaching to and entering host cells, halting infection at an early stage.
k.
Transmembrane protease serine 2 (TMPRSS2) is a host cell protease that primes the spike protein, facilitating cellular entry. TMPRSS2 activity helps enable cleavage of the spike protein required for membrane fusion and virus entry. Inhibition may especially protect respiratory epithelial cells, buy may have physiological effects.
l.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
m.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
n.
A549-AT is a human lung carcinoma cell line stably transfected with ACE2 and TMPRSS2 receptors. Unlike the parental line, this overexpression ensures stable infection and enhanced viral entry, allowing for the evaluation of antiviral efficacy against various SARS-CoV-2 variants.
o.
293T is a human embryonic kidney cell line that can be engineered for high ACE2 expression and SARS-CoV-2 susceptibility. 293T cells are easily transfected and support high protein expression.
p.
HEK293-hACE2 is a human embryonic kidney cell line with high ACE2 expression and SARS-CoV-2 susceptibility. Cells have been transfected with a plasmid to express the human ACE2 (hACE2) protein.
q.
293T/hACE2/TMPRSS2 is a human embryonic kidney cell line engineered for high ACE2 and TMPRSS2 expression, which mimics key aspects of human infection. 293T/hACE2/TMPRSS2 cells are very susceptible to SARS-CoV-2 infection.
r.
Vero E6 is an African green monkey kidney cell line with low/no ACE2 expression and high SARS-CoV-2 susceptibility. The cell line is easy to maintain and supports robust viral replication, however the monkey origin may not accurately represent human responses.
s.
SH-SY5Y is a human neuroblastoma cell line that exhibits neuronal phenotypes. It is commonly used as an in vitro model for studying neurotoxicity, neurodegenerative diseases, and neuronal differentiation.
Mohd Abd Razak et al., 16 Sep 2023, peer-reviewed, 11 authors.
Contact: ridzuan.ar@moh.gov.my.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds
Natural Product Communications, doi:10.1177/1934578x231188861
Since the COVID-19 pandemic in 2020, many reports have highlighted several potential anti-SARS-CoV-2 drug candidates, including phenolic compounds. Therefore, this study aimed to evaluate the anti-SARS-CoV-2 activity of nine common phenolic compounds found in plants using the in vitro cellular infection model. The anti-SARS-CoV-2 activity of curcumin, quercetin, gallic acid, catechin, rutin, kaempferol, naringenin, coumaric acid and caffeic acid were evaluated on SARS-CoV-2-infected Vero E6 cells by using a cytopathic effect (CPE)-based assay. The anti-SARS-CoV-2 activity in human lung cells, A549 expressing human ACE2 and TMPRSS2, was evaluated by the RT-qPCR technique. S1-ACE2 interaction and 3CL protease activity assays were also performed for the potent compound. Of the nine phenolic compounds, only curcumin inhibited the SARS-CoV-2 induced CPE activity (EC 50 of 13.63 µM) in Vero E6 cells, but with a low selective index (SI) value. Interestingly, curcumin exhibited potent anti-SARS-CoV-2 activity in A549 cells with an EC 50 of 4.57 µM and an SI value of 7.96. S1-ACE2 interaction and 3CL protease inhibitory activities of curcumin were also observed. In conclusion, curcumin showed a moderate in vitro anti-SARS-CoV-2 activity. The true potential of curcumin as an anti-SARS-CoV-2 candidate could be further evaluated in a COVID-19 animal model.
Additional Information The data presented in this study are available on request from the corresponding author.
Declaration of Conflicting Interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval Ethical Approval is not applicable for this article.
ORCID iD Mohd Ridzuan Mohd Abd Razak https://orcid.org/0000-0002-9589-5892
Statement of Human and Animal Rights This article does not contain any studies with human or animal subjects.
Informed Consent There are no human subjects in this article and informed consent is not applicable.
References
Abdallah, El-Halawany, Sirwi, Repurposing of some natural product isolates as SARS-COV-2 main protease inhibitors via in vitro cell free and cell-based antiviral assessments and molecular modeling approaches, Pharmaceuticals
Abu-Raddad, Chemaitelly, Butt, National Study Group for C-V. Effectiveness of the BNT162b2 COVID-19 vaccine against the B.1.1.7 and B.1.351 variants, N Engl J Med
Accorsi, Britton, Ke, Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and Delta variants, JAMA
Agrawal, Agrawal, Blunden, Naringenin as a possible candidate against SARS-CoV-2 infection and in the pathogenesis of COVID-19, Nat Prod Commun, doi:10.1177/1934578X211066723
Agrawal, Agrawal, Blunden, Quercetin: antiviral significance and possible COVID-19 integrative considerations, Nat Prod Commun, doi:10.1177/1934578X20976293
Agrawal, Agrawal, Blunden, Rutin: a potential antiviral for repurposing as a SARS-CoV-2 main protease (mpro) inhibitor, Nat Prod Commun, doi:10.1177/1934578X21991723
Alzaabi, Hamdy, Ashmawy, Flavonoids are promising safe therapy against COVID-19, Phytochem Rev
Bahun, Jukic, Oblak, Inhibition of the SARS-CoV-2 3CL(pro) main protease by plant polyphenols, Food Chem
Bormann, Alt, Schipper, Turmeric root and its bioactive ingredient curcumin effectively neutralize SARS-CoV-2 in vitro, Viruses
Chemaitelly, Tang, Hasan, Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar, N Engl J Med
Chen, Shinn, Itkin, Drug repurposing screen for compounds inhibiting the cytopathic effect of SARS-CoV-2, Front Pharmacol, doi:10.3389/fphar.2020.592737
Choy, Wong, Kaewpreedee, Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro, Antiviral Res
Couzin-Frankel, Antiviral pills could change pandemic's course, Science
Drozdzal, Rosik, Lechowicz, An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment, Drug Resist Updat
El Gizawy, Boshra, Mostafa, bioactive constituents exert anti-SARS-CoV-2 and antiinflammatory activities: molecular docking and dynamics, in vitro, and in vivo studies, Molecules
Elfiky, Natural products may interfere with SARS-CoV-2 attachment to the host cell, J Biomol Struct Dyn
Fischer Wa 2nd, Eron, Jr, Holman, A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci Transl Med
Goc, Sumera, Rath, Niedzwiecki, Phenolic compounds disrupt spike-mediated receptor-binding and entry of SARS-CoV-2 pseudo-virions, PLoS One
Gordon, Jang, Bouhaddou, A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature
Harvey, Carabelli, Jackson, SARS-CoV-2 variants, spike mutations and immune escape, Nat Rev Microbiol
Hoffmann, Mosbauer, Hofmann-Winkler, Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2, Nature
Jena, Kanungo, Nayak, Chainy, Dandapat, Catechin and curcumin interact with S protein of SARS-CoV2 and ACE2 of human cell membrane: insights from computational studies, Sci Rep
Kanjanasirirat, Suksatu, Manopwisedjaroen, High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents, Sci Rep
Mangiavacchi, Botwina, Menichetti, Selenofunctionalization of quercetin improves the non-covalent inhibition of M(pro) and its antiviral activity in cells against SARS-CoV-2, Int J Mol Sci
Marin-Palma, Tabares-Guevara, Zapata-Cardona, Curcumin inhibits in vitro SARS-CoV-2 infection in Vero E6 cells through multiple antiviral mechanisms, Molecules
Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J Immunol Methods
Nguyen, Jung, Kim, The inhibitory effects of plant derivate polyphenols on the main protease of SARS coronavirus 2 and their structure-activity relationship, Molecules
Polack, Thomas, Kitchin, Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine, N Engl J Med
Rebello, Beyl, Lertora, Safety and pharmacokinetics of naringenin: a randomized, controlled, single-ascending-dose clinical trial, Diabetes Obes Metab
Sa-Ngiamsuntorn, Suksatu, Pewkliang, Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives, J Nat Prod
Sancineto, Ostacolo, Ortega-Alarcon, L-Arginine improves solubility and Anti-SARS-CoV-2 Mpro activity of rutin but not the antiviral activity in cells, Molecules
Stohs, Ji, Bucci, Preuss, A comparative pharmacokinetic assessment of a novel highly bioavailable curcumin formulation with 95% curcumin: a randomized, double-blind, crossover study, J Am Coll Nutr
Teli, Shah, Chhabria, In silico screening of natural compounds as potential inhibitors of SARS-CoV-2 main protease and spike RBD: targets for COVID-19, Front Mol Biosci, doi:10.3389/fmolb.2020.599079
Vahedian-Azimi, Abbasifard, Rahimi-Bashar, Effectiveness of curcumin on outcomes of hospitalized COVID-19 patients: a systematic review of clinical trials, Nutrients
Vangeel, Chiu, Jonghe, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antiviral Res
Voysey, Clemens, Madhi, Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, Lancet
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Zhang, Zeng, Pan, Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial, Lancet Infect Dis
Zhang, Zhang, Li, A cell-based large-scale screening of natural compounds for inhibitors of SARS-CoV-2, Signal Transduct Target Ther
DOI record:
{
"DOI": "10.1177/1934578x231188861",
"ISSN": [
"1934-578X",
"1555-9475"
],
"URL": "http://dx.doi.org/10.1177/1934578X231188861",
"abstract": "<jats:p> Since the COVID-19 pandemic in 2020, many reports have highlighted several potential anti-SARS-CoV-2 drug candidates, including phenolic compounds. Therefore, this study aimed to evaluate the anti-SARS-CoV-2 activity of nine common phenolic compounds found in plants using the in vitro cellular infection model. The anti-SARS-CoV-2 activity of curcumin, quercetin, gallic acid, catechin, rutin, kaempferol, naringenin, coumaric acid and caffeic acid were evaluated on SARS-CoV-2-infected Vero E6 cells by using a cytopathic effect (CPE)-based assay. The anti-SARS-CoV-2 activity in human lung cells, A549 expressing human ACE2 and TMPRSS2, was evaluated by the RT-qPCR technique. S1-ACE2 interaction and 3CL protease activity assays were also performed for the potent compound. Of the nine phenolic compounds, only curcumin inhibited the SARS-CoV-2 induced CPE activity (EC<jats:sub>50</jats:sub> of 13.63 µM) in Vero E6 cells, but with a low selective index (SI) value. Interestingly, curcumin exhibited potent anti-SARS-CoV-2 activity in A549 cells with an EC<jats:sub>50</jats:sub> of 4.57 µM and an SI value of 7.96. S1-ACE2 interaction and 3CL protease inhibitory activities of curcumin were also observed. In conclusion, curcumin showed a moderate in vitro anti-SARS-CoV-2 activity. The true potential of curcumin as an anti-SARS-CoV-2 candidate could be further evaluated in a COVID-19 animal model. </jats:p>",
"alternative-id": [
"10.1177/1934578X231188861"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9589-5892",
"affiliation": [
{
"name": "Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"authenticated-orcid": false,
"family": "Mohd Abd Razak",
"given": "Mohd Ridzuan",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Md Jelas",
"given": "Nur Hana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Muhammad",
"given": "Amirrudin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Padlan",
"given": "Noorsofiana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Sa’at",
"given": "Muhammad Nor Farhan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Azizan",
"given": "Muhammad Afif",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Rosli",
"given": "Siti Nur Zawani",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Ellan",
"given": "E. Kavithambigai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Zainol",
"given": "Murizal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Thayan",
"given": "Ravindran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Syed Mohamed",
"given": "Ami Fazlin",
"sequence": "additional"
}
],
"container-title": "Natural Product Communications",
"container-title-short": "Natural Product Communications",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2023,
9,
16
]
],
"date-time": "2023-09-16T10:05:15Z",
"timestamp": 1694858715000
},
"deposited": {
"date-parts": [
[
2023,
9,
16
]
],
"date-time": "2023-09-16T10:05:20Z",
"timestamp": 1694858720000
},
"funder": [
{
"DOI": "10.13039/501100013885",
"award": [
"NMRR-20-874-54715 (20-035)"
],
"doi-asserted-by": "publisher",
"name": "Kementerian Kesihatan Malaysia"
}
],
"indexed": {
"date-parts": [
[
2023,
9,
17
]
],
"date-time": "2023-09-17T14:42:47Z",
"timestamp": 1694961767447
},
"is-referenced-by-count": 0,
"issue": "9",
"issued": {
"date-parts": [
[
2023,
9
]
]
},
"journal-issue": {
"issue": "9",
"published-print": {
"date-parts": [
[
2023,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
9,
1
]
],
"date-time": "2023-09-01T00:00:00Z",
"timestamp": 1693526400000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/1934578X231188861",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/1934578X231188861",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/1934578X231188861",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"prefix": "10.1177",
"published": {
"date-parts": [
[
2023,
9
]
]
},
"published-online": {
"date-parts": [
[
2023,
9,
16
]
]
},
"published-print": {
"date-parts": [
[
2023,
9
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1016/S1473-3099(20)30843-4",
"doi-asserted-by": "publisher",
"key": "bibr1-1934578X231188861"
},
{
"DOI": "10.1016/S0140-6736(20)32661-1",
"doi-asserted-by": "publisher",
"key": "bibr2-1934578X231188861"
},
{
"DOI": "10.1056/NEJMoa2034577",
"doi-asserted-by": "publisher",
"key": "bibr3-1934578X231188861"
},
{
"DOI": "10.1056/NEJMc2104974",
"doi-asserted-by": "publisher",
"key": "bibr4-1934578X231188861"
},
{
"DOI": "10.1038/s41579-021-00573-0",
"doi-asserted-by": "publisher",
"key": "bibr5-1934578X231188861"
},
{
"DOI": "10.1056/NEJMoa2114114",
"doi-asserted-by": "publisher",
"key": "bibr6-1934578X231188861"
},
{
"DOI": "10.1001/jama.2022.0470",
"doi-asserted-by": "publisher",
"key": "bibr7-1934578X231188861"
},
{
"DOI": "10.1016/j.drup.2021.100794",
"doi-asserted-by": "publisher",
"key": "bibr8-1934578X231188861"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"doi-asserted-by": "publisher",
"key": "bibr9-1934578X231188861"
},
{
"DOI": "10.1126/science.acx9605",
"doi-asserted-by": "publisher",
"key": "bibr10-1934578X231188861"
},
{
"DOI": "10.1016/j.antiviral.2022.105252",
"doi-asserted-by": "publisher",
"key": "bibr11-1934578X231188861"
},
{
"DOI": "10.3390/ph14030213",
"doi-asserted-by": "publisher",
"key": "bibr12-1934578X231188861"
},
{
"DOI": "10.3389/fphar.2020.592737",
"doi-asserted-by": "publisher",
"key": "bibr13-1934578X231188861"
},
{
"DOI": "10.1021/acs.jnatprod.0c01324",
"doi-asserted-by": "publisher",
"key": "bibr14-1934578X231188861"
},
{
"DOI": "10.1038/s41392-020-00343-z",
"doi-asserted-by": "publisher",
"key": "bibr15-1934578X231188861"
},
{
"author": "Agrawal PK",
"first-page": "1934578X",
"issue": "12",
"journal-title": "Nat Prod Commun.",
"key": "bibr16-1934578X231188861",
"volume": "16",
"year": "2021"
},
{
"author": "Agrawal PK",
"first-page": "1934578X",
"issue": "4",
"journal-title": "Nat Prod Commun.",
"key": "bibr17-1934578X231188861",
"volume": "16",
"year": "2021"
},
{
"author": "Agrawal PK",
"first-page": "1934578X",
"issue": "12",
"journal-title": "Nat Prod Commun.",
"key": "bibr18-1934578X231188861",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.3390/molecules26226900",
"doi-asserted-by": "publisher",
"key": "bibr19-1934578X231188861"
},
{
"DOI": "10.1016/j.foodchem.2021.131594",
"doi-asserted-by": "publisher",
"key": "bibr20-1934578X231188861"
},
{
"DOI": "10.3390/molecules26071924",
"doi-asserted-by": "publisher",
"key": "bibr21-1934578X231188861"
},
{
"DOI": "10.3390/ijms22137048",
"doi-asserted-by": "publisher",
"key": "bibr22-1934578X231188861"
},
{
"DOI": "10.3390/molecules26196062",
"doi-asserted-by": "publisher",
"key": "bibr23-1934578X231188861"
},
{
"DOI": "10.1007/s11101-021-09759-z",
"doi-asserted-by": "publisher",
"key": "bibr24-1934578X231188861"
},
{
"DOI": "10.1038/s41598-021-81462-7",
"doi-asserted-by": "publisher",
"key": "bibr25-1934578X231188861"
},
{
"DOI": "10.3390/v13101914",
"doi-asserted-by": "publisher",
"key": "bibr26-1934578X231188861"
},
{
"author": "Elfiky AA",
"first-page": "3194",
"issue": "9",
"journal-title": "J Biomol Struct Dyn",
"key": "bibr27-1934578X231188861",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "bibr28-1934578X231188861"
},
{
"DOI": "10.1016/0022-1759(83)90303-4",
"doi-asserted-by": "publisher",
"key": "bibr29-1934578X231188861"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"doi-asserted-by": "publisher",
"key": "bibr30-1934578X231188861"
},
{
"DOI": "10.1371/journal.pone.0253489",
"doi-asserted-by": "publisher",
"key": "bibr31-1934578X231188861"
},
{
"DOI": "10.3389/fmolb.2020.599079",
"doi-asserted-by": "publisher",
"key": "bibr32-1934578X231188861"
},
{
"DOI": "10.1080/07315724.2017.1358118",
"doi-asserted-by": "publisher",
"key": "bibr33-1934578X231188861"
},
{
"DOI": "10.3390/nu14020256",
"doi-asserted-by": "publisher",
"key": "bibr34-1934578X231188861"
},
{
"DOI": "10.3390/molecules26195844",
"doi-asserted-by": "publisher",
"key": "bibr35-1934578X231188861"
},
{
"DOI": "10.1111/dom.13868",
"doi-asserted-by": "publisher",
"key": "bibr36-1934578X231188861"
},
{
"DOI": "10.1016/j.antiviral.2020.104786",
"doi-asserted-by": "publisher",
"key": "bibr37-1934578X231188861"
},
{
"DOI": "10.1038/s41598-020-77003-3",
"doi-asserted-by": "publisher",
"key": "bibr38-1934578X231188861"
},
{
"DOI": "10.1038/s41586-020-2575-3",
"doi-asserted-by": "publisher",
"key": "bibr39-1934578X231188861"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "http://journals.sagepub.com/doi/10.1177/1934578X231188861"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Complementary and alternative medicine",
"Plant Science",
"Drug Discovery",
"Pharmacology",
"General Medicine"
],
"subtitle": [],
"title": "In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "18"
}
