Phenolic compounds disrupt spike-mediated receptor-binding and entry of SARS-CoV-2 pseudo-virions
et al., PLOS ONE, doi:10.1371/journal.pone.0253489, Jun 2021
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
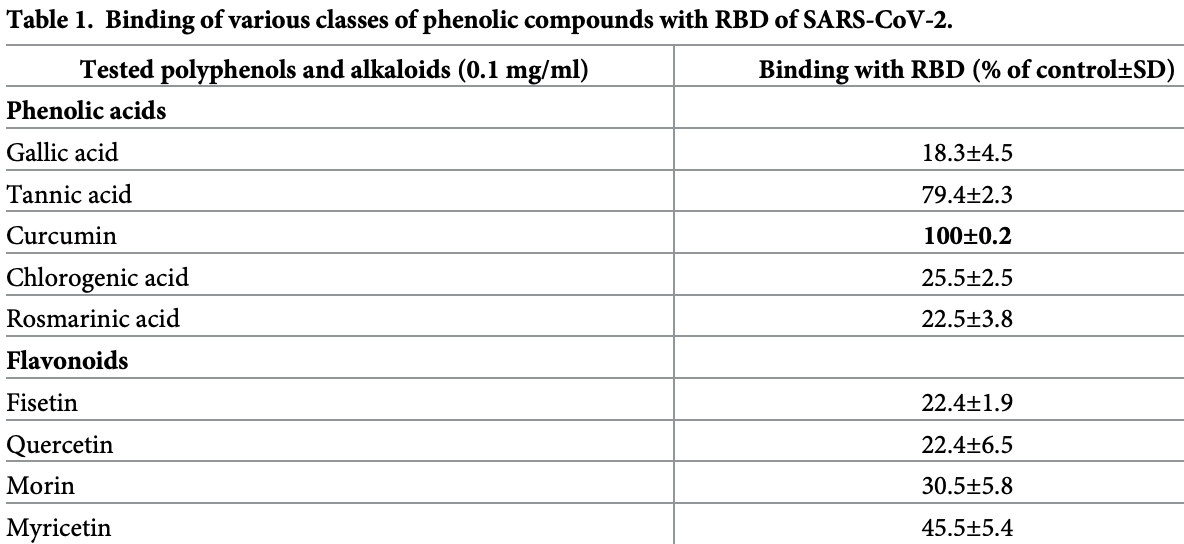
In vitro study of 56 polyphenols showing that curcumin has high binding affinity to the RBD of the SARS-CoV-2 spike protein, inhibits ACE2 at non-toxic concentrations, and decreases activity of TMPRSS2. Promising results were also seen for brazilin and theaflavin-3,3’-digallate.
62 preclinical studies support the efficacy of curcumin for COVID-19:
In silico studies predict inhibition of SARS-CoV-2 with curcumin or metabolites via binding to the spikeA,1,5,6,11,16,18,24,27 (and specifically the receptor binding domainB,2,4,14,17,20 ), MproC,4-6,11,13,15-17,19,20,22,25,27,28,30,48 , RNA-dependent RNA polymeraseD,4-6,17,26 , PLproE,6, ACE2F,2,18,19,21 , nucleocapsidG,12,29 , nsp10H,29, and helicaseI,36 proteins, and inhibition of spike-ACE2 interactionJ,3.
In vitro studies demonstrate inhibition of the spikeA,41 (and specifically the receptor binding domainB,51), MproC,23,41,48,50 , ACE2F,51, and TMPRSS2K,51 proteins, and inhibition of spike-ACE2 interactionJ,3,34 .
In vitro studies demonstrate efficacy in Calu-3L,49, A549M,41, A549-ATN,31, 293TO,7, HEK293-hACE2P,23,39 , 293T/hACE2/TMPRSS2Q,40, Vero E6R,1,13,17,27,39,41,43,45,47,49 , and SH-SY5YS,38 cells.
Curcumin decreases pro-inflammatory cytokines induced by SARS-CoV-2 in peripheral blood mononuclear cells47, alleviates SARS-CoV-2 spike protein-induced mitochondrial membrane damage and oxidative stress7, may limit COVID-19 induced cardiac damage by inhibiting the NF-κB signaling pathway which mediates the profibrotic effects of the SARS-CoV-2 spike protein on cardiac fibroblasts35, is predicted to inhibit the interaction between the SARS-CoV-2 spike protein receptor binding domain and the human ACE2 receptor for the delta and omicron variants14, lowers ACE2 and STAT3, curbing lung inflammation and ARDS in preclinical COVID-19 models32, inhibits SARS-CoV-2 ORF3a ion channel activity, which contributes to viral pathogenicity and cytotoxicity42, has direct virucidal action by disrupting viral envelope integrity44, may inhibit viral replication and modulate inflammatory pathways like NF-κB via SIRT1 activation52, and can function as a photosensitizer in photodynamic therapy to generate reactive oxygen species that damage the virus44.
1.
Marzouk et al., Computational and Experimental Insights into the Antiviral Mechanism of Turmeric (Curcuma longa) against SARS-CoV-2 D614G, BIO Web of Conferences, doi:10.1051/bioconf/202519804002.
2.
Wu et al., Utilizing natural compounds as ligands to disrupt the binding of SARS-CoV-2 receptor-binding domain to angiotensin-converting enzyme 2, impeding viral infection, Phytochemistry Letters, doi:10.1016/j.phytol.2025.102999.
3.
Najimi et al., Phytochemical Inhibitors of SARS‐CoV‐2 Entry: Targeting the ACE2‐RBD Interaction with l‐Tartaric Acid, l‐Ascorbic Acid, and Curcuma longa Extract, ChemistrySelect, doi:10.1002/slct.202406035.
4.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
5.
Al balawi et al., Assessing multi-target antiviral and antioxidant activities of natural compounds against SARS-CoV-2: an integrated in vitro and in silico study, Bioresources and Bioprocessing, doi:10.1186/s40643-024-00822-z.
6.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
7.
Zhang et al., Computational Discovery of Mitochondrial Dysfunction Biomarkers in Severe SARS-CoV-2 Infection: Facilitating Pytomedicine Screening, Phytomedicine, doi:10.1016/j.phymed.2024.155784.
8.
Öztürkkan et al., In Silico investigation of the effects of curcuminoids on the spike protein of the omicron variant of SARS-CoV-2, Baku State University Journal of Chemistry and Material Sciences, 1:2, bsuj.bsu.edu.az/uploads/pdf/ec4204d62f7802de54e6092bf7860029.pdf.
9.
Yunze et al., Therapeutic effect and potential mechanism of curcumin, an active ingredient in Tongnao Decoction, on COVID-19 combined with stroke: a network pharmacology study and GEO database mining, Research Square, doi:10.21203/rs.3.rs-4329762/v1.
10.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
11.
Boseila et al., Throat spray formulated with virucidal Pharmaceutical excipients as an effective early prophylactic or treatment strategy against pharyngitis post-exposure to SARS CoV-2, European Journal of Pharmaceutics and Biopharmaceutics, doi:10.1016/j.ejpb.2024.114279.
12.
Hidayah et al., Bioinformatics study of curcumin, demethoxycurcumin, bisdemethoxycurcumin and cyclocurcumin compounds in Curcuma longa as an antiviral agent via nucleocapsid on SARS-CoV-2 inhibition, International Conference on Organic and Applied Chemistry, doi:10.1063/5.0197724.
13.
Singh et al., Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 M Pro protein - An in-silico and cell-based approach, Research Square, doi:10.21203/rs.3.rs-3888947/v1.
14.
Kant et al., Structure-based drug discovery to identify SARS-CoV2 spike protein–ACE2 interaction inhibitors, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2023.2300060.
15.
Naderi Beni et al., In silico studies of anti-oxidative and hot temperament-based phytochemicals as natural inhibitors of SARS-CoV-2 Mpro, PLOS ONE, doi:10.1371/journal.pone.0295014.
16.
Moschovou et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, International Journal of Molecular Sciences, doi:10.3390/ijms242115894.
17.
Eleraky et al., Curcumin Transferosome-Loaded Thermosensitive Intranasal in situ Gel as Prospective Antiviral Therapy for SARS-Cov-2, International Journal of Nanomedicine, doi:10.2147/IJN.S423251.
18.
Singh (B) et al., Computational studies to analyze effect of curcumin inhibition on coronavirus D614G mutated spike protein, The Seybold Report, doi:10.17605/OSF.IO/TKEXJ.
19.
Thapa et al., In-silico Approach for Predicting the Inhibitory Effect of Home Remedies on Severe Acute Respiratory Syndrome Coronavirus-2, Makara Journal of Science, doi:10.7454/mss.v27i3.1609.
20.
Srivastava et al., Paradigm of Well-Orchestrated Pharmacokinetic Properties of Curcuminoids Relative to Conventional Drugs for the Inactivation of SARS-CoV-2 Receptors: An In Silico Approach, Stresses, doi:10.3390/stresses3030043.
21.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
22.
Winih Kinasih et al., Analisis in silico interaksi senyawa kurkuminoid terhadap enzim main protease 6LU7 dari SARS-CoV-2, Duta Pharma Journal, doi:10.47701/djp.v3i1.2904.
23.
Wu (B) et al., Potential Mechanism of Curcumin and Resveratrol against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-2780614/v1.
24.
Nag et al., Curcumin inhibits spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in silico study, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2022.105552.
25.
Rampogu et al., Molecular Docking and Molecular Dynamics Simulations Discover Curcumin Analogue as a Plausible Dual Inhibitor for SARS-CoV-2, International Journal of Molecular Sciences, doi:10.3390/ijms23031771.
26.
Singh (C) et al., Potential of turmeric-derived compounds against RNA-dependent RNA polymerase of SARS-CoV-2: An in-silico approach, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2021.104965.
27.
Kandeil et al., Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2, Pathogens, doi:10.3390/pathogens10060758.
28.
Rajagopal et al., Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-020-00126-x.
29.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
30.
Sekiou et al., In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2, ChemRxiv, doi:10.26434/chemrxiv.12181404.v1.
31.
Grüneberg et al., Dose-dependent antiviral effects of glycyrrhizin, curcumin, and harmaline against clinical SARS-CoV-2 isolates, including D614G, Omicron BA.5, and Omicron XBB.1, BMC Complementary Medicine and Therapies, doi:10.1186/s12906-026-05253-1.
32.
Aktay et al., Oral Administration of Water-Soluble Curcumin Complex Prevents ARDS With the Potential for COVID-19 Treatment, Phytotherapy Research, doi:10.1002/ptr.70046.
33.
Olubiyi et al., Novel dietary herbal preparations with inhibitory activities against multiple SARS-CoV-2 targets: A multidisciplinary investigation into antiviral activities, Food Chemistry Advances, doi:10.1016/j.focha.2025.100969.
34.
Emam et al., Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors, AMB Express, doi:10.1186/s13568-024-01739-8.
35.
Van Tin et al., Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling, Cells, doi:10.3390/cells13161331.
36.
Li et al., Thermal shift assay (TSA)-based drug screening strategy for rapid discovery of inhibitors against the Nsp13 helicase of SARS-CoV-2, Animals and Zoonoses, doi:10.1016/j.azn.2024.06.001.
37.
Kamble et al., Nanoparticulate curcumin spray imparts prophylactic and therapeutic properties against SARS-CoV-2, Emergent Materials, doi:10.1007/s42247-024-00754-6.
38.
Nicoliche et al., Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2, Scientific Reports, doi:10.1038/s41598-024-61662-7.
39.
Nittayananta et al., A novel film spray containing curcumin inhibits SARS-CoV-2 and influenza virus infection and enhances mucosal immunity, Virology Journal, doi:10.1186/s12985-023-02282-x.
40.
Septisetyani et al., Curcumin and turmeric extract inhibited SARS-CoV-2 pseudovirus cell entry and Spike mediated cell fusion, bioRxiv, doi:10.1101/2023.09.28.560070.
41.
Mohd Abd Razak et al., In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds, Natural Product Communications, doi:10.1177/1934578X231188861.
42.
Fam et al., Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites, Scientific Reports, doi:10.1038/s41598-023-31764-9.
43.
Teshima et al., Antiviral activity of curcumin and its analogs selected by an artificial intelligence-supported activity prediction system in SARS-CoV-2-infected VeroE6 cells, Natural Product Research, doi:10.1080/14786419.2023.2194647.
44.
Zupin et al., Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer, Viruses, doi:10.3390/v14102132.
45.
Leka et al., In vitro antiviral activity against SARS-CoV-2 of common herbal medicinal extracts and their bioactive compounds, Phytotherapy Research, doi:10.1002/ptr.7463.
46.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
47.
Marín-Palma et al., Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms, Molecules, doi:10.3390/molecules26226900.
48.
Bahun et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594.
49.
Bormann et al., Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro, Viruses, doi:10.3390/v13101914.
50.
Guijarro-Real et al., Potential In Vitro Inhibition of Selected Plant Extracts against SARS-CoV-2 Chymotripsin-Like Protease (3CLPro) Activity, Foods, doi:10.3390/foods10071503.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The receptor binding domain is a specific region of the spike protein that binds ACE2 and is a major target of neutralizing antibodies. Focusing on the precise binding site allows highly specific disruption of viral attachment with reduced potential for off-target effects.
c.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
d.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
e.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
f.
The angiotensin converting enzyme 2 (ACE2) protein is a host cell transmembrane protein that serves as the cellular receptor for the SARS-CoV-2 spike protein. ACE2 is expressed on many cell types, including epithelial cells in the lungs, and allows the virus to enter and infect host cells. Inhibition may affect ACE2's physiological function in blood pressure control.
g.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
h.
Non-structural protein 10 (nsp10) serves as an RNA chaperone and stabilizes conformations of nsp12 and nsp14 in the replicase-transcriptase complex, which synthesizes new viral RNAs. Nsp10 disruption may destabilize replicase-transcriptase complex activity.
i.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
j.
The interaction between the SARS-CoV-2 spike protein and the human ACE2 receptor is a primary method of viral entry, inhibiting this interaction can prevent the virus from attaching to and entering host cells, halting infection at an early stage.
k.
Transmembrane protease serine 2 (TMPRSS2) is a host cell protease that primes the spike protein, facilitating cellular entry. TMPRSS2 activity helps enable cleavage of the spike protein required for membrane fusion and virus entry. Inhibition may especially protect respiratory epithelial cells, buy may have physiological effects.
l.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
m.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
n.
A549-AT is a human lung carcinoma cell line stably transfected with ACE2 and TMPRSS2 receptors. Unlike the parental line, this overexpression ensures stable infection and enhanced viral entry, allowing for the evaluation of antiviral efficacy against various SARS-CoV-2 variants.
o.
293T is a human embryonic kidney cell line that can be engineered for high ACE2 expression and SARS-CoV-2 susceptibility. 293T cells are easily transfected and support high protein expression.
p.
HEK293-hACE2 is a human embryonic kidney cell line with high ACE2 expression and SARS-CoV-2 susceptibility. Cells have been transfected with a plasmid to express the human ACE2 (hACE2) protein.
q.
293T/hACE2/TMPRSS2 is a human embryonic kidney cell line engineered for high ACE2 and TMPRSS2 expression, which mimics key aspects of human infection. 293T/hACE2/TMPRSS2 cells are very susceptible to SARS-CoV-2 infection.
r.
Vero E6 is an African green monkey kidney cell line with low/no ACE2 expression and high SARS-CoV-2 susceptibility. The cell line is easy to maintain and supports robust viral replication, however the monkey origin may not accurately represent human responses.
s.
SH-SY5Y is a human neuroblastoma cell line that exhibits neuronal phenotypes. It is commonly used as an in vitro model for studying neurotoxicity, neurodegenerative diseases, and neuronal differentiation.
Goc et al., 17 Jun 2021, peer-reviewed, 4 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Phenolic compounds disrupt spike-mediated receptor-binding and entry of SARS-CoV-2 pseudo-virions
PLOS ONE, doi:10.1371/journal.pone.0253489
In the pursuit of suitable and effective solutions to SARS-CoV-2 infection, we investigated the efficacy of several phenolic compounds in controlling key cellular mechanisms involved in its infectivity. The way the SARS-CoV-2 virus infects the cell is a complex process and comprises four main stages: attachment to the cognate receptor, cellular entry, replication and cellular egress. Since, this is a multi-part process, it creates many opportunities to develop effective interventions. Targeting binding of the virus to the host receptor in order to prevent its entry has been of particular interest. Here, we provide experimental evidence that, among 56 tested polyphenols, including plant extracts, brazilin, theaflavin-3,3'-digallate, and curcumin displayed the highest binding with the receptor-binding domain of spike protein, inhibiting viral attachment to the human angiotensin-converting enzyme 2 receptor, and thus cellular entry of pseudo-typed SARS-CoV-2 virions. Both, theaflavin-3,3'-digallate at 25 μg/ml and curcumin above 10 μg/ml concentration, showed binding with the angiotensin-converting enzyme 2 receptor reducing at the same time its activity in both cell-free and cell-based assays. Our study also demonstrates that brazilin and theaflavin-3,3'-digallate, and to a still greater extent, curcumin, decrease the activity of transmembrane serine protease 2 both in cell-free and cell-based assays. Similar pattern was observed with cathepsin L, although only theaflavin-3,3'-digallate showed a modest diminution of cathepsin L expression at protein level. Finally, each of these three compounds moderately increased endosomal/lysosomal pH. In conclusion, this study demonstrates pleiotropic anti-SARS-CoV-2 efficacy of specific polyphenols and their prospects for further scientific and clinical investigations.
Supporting information
S1 File. Raw Western blot images. (PPTX)
Author Contributions Conceptualization: Anna Goc.
Data curation: Matthias Rath, Aleksandra Niedzwiecki. Formal analysis: Anna Goc, Waldemar Sumera. Visualization: Anna Goc, Waldemar Sumera, Aleksandra Niedzwiecki. Writing -original draft: Anna Goc, Waldemar Sumera, Matthias Rath, Aleksandra Niedzwiecki. Writing -review & editing: Anna Goc, Waldemar Sumera, Aleksandra Niedzwiecki.
References
Anggakusuma, Colpitts, Schang, Rachmawati, Frentzen et al., Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells, Gut, doi:10.1136/gutjnl-2012-304299
Belouzard, Chu, Whittaker, Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinctsites, Proc Natl Acad Sci, doi:10.1073/pnas.0809524106
Bertram, Dijkman, Hajan, Heurich, Gierer et al., TMPRSS2 activates the human coronavirus 229E for cathepsin-independent host cell entry and is expressed in viral target cells in the respiratory epithelium, J Virol, doi:10.1128/JVI.03372-12
Bertram, Glowacka, Muller, Lavender, Gnirss et al., Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease, J Virol, doi:10.1128/JVI.05300-11
Bosch, Van Der Zee, De Haan, Rottier, The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex, J Virol, doi:10.1128/jvi.77.16.8801-8811.2003
Chen, Chen, Wen, Ou, Chiou et al., Inhibition of enveloped viruses infectivity by curcumin, PLoS One, doi:10.1371/journal.pone.0062482
Chen, Du, Potential natural compounds for preventing 2019-nCoV infection
Chen, Liu, Guo, Emerging coronaviruses: genome structure, replication, and pathogenesis, J Med Virol, doi:10.1002/jmv.26234
Chowdhury, Sahuc, Rouille, Rivière, Bonneau et al., Polyphenols of black tea, inhibit entry of hepatitis C virus in cell culture, PLoS One, doi:10.1371/journal.pone.0198226
Cory, Passarelli, Szeto, Tamez, Mattei, The Role of polyphenols in human health and food systems: A mini-review, Front Nutr, doi:10.3389/fnut.2018.00087
Coutard, Valle, De Lamballerie, Canard, Seidah et al., The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade, Antiviral Res, doi:10.1016/j.antiviral.2020.104742
Cui, Zhou, Huang, Zhang, Wang et al., Identification of theaflavin-3,3'-digallate as a novel Zika Virus protease inhibitor, Front Pharmacol, doi:10.3389/fphar.2020.514313
Du, He, Zhou, Liu, Zheng et al., The spike protein of SARS-CoV-a target for vaccine and therapeutic development, Nat Rev Microbiol, doi:10.1038/nrmicro2090
Glowacka, Bertram, Muller, Allen, Soilleux et al., Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response, J Virol, doi:10.1128/JVI.02232-10
Goc, Matthias, Niedzwiecki, Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry, Sci. Rep, doi:10.1038/s41598-021-84850-1
Gorbalenya, Baker, Baric, De Groot, Drosten et al., The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2, Nat Microbiol, doi:10.1038/s41564-020-0695-z
Guan, Zheng, He, Liu, Zhuang et al., Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China, Science, doi:10.1126/science.1087139
Hoffmann, Kleine-Weber, Schroeder, Kru ¨ger, Herrler et al., SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Iwata-Yoshikawa, Okamura, Shimizu, Hasegawa, Takeda et al., TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection, J Virol, doi:10.1128/JVI.01815-18
Jaimes, Andre, Chappie, Jean, Millet et al., Phylogenetic analysis and structural modeling of SARS-CoV-2 spike protein reveals an evolutionary distinct and proteolytically sensitive activation loop, J Mol Biol, doi:10.1016/j.jmb.2020.04.009
Jaruga, Bielak-Zmijewska, Sikora, Skierski, Radziszewska et al., Glutathioneindependent mechanism of apoptosis inhibition by curcumin in rat thymocytes, Biochem Pharmacol, doi:10.1016/s0006-2952%2898%2900144-0
Jena, Kanungo, Nayak, Chainy, Dandapat, Catechin and curcumin interact with S protein of SARS-CoV2 and ACE2 of human cell membrane: insights from computational study, Sci Rep, doi:10.1038/s41598-021-81462-7
Liu, Luo, Libby, Shi, Cathepsin L-selective inhibitors: A potentially promising treatment for COVID-19 patients, Pharmacol Ther
Lung, Lin, Yang, Chou, Chang et al., The potential SARS-CoV-2 entry inhibitor, doi:10.1101/2020.03.26.009803v1
Maiti, Banerjee, Epigallocatechin-gallate and theaflavin-gallate interaction in SARS CoV-2 spike protein central-channel with reference to the hydroxychloroquine interaction. Bioinformatics and molecular docking study, Drug Dev Res, doi:10.1002/ddr.21730
Masters, Perlman, Fields Virology
Mollica, Rizzo, Massari, The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer, Future Oncol, doi:10.2217/fon-2020-0571
Nelson, Dyall, Hoenen, Barnes, Zhou, The phosphatidylinositol-3-phosphate 5-kinase inhibitor apilimod blocks filoviral entry and infection, PLoS Negl Trop Dis, doi:10.1371/journal.pntd.0005540
Ohkuma, Poole, Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents, Proc Natl Acad Sci, doi:10.1073/pnas.75.7.3327
Ou, Liu, Lei, Li, Mi et al., Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV, Nat Commun, doi:10.1038/s41467-020-15562-9
Pandey, Rizvi, Plant polyphenols as dietary antioxidants in human health and disease, Oxid Med Cell Longev, doi:10.4161/oxim.2.5.9498
Paraiso, Revel, Stevens, Potential use of polyphenols in the battle against COVID-19, Curr Opin Food Sci, doi:10.1016/j.cofs.2020.08.004
Patel, Rajendran, Shah, Patel, Pakala et al., Virtual screening of curcumin and its analogs against the spike surface glycoprotein of SARS-CoV-2 and SARS-CoV, J Biomol Struct Dyn, doi:10.1080/07391102.2020.1868338
Patil, Saraogi, Natural products as potential drug permeation enhancer in transdermal drug delivery system, Arch Dermatol Res, doi:10.1007/s00403-014-1445-y
Perrotta, Matera, Cazzola, Bianco, Severe respiratory SARS-CoV2 infection: Does ACE2 receptor matter?, Respir Med, doi:10.1016/j.rmed.2020.105996
Ravish, Raghav, Curcumin as inhibitor of mammalian cathepsin B, cathepsin H, acid phosphatase and alkaline phosphatase: a correlation with pharmacological activities, Med Chem Res
Rehman, Al-Ajmi, Hussain, Natural compounds as inhibitors of SARS-CoV-2 main protease (3CLpro): A molecular docking and simulation approach to combat COVID-19, Curr Pharm Des, doi:10.2174/1381612826999201116195851
Shrimp, Kales, Sanderson, Simeonov, Shen et al., An enzymatic TMPRSS2 assay for assessment of clinical candidates and discovery of inhibitors as potential treatment of COVID-19, ACS Pharmacol Transl Sci, doi:10.1021/acsptsci.0c00106
Simmons, Gosalia, Rennekamp, Reeves, Diamond et al., Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry, Proc Natl Acad Sci, doi:10.1073/pnas.0505577102
Taggart, Lowe, Greene, Mulgrew, Neill et al., Cathepsin B, L, and S cleave and inactivate secretory leucoprotease inhibitor, J Biol Chem, doi:10.1074/jbc.M103220200
Upadhyay, Dixit, Role of polyphenols and other phytochemicals on molecular signaling, Oxid Med Cell Longev, doi:10.1155/2015/504253
Verma, Twilley, Esmear, Oosthuizen, Reid et al., Anti-SARS-CoV natural products with the potential to inhibit SARS-CoV-2 (COVID-19), Front Pharmacol, doi:10.3389/fphar.2020.561334
Wan, Shang, Graham, Baric, Li, Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS, J Virol, doi:10.1128/JVI.00127-20
Wolters, Chapman, Importance of lysosomal cysteine proteases in lung disease, Respir Res, doi:10.1186/rr29
Wu, Liu, Yang, Zhang, Zhong et al., Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods, Acta Pharm Sin B, doi:10.1016/j.apsb.2020.02.008
Xia, Lan, Su, Wang, Xu et al., The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin, Signal Transduct Target Ther, doi:10.1038/s41392-020-0184-0
Xu, Chen, Wang, Feng, Zhou et al., Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission, Sci China Life Sci, doi:10.1007/s11427-020-1637-5
Yoo, Lee, Kim, Kang, Park, Curcumin inhibits the upregulation of cathepsin L by palmitate in fat, Endocrine Abstracts
Zhang, Huang, Yang, Liu, Wang et al., Antifibrotic effects of curcumin are associated with overexpression of cathepsins K and L in bleomycin treated mice and human fibroblasts, Respir Res, doi:10.1186/1465-9921-12-154
Zhang, Shen, Wang, Cheng, Discovery of anti-SARS-CoV-2 agents from commercially available flavor via docking screening
Zhou, Yang, Wang, Hu, Zhang et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature, doi:10.1038/s41586-020-2012-7
´szti-Gere, Czimmermann, Ujhelyi, Balla, Maiwald et al., In vitro characterization of TMPRSS2 inhibition in IPEC-J2 cells, J Enzyme Inhib Med Chem, doi:10.1080/14756366.2016.1193732
DOI record:
{
"DOI": "10.1371/journal.pone.0253489",
"ISSN": [
"1932-6203"
],
"URL": "http://dx.doi.org/10.1371/journal.pone.0253489",
"abstract": "<jats:p>In the pursuit of suitable and effective solutions to SARS-CoV-2 infection, we investigated the efficacy of several phenolic compounds in controlling key cellular mechanisms involved in its infectivity. The way the SARS-CoV-2 virus infects the cell is a complex process and comprises four main stages: attachment to the cognate receptor, cellular entry, replication and cellular egress. Since, this is a multi-part process, it creates many opportunities to develop effective interventions. Targeting binding of the virus to the host receptor in order to prevent its entry has been of particular interest. Here, we provide experimental evidence that, among 56 tested polyphenols, including plant extracts, brazilin, theaflavin-3,3’-digallate, and curcumin displayed the highest binding with the receptor-binding domain of spike protein, inhibiting viral attachment to the human angiotensin-converting enzyme 2 receptor, and thus cellular entry of pseudo-typed SARS-CoV-2 virions. Both, theaflavin-3,3’-digallate at 25 μg/ml and curcumin above 10 μg/ml concentration, showed binding with the angiotensin-converting enzyme 2 receptor reducing at the same time its activity in both cell-free and cell-based assays. Our study also demonstrates that brazilin and theaflavin-3,3’-digallate, and to a still greater extent, curcumin, decrease the activity of transmembrane serine protease 2 both in cell-free and cell-based assays. Similar pattern was observed with cathepsin L, although only theaflavin-3,3’-digallate showed a modest diminution of cathepsin L expression at protein level. Finally, each of these three compounds moderately increased endosomal/lysosomal pH. In conclusion, this study demonstrates pleiotropic anti-SARS-CoV-2 efficacy of specific polyphenols and their prospects for further scientific and clinical investigations.</jats:p>",
"author": [
{
"ORCID": "http://orcid.org/0000-0001-8736-2941",
"affiliation": [],
"authenticated-orcid": true,
"family": "Goc",
"given": "Anna",
"sequence": "first"
},
{
"affiliation": [],
"family": "Sumera",
"given": "Waldemar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rath",
"given": "Matthias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Niedzwiecki",
"given": "Aleksandra",
"sequence": "additional"
}
],
"container-title": "PLOS ONE",
"container-title-short": "PLoS ONE",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"www.plosone.org"
]
},
"created": {
"date-parts": [
[
2021,
6,
17
]
],
"date-time": "2021-06-17T17:35:12Z",
"timestamp": 1623951312000
},
"deposited": {
"date-parts": [
[
2021,
6,
17
]
],
"date-time": "2021-06-17T17:36:00Z",
"timestamp": 1623951360000
},
"editor": [
{
"affiliation": [],
"family": "Lawson",
"given": "Victoria",
"sequence": "first"
}
],
"indexed": {
"date-parts": [
[
2023,
8,
31
]
],
"date-time": "2023-08-31T18:23:17Z",
"timestamp": 1693506197925
},
"is-referenced-by-count": 38,
"issue": "6",
"issued": {
"date-parts": [
[
2021,
6,
17
]
]
},
"journal-issue": {
"issue": "6",
"published-online": {
"date-parts": [
[
2021,
6,
17
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
6,
17
]
],
"date-time": "2021-06-17T00:00:00Z",
"timestamp": 1623888000000
}
}
],
"link": [
{
"URL": "https://dx.plos.org/10.1371/journal.pone.0253489",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "340",
"original-title": [],
"page": "e0253489",
"prefix": "10.1371",
"published": {
"date-parts": [
[
2021,
6,
17
]
]
},
"published-online": {
"date-parts": [
[
2021,
6,
17
]
]
},
"publisher": "Public Library of Science (PLoS)",
"reference": [
{
"key": "pone.0253489.ref001",
"unstructured": "World Health Organization. Coronavirus disease (COVID-19). 2020. [cited 2021 January 28]. https://covid19.who.int."
},
{
"key": "pone.0253489.ref002",
"unstructured": "International Committee on Taxonomy of Viruses. Severe acute respiratory syndrome-related coronavirus. 2020. [cited 2021 January 28]. https://talk.ictvonline.org/taxonomy."
},
{
"DOI": "10.1007/s11427-020-1637-5",
"article-title": "Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission",
"author": "X Xu",
"doi-asserted-by": "crossref",
"first-page": "457",
"journal-title": "Sci China Life Sci",
"key": "pone.0253489.ref003",
"volume": "63",
"year": "2020"
},
{
"author": "PS Masters",
"first-page": "825",
"key": "pone.0253489.ref004",
"volume-title": "Fields Virology",
"year": "2013"
},
{
"DOI": "10.1038/s41564-020-0695-z",
"article-title": "The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2",
"author": "AE Gorbalenya",
"doi-asserted-by": "crossref",
"first-page": "536",
"journal-title": "Nat Microbiol",
"key": "pone.0253489.ref005",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26234",
"article-title": "Emerging coronaviruses: genome structure, replication, and pathogenesis",
"author": "Y Chen",
"doi-asserted-by": "crossref",
"first-page": "2249",
"journal-title": "J Med Virol",
"key": "pone.0253489.ref006",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1126/science.1087139",
"article-title": "Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China",
"author": "Y Guan",
"doi-asserted-by": "crossref",
"first-page": "276",
"journal-title": "Science",
"key": "pone.0253489.ref007",
"volume": "302",
"year": "2003"
},
{
"DOI": "10.1016/j.jmb.2020.04.009",
"article-title": "Phylogenetic analysis and structural modeling of SARS-CoV-2 spike protein reveals an evolutionary distinct and proteolytically sensitive activation loop",
"author": "JA Jaimes",
"doi-asserted-by": "crossref",
"first-page": "3309",
"journal-title": "J Mol Biol",
"key": "pone.0253489.ref008",
"volume": "432",
"year": "2020"
},
{
"DOI": "10.1038/nrmicro2090",
"article-title": "The spike protein of SARS-CoV—a target for vaccine and therapeutic development",
"author": "L Du",
"doi-asserted-by": "crossref",
"first-page": "226",
"journal-title": "Nat Rev Microbiol",
"key": "pone.0253489.ref009",
"volume": "7",
"year": "2009"
},
{
"DOI": "10.1038/s41586-020-2012-7",
"article-title": "A pneumonia outbreak associated with a new coronavirus of probable bat origin",
"author": "P Zhou",
"doi-asserted-by": "crossref",
"first-page": "270",
"journal-title": "Nature",
"key": "pone.0253489.ref010",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "M Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "pone.0253489.ref011",
"volume": "181",
"year": "2020"
},
{
"article-title": "Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS",
"author": "Y Wan",
"first-page": "e00127",
"journal-title": "J Virol",
"key": "pone.0253489.ref012",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-15562-9",
"article-title": "Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV",
"author": "X Ou",
"doi-asserted-by": "crossref",
"first-page": "1620",
"journal-title": "Nat Commun",
"key": "pone.0253489.ref013",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1073/pnas.0809524106",
"article-title": "Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinctsites",
"author": "S Belouzard",
"doi-asserted-by": "crossref",
"first-page": "5871",
"journal-title": "Proc Natl Acad Sci. USA",
"key": "pone.0253489.ref014",
"volume": "106",
"year": "2009"
},
{
"DOI": "10.1016/j.antiviral.2020.104742",
"article-title": "The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade",
"author": "B Coutard",
"doi-asserted-by": "crossref",
"first-page": "104742",
"journal-title": "Antiviral Res",
"key": "pone.0253489.ref015",
"volume": "176",
"year": "2020"
},
{
"DOI": "10.1038/s41392-020-0184-0",
"article-title": "The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin",
"author": "S Xia",
"doi-asserted-by": "crossref",
"first-page": "92",
"journal-title": "Signal Transduct Target Ther",
"key": "pone.0253489.ref016",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1128/JVI.77.16.8801-8811.2003",
"article-title": "The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex",
"author": "BJ Bosch",
"doi-asserted-by": "crossref",
"first-page": "8801",
"journal-title": "J Virol",
"key": "pone.0253489.ref017",
"volume": "77",
"year": "2003"
},
{
"DOI": "10.1016/j.pharmthera.2020.107587",
"article-title": "Cathepsin L-selective inhibitors: A potentially promising treatment for COVID-19 patients",
"author": "T Liu",
"doi-asserted-by": "crossref",
"first-page": "107587",
"journal-title": "Pharmacol Ther",
"key": "pone.0253489.ref018",
"volume": "213",
"year": "2011"
},
{
"DOI": "10.1128/JVI.02232-10",
"article-title": "Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response",
"author": "I Glowacka",
"doi-asserted-by": "crossref",
"first-page": "4122",
"journal-title": "J Virol",
"key": "pone.0253489.ref019",
"volume": "85",
"year": "2011"
},
{
"DOI": "10.1186/rr29",
"article-title": "Importance of lysosomal cysteine proteases in lung disease",
"author": "PJ Wolters",
"doi-asserted-by": "crossref",
"first-page": "170",
"journal-title": "Respir Res",
"key": "pone.0253489.ref020",
"volume": "1",
"year": "2000"
},
{
"DOI": "10.1073/pnas.0505577102",
"article-title": "Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry",
"author": "G Simmons",
"doi-asserted-by": "crossref",
"first-page": "11876",
"journal-title": "Proc Natl Acad Sci. USA",
"key": "pone.0253489.ref021",
"volume": "102",
"year": "2005"
},
{
"DOI": "10.1074/jbc.M103220200",
"article-title": "Cathepsin B, L, and S cleave and inactivate secretory leucoprotease inhibitor",
"author": "CC Taggart",
"doi-asserted-by": "crossref",
"first-page": "33345",
"journal-title": "J Biol Chem",
"key": "pone.0253489.ref022",
"volume": "276",
"year": "2001"
},
{
"article-title": "TMPRSS2 contributes to virus spread and immunopathology in the airways of murine models after coronavirus infection",
"author": "N Iwata-Yoshikawa",
"first-page": "01815",
"journal-title": "J Virol",
"key": "pone.0253489.ref023",
"volume": "9",
"year": "2019"
},
{
"DOI": "10.1016/j.rmed.2020.105996",
"article-title": "Severe respiratory SARS-CoV2 infection: Does ACE2 receptor matter?",
"author": "F Perrotta",
"doi-asserted-by": "crossref",
"first-page": "105996",
"journal-title": "Respir Med",
"key": "pone.0253489.ref024",
"volume": "168",
"year": "2020"
},
{
"DOI": "10.2217/fon-2020-0571",
"article-title": "The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer",
"author": "V Mollica",
"doi-asserted-by": "crossref",
"first-page": "2029",
"journal-title": "Future Oncol",
"key": "pone.0253489.ref025",
"volume": "16",
"year": "2020"
},
{
"DOI": "10.1128/JVI.03372-12",
"article-title": "TMPRSS2 activates the human coronavirus 229E for cathepsin-independent host cell entry and is expressed in viral target cells in the respiratory epithelium",
"author": "S Bertram",
"doi-asserted-by": "crossref",
"first-page": "6150",
"journal-title": "J Virol",
"key": "pone.0253489.ref026",
"volume": "87",
"year": "2013"
},
{
"DOI": "10.1128/JVI.05300-11",
"article-title": "Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease",
"author": "S Bertram",
"doi-asserted-by": "crossref",
"first-page": "13363",
"journal-title": "J Virol",
"key": "pone.0253489.ref027",
"volume": "85",
"year": "2011"
},
{
"DOI": "10.1007/s00403-014-1445-y",
"article-title": "Natural products as potential drug permeation enhancer in transdermal drug delivery system",
"author": "UK Patil",
"doi-asserted-by": "crossref",
"first-page": "419",
"journal-title": "Arch Dermatol Res",
"key": "pone.0253489.ref028",
"volume": "306",
"year": "2014"
},
{
"DOI": "10.1155/2015/504253",
"article-title": "Role of polyphenols and other phytochemicals on molecular signaling",
"author": "S Upadhyay",
"doi-asserted-by": "crossref",
"first-page": "504253",
"journal-title": "Oxid Med Cell Longev",
"key": "pone.0253489.ref029",
"volume": "2015",
"year": "2015"
},
{
"DOI": "10.4161/oxim.2.5.9498",
"article-title": "Plant polyphenols as dietary antioxidants in human health and disease",
"author": "KB Pandey",
"doi-asserted-by": "crossref",
"first-page": "270",
"journal-title": "Oxid Med Cell Longev",
"key": "pone.0253489.ref030",
"volume": "2",
"year": "2009"
},
{
"DOI": "10.3389/fnut.2018.00087",
"article-title": "The Role of polyphenols in human health and food systems: A mini-review",
"author": "H Cory",
"doi-asserted-by": "crossref",
"first-page": "87",
"journal-title": "Front Nutr",
"key": "pone.0253489.ref031",
"volume": "5",
"year": "2018"
},
{
"DOI": "10.1016/j.cofs.2020.08.004",
"article-title": "Potential use of polyphenols in the battle against COVID-19",
"author": "IL Paraiso",
"doi-asserted-by": "crossref",
"first-page": "149",
"journal-title": "Curr Opin Food Sci",
"key": "pone.0253489.ref032",
"volume": "32",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2020.561334",
"article-title": "Anti-SARS-CoV natural products with the potential to inhibit SARS-CoV-2 (COVID-19)",
"author": "S Verma",
"doi-asserted-by": "crossref",
"first-page": "561334",
"journal-title": "Front Pharmacol",
"key": "pone.0253489.ref033",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.apsb.2020.02.008",
"article-title": "Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods",
"author": "C Wu",
"doi-asserted-by": "crossref",
"first-page": "766",
"journal-title": "Acta Pharm Sin B",
"key": "pone.0253489.ref034",
"volume": "10",
"year": "2020"
},
{
"article-title": "Natural compounds as inhibitors of SARS-CoV-2 main protease (3CLpro): A molecular docking and simulation approach to combat COVID-19",
"author": "T Rehman",
"journal-title": "Curr Pharm Des",
"key": "pone.0253489.ref035",
"year": "2020"
},
{
"author": "H Chen",
"journal-title": "Potential natural compounds for preventing 2019-nCoV infection",
"key": "pone.0253489.ref036",
"year": "2020"
},
{
"DOI": "10.1080/07391102.2020.1868338",
"article-title": "Virtual screening of curcumin and its analogs against the spike surface glycoprotein of SARS-CoV-2 and SARS-CoV",
"author": "A Patel",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "J Biomol Struct Dyn",
"key": "pone.0253489.ref037",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1038/s41598-021-81462-7",
"article-title": "Catechin and curcumin interact with S protein of SARS-CoV2 and ACE2 of human cell membrane: insights from computational study",
"author": "AB Jena",
"doi-asserted-by": "crossref",
"first-page": "2043",
"journal-title": "Sci Rep",
"key": "pone.0253489.ref038",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1136/gutjnl-2012-304299",
"article-title": "Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells",
"author": "Anggakusuma",
"doi-asserted-by": "crossref",
"first-page": "1137",
"journal-title": "Gut",
"key": "pone.0253489.ref039",
"volume": "63",
"year": "2014"
},
{
"DOI": "10.1371/journal.pone.0062482",
"article-title": "Inhibition of enveloped viruses infectivity by curcumin",
"author": "TY Chen",
"doi-asserted-by": "crossref",
"first-page": "e62482",
"journal-title": "PLoS One",
"key": "pone.0253489.ref040",
"volume": "8",
"year": "2013"
},
{
"DOI": "10.1016/S0006-2952(98)00144-0",
"article-title": "Glutathione-independent mechanism of apoptosis inhibition by curcumin in rat thymocytes",
"author": "E Jaruga",
"doi-asserted-by": "crossref",
"first-page": "961",
"journal-title": "Biochem Pharmacol",
"key": "pone.0253489.ref041",
"volume": "56",
"year": "1998"
},
{
"DOI": "10.1371/journal.pone.0198226",
"article-title": "Polyphenols of black tea, inhibit entry of hepatitis C virus in cell culture",
"author": "P Chowdhury",
"doi-asserted-by": "crossref",
"first-page": "e0198226",
"journal-title": "PLoS One",
"key": "pone.0253489.ref042",
"volume": "13",
"year": "2018"
},
{
"DOI": "10.3389/fphar.2020.514313",
"article-title": "Identification of theaflavin—3,3’-digallate as a novel Zika Virus protease inhibitor",
"author": "X Cui",
"doi-asserted-by": "crossref",
"first-page": "514313",
"journal-title": "Front Pharmacol",
"key": "pone.0253489.ref043",
"volume": "11",
"year": "2020"
},
{
"author": "J Lung",
"journal-title": "The potential SARS-CoV-2 entry inhibitor",
"key": "pone.0253489.ref044",
"year": "2020"
},
{
"article-title": "Epigallocatechin-gallate and theaflavin-gallate interaction in SARS CoV-2 spike protein central-channel with reference to the hydroxychloroquine interaction. Bioinformatics and molecular docking study",
"author": "S Maiti",
"journal-title": "Drug Dev Res",
"key": "pone.0253489.ref045",
"year": "2020"
},
{
"author": "JJ Zhang",
"journal-title": "Discovery of anti-SARS-CoV-2 agents from commercially available flavor via docking screening",
"key": "pone.0253489.ref046",
"year": "2020"
},
{
"DOI": "10.1073/pnas.75.7.3327",
"article-title": "Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents",
"author": "S Ohkuma",
"doi-asserted-by": "crossref",
"first-page": "3327",
"journal-title": "Proc Natl Acad Sci. USA",
"key": "pone.0253489.ref047",
"volume": "75",
"year": "1978"
},
{
"DOI": "10.1007/s00044-013-0872-1",
"article-title": "Curcumin as inhibitor of mammalian cathepsin B, cathepsin H, acid phosphatase and alkaline phosphatase: a correlation with pharmacological activities",
"author": "I Ravish",
"doi-asserted-by": "crossref",
"first-page": "2847",
"journal-title": "Med Chem Res",
"key": "pone.0253489.ref048",
"volume": "23",
"year": "2014"
},
{
"DOI": "10.1186/1465-9921-12-154",
"article-title": "Antifibrotic effects of curcumin are associated with overexpression of cathepsins K and L in bleomycin treated mice and human fibroblasts",
"author": "D Zhang",
"doi-asserted-by": "crossref",
"first-page": "154",
"journal-title": "Respir Res",
"key": "pone.0253489.ref049",
"volume": "12",
"year": "2011"
},
{
"article-title": "Curcumin inhibits the upregulation of cathepsin L by palmitate in fat",
"author": "J Yoo",
"first-page": "P1226",
"journal-title": "Endocrine Abstracts",
"key": "pone.0253489.ref050",
"volume": "29",
"year": "2012"
},
{
"DOI": "10.1038/s41598-021-84850-1",
"article-title": "Polyunsaturated ω-3 fatty acids inhibit ACE2-controlled SARS-CoV-2 binding and cellular entry",
"author": "A Goc",
"doi-asserted-by": "crossref",
"first-page": "5207",
"journal-title": "Sci. Rep",
"key": "pone.0253489.ref051",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1080/14756366.2016.1193732",
"article-title": "In vitro characterization of TMPRSS2 inhibition in IPEC-J2 cells",
"author": "R Pászti-Gere",
"doi-asserted-by": "crossref",
"first-page": "123",
"journal-title": "J Enzyme Inhib Med Chem",
"key": "pone.0253489.ref052",
"volume": "31",
"year": "2016"
},
{
"DOI": "10.1021/acsptsci.0c00106",
"article-title": "An enzymatic TMPRSS2 assay for assessment of clinical candidates and discovery of inhibitors as potential treatment of COVID-19",
"author": "JH Shrimp",
"doi-asserted-by": "crossref",
"first-page": "997",
"journal-title": "ACS Pharmacol Transl Sci",
"key": "pone.0253489.ref053",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1371/journal.pntd.0005540",
"article-title": "The phosphatidylinositol-3-phosphate 5-kinase inhibitor apilimod blocks filoviral entry and infection",
"author": "EA Nelson",
"doi-asserted-by": "crossref",
"first-page": "e0005540",
"journal-title": "PLoS Negl Trop Dis",
"key": "pone.0253489.ref054",
"volume": "11",
"year": "2017"
}
],
"reference-count": 54,
"references-count": 54,
"relation": {},
"resource": {
"primary": {
"URL": "https://dx.plos.org/10.1371/journal.pone.0253489"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Phenolic compounds disrupt spike-mediated receptor-binding and entry of SARS-CoV-2 pseudo-virions",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1371/journal.pone.corrections_policy",
"volume": "16"
}
