Potential of turmeric-derived compounds against RNA-dependent RNA polymerase of SARS-CoV-2: An in-silico approach
et al., Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2021.104965, Oct 2021
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
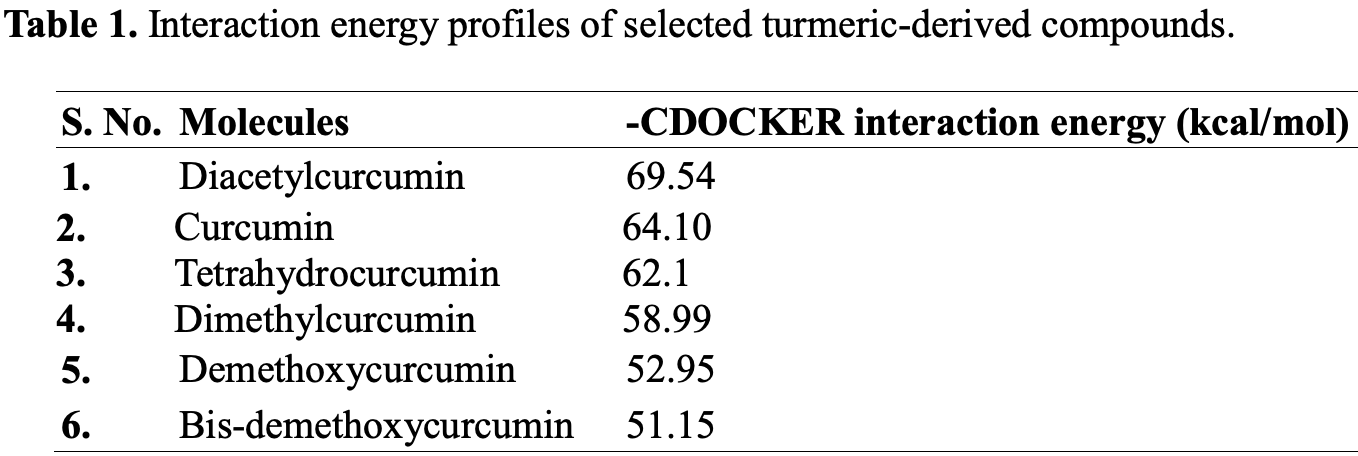
In silico study showing strong binding affinity of curcumin and diacetylcurcumin with SARS-CoV-2 RNA-dependent RNA polymerase. Comparison with remdesivir and favipiravir suggested greater potential of these compounds as an RdRp inhibitor.
62 preclinical studies support the efficacy of curcumin for COVID-19:
In silico studies predict inhibition of SARS-CoV-2 with curcumin or metabolites via binding to the spikeA,1,5,6,11,16,18,24,27 (and specifically the receptor binding domainB,2,4,14,17,20 ), MproC,4-6,11,13,15-17,19,20,22,25,27,28,30,48 , RNA-dependent RNA polymeraseD,4-6,17,26 , PLproE,6, ACE2F,2,18,19,21 , nucleocapsidG,12,29 , nsp10H,29, and helicaseI,36 proteins, and inhibition of spike-ACE2 interactionJ,3.
In vitro studies demonstrate inhibition of the spikeA,41 (and specifically the receptor binding domainB,51), MproC,23,41,48,50 , ACE2F,51, and TMPRSS2K,51 proteins, and inhibition of spike-ACE2 interactionJ,3,34 .
In vitro studies demonstrate efficacy in Calu-3L,49, A549M,41, A549-ATN,31, 293TO,7, HEK293-hACE2P,23,39 , 293T/hACE2/TMPRSS2Q,40, Vero E6R,1,13,17,27,39,41,43,45,47,49 , and SH-SY5YS,38 cells.
Curcumin decreases pro-inflammatory cytokines induced by SARS-CoV-2 in peripheral blood mononuclear cells47, alleviates SARS-CoV-2 spike protein-induced mitochondrial membrane damage and oxidative stress7, may limit COVID-19 induced cardiac damage by inhibiting the NF-κB signaling pathway which mediates the profibrotic effects of the SARS-CoV-2 spike protein on cardiac fibroblasts35, is predicted to inhibit the interaction between the SARS-CoV-2 spike protein receptor binding domain and the human ACE2 receptor for the delta and omicron variants14, lowers ACE2 and STAT3, curbing lung inflammation and ARDS in preclinical COVID-19 models32, inhibits SARS-CoV-2 ORF3a ion channel activity, which contributes to viral pathogenicity and cytotoxicity42, has direct virucidal action by disrupting viral envelope integrity44, may inhibit viral replication and modulate inflammatory pathways like NF-κB via SIRT1 activation52, and can function as a photosensitizer in photodynamic therapy to generate reactive oxygen species that damage the virus44.
1.
Marzouk et al., Computational and Experimental Insights into the Antiviral Mechanism of Turmeric (Curcuma longa) against SARS-CoV-2 D614G, BIO Web of Conferences, doi:10.1051/bioconf/202519804002.
2.
Wu et al., Utilizing natural compounds as ligands to disrupt the binding of SARS-CoV-2 receptor-binding domain to angiotensin-converting enzyme 2, impeding viral infection, Phytochemistry Letters, doi:10.1016/j.phytol.2025.102999.
3.
Najimi et al., Phytochemical Inhibitors of SARS‐CoV‐2 Entry: Targeting the ACE2‐RBD Interaction with l‐Tartaric Acid, l‐Ascorbic Acid, and Curcuma longa Extract, ChemistrySelect, doi:10.1002/slct.202406035.
4.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
5.
Al balawi et al., Assessing multi-target antiviral and antioxidant activities of natural compounds against SARS-CoV-2: an integrated in vitro and in silico study, Bioresources and Bioprocessing, doi:10.1186/s40643-024-00822-z.
6.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
7.
Zhang et al., Computational Discovery of Mitochondrial Dysfunction Biomarkers in Severe SARS-CoV-2 Infection: Facilitating Pytomedicine Screening, Phytomedicine, doi:10.1016/j.phymed.2024.155784.
8.
Öztürkkan et al., In Silico investigation of the effects of curcuminoids on the spike protein of the omicron variant of SARS-CoV-2, Baku State University Journal of Chemistry and Material Sciences, 1:2, bsuj.bsu.edu.az/uploads/pdf/ec4204d62f7802de54e6092bf7860029.pdf.
9.
Yunze et al., Therapeutic effect and potential mechanism of curcumin, an active ingredient in Tongnao Decoction, on COVID-19 combined with stroke: a network pharmacology study and GEO database mining, Research Square, doi:10.21203/rs.3.rs-4329762/v1.
10.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
11.
Boseila et al., Throat spray formulated with virucidal Pharmaceutical excipients as an effective early prophylactic or treatment strategy against pharyngitis post-exposure to SARS CoV-2, European Journal of Pharmaceutics and Biopharmaceutics, doi:10.1016/j.ejpb.2024.114279.
12.
Hidayah et al., Bioinformatics study of curcumin, demethoxycurcumin, bisdemethoxycurcumin and cyclocurcumin compounds in Curcuma longa as an antiviral agent via nucleocapsid on SARS-CoV-2 inhibition, International Conference on Organic and Applied Chemistry, doi:10.1063/5.0197724.
13.
Singh et al., Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 M Pro protein - An in-silico and cell-based approach, Research Square, doi:10.21203/rs.3.rs-3888947/v1.
14.
Kant et al., Structure-based drug discovery to identify SARS-CoV2 spike protein–ACE2 interaction inhibitors, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2023.2300060.
15.
Naderi Beni et al., In silico studies of anti-oxidative and hot temperament-based phytochemicals as natural inhibitors of SARS-CoV-2 Mpro, PLOS ONE, doi:10.1371/journal.pone.0295014.
16.
Moschovou et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, International Journal of Molecular Sciences, doi:10.3390/ijms242115894.
17.
Eleraky et al., Curcumin Transferosome-Loaded Thermosensitive Intranasal in situ Gel as Prospective Antiviral Therapy for SARS-Cov-2, International Journal of Nanomedicine, doi:10.2147/IJN.S423251.
18.
Singh (B) et al., Computational studies to analyze effect of curcumin inhibition on coronavirus D614G mutated spike protein, The Seybold Report, doi:10.17605/OSF.IO/TKEXJ.
19.
Thapa et al., In-silico Approach for Predicting the Inhibitory Effect of Home Remedies on Severe Acute Respiratory Syndrome Coronavirus-2, Makara Journal of Science, doi:10.7454/mss.v27i3.1609.
20.
Srivastava et al., Paradigm of Well-Orchestrated Pharmacokinetic Properties of Curcuminoids Relative to Conventional Drugs for the Inactivation of SARS-CoV-2 Receptors: An In Silico Approach, Stresses, doi:10.3390/stresses3030043.
21.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
22.
Winih Kinasih et al., Analisis in silico interaksi senyawa kurkuminoid terhadap enzim main protease 6LU7 dari SARS-CoV-2, Duta Pharma Journal, doi:10.47701/djp.v3i1.2904.
23.
Wu (B) et al., Potential Mechanism of Curcumin and Resveratrol against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-2780614/v1.
24.
Nag et al., Curcumin inhibits spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in silico study, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2022.105552.
25.
Rampogu et al., Molecular Docking and Molecular Dynamics Simulations Discover Curcumin Analogue as a Plausible Dual Inhibitor for SARS-CoV-2, International Journal of Molecular Sciences, doi:10.3390/ijms23031771.
26.
Singh (C) et al., Potential of turmeric-derived compounds against RNA-dependent RNA polymerase of SARS-CoV-2: An in-silico approach, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2021.104965.
27.
Kandeil et al., Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2, Pathogens, doi:10.3390/pathogens10060758.
28.
Rajagopal et al., Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-020-00126-x.
29.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
30.
Sekiou et al., In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2, ChemRxiv, doi:10.26434/chemrxiv.12181404.v1.
31.
Grüneberg et al., Dose-dependent antiviral effects of glycyrrhizin, curcumin, and harmaline against clinical SARS-CoV-2 isolates, including D614G, Omicron BA.5, and Omicron XBB.1, BMC Complementary Medicine and Therapies, doi:10.1186/s12906-026-05253-1.
32.
Aktay et al., Oral Administration of Water-Soluble Curcumin Complex Prevents ARDS With the Potential for COVID-19 Treatment, Phytotherapy Research, doi:10.1002/ptr.70046.
33.
Olubiyi et al., Novel dietary herbal preparations with inhibitory activities against multiple SARS-CoV-2 targets: A multidisciplinary investigation into antiviral activities, Food Chemistry Advances, doi:10.1016/j.focha.2025.100969.
34.
Emam et al., Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors, AMB Express, doi:10.1186/s13568-024-01739-8.
35.
Van Tin et al., Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling, Cells, doi:10.3390/cells13161331.
36.
Li et al., Thermal shift assay (TSA)-based drug screening strategy for rapid discovery of inhibitors against the Nsp13 helicase of SARS-CoV-2, Animals and Zoonoses, doi:10.1016/j.azn.2024.06.001.
37.
Kamble et al., Nanoparticulate curcumin spray imparts prophylactic and therapeutic properties against SARS-CoV-2, Emergent Materials, doi:10.1007/s42247-024-00754-6.
38.
Nicoliche et al., Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2, Scientific Reports, doi:10.1038/s41598-024-61662-7.
39.
Nittayananta et al., A novel film spray containing curcumin inhibits SARS-CoV-2 and influenza virus infection and enhances mucosal immunity, Virology Journal, doi:10.1186/s12985-023-02282-x.
40.
Septisetyani et al., Curcumin and turmeric extract inhibited SARS-CoV-2 pseudovirus cell entry and Spike mediated cell fusion, bioRxiv, doi:10.1101/2023.09.28.560070.
41.
Mohd Abd Razak et al., In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds, Natural Product Communications, doi:10.1177/1934578X231188861.
42.
Fam et al., Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites, Scientific Reports, doi:10.1038/s41598-023-31764-9.
43.
Teshima et al., Antiviral activity of curcumin and its analogs selected by an artificial intelligence-supported activity prediction system in SARS-CoV-2-infected VeroE6 cells, Natural Product Research, doi:10.1080/14786419.2023.2194647.
44.
Zupin et al., Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer, Viruses, doi:10.3390/v14102132.
45.
Leka et al., In vitro antiviral activity against SARS-CoV-2 of common herbal medicinal extracts and their bioactive compounds, Phytotherapy Research, doi:10.1002/ptr.7463.
46.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
47.
Marín-Palma et al., Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms, Molecules, doi:10.3390/molecules26226900.
48.
Bahun et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594.
49.
Bormann et al., Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro, Viruses, doi:10.3390/v13101914.
50.
Guijarro-Real et al., Potential In Vitro Inhibition of Selected Plant Extracts against SARS-CoV-2 Chymotripsin-Like Protease (3CLPro) Activity, Foods, doi:10.3390/foods10071503.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The receptor binding domain is a specific region of the spike protein that binds ACE2 and is a major target of neutralizing antibodies. Focusing on the precise binding site allows highly specific disruption of viral attachment with reduced potential for off-target effects.
c.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
d.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
e.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
f.
The angiotensin converting enzyme 2 (ACE2) protein is a host cell transmembrane protein that serves as the cellular receptor for the SARS-CoV-2 spike protein. ACE2 is expressed on many cell types, including epithelial cells in the lungs, and allows the virus to enter and infect host cells. Inhibition may affect ACE2's physiological function in blood pressure control.
g.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
h.
Non-structural protein 10 (nsp10) serves as an RNA chaperone and stabilizes conformations of nsp12 and nsp14 in the replicase-transcriptase complex, which synthesizes new viral RNAs. Nsp10 disruption may destabilize replicase-transcriptase complex activity.
i.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
j.
The interaction between the SARS-CoV-2 spike protein and the human ACE2 receptor is a primary method of viral entry, inhibiting this interaction can prevent the virus from attaching to and entering host cells, halting infection at an early stage.
k.
Transmembrane protease serine 2 (TMPRSS2) is a host cell protease that primes the spike protein, facilitating cellular entry. TMPRSS2 activity helps enable cleavage of the spike protein required for membrane fusion and virus entry. Inhibition may especially protect respiratory epithelial cells, buy may have physiological effects.
l.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
m.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
n.
A549-AT is a human lung carcinoma cell line stably transfected with ACE2 and TMPRSS2 receptors. Unlike the parental line, this overexpression ensures stable infection and enhanced viral entry, allowing for the evaluation of antiviral efficacy against various SARS-CoV-2 variants.
o.
293T is a human embryonic kidney cell line that can be engineered for high ACE2 expression and SARS-CoV-2 susceptibility. 293T cells are easily transfected and support high protein expression.
p.
HEK293-hACE2 is a human embryonic kidney cell line with high ACE2 expression and SARS-CoV-2 susceptibility. Cells have been transfected with a plasmid to express the human ACE2 (hACE2) protein.
q.
293T/hACE2/TMPRSS2 is a human embryonic kidney cell line engineered for high ACE2 and TMPRSS2 expression, which mimics key aspects of human infection. 293T/hACE2/TMPRSS2 cells are very susceptible to SARS-CoV-2 infection.
r.
Vero E6 is an African green monkey kidney cell line with low/no ACE2 expression and high SARS-CoV-2 susceptibility. The cell line is easy to maintain and supports robust viral replication, however the monkey origin may not accurately represent human responses.
s.
SH-SY5Y is a human neuroblastoma cell line that exhibits neuronal phenotypes. It is commonly used as an in vitro model for studying neurotoxicity, neurodegenerative diseases, and neuronal differentiation.
Singh et al., 22 Oct 2021, peer-reviewed, 3 authors.
In silico studies are an important part of preclinical research, however results may be very different in vivo.
Potential of turmeric-derived compounds against RNA‐dependent RNA polymerase of SARS‐CoV‐2: An in-silico approach
Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2021.104965
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests All authors hereby declare that they have no competing interests related to this work. J o u r n a l P r e -p r o o f
References
Bhardwaj, Singh, Das, Purohit, Evaluation of acridinedione analogs as potential SARS-CoV-2 main protease inhibitors and their comparison with repurposed anti-viral drugs, Comput. Biol. Med, doi:10.1016/j.compbiomed.2020.104117
Bhardwaj, Singh, Sharma, Rajendran, Purohit et al., Bioactive molecules of Tea as potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2, Front. Med, doi:10.3389/FMED.2021.684020.JournalPre-proof
Bhardwaj, Singh, Sharma, Rajendran, Purohit et al., Identification of bioactive molecules from tea plant as SARS-CoV-2 main protease inhibitors, J. Biomol. Struct. Dyn, doi:10.1080/07391102.2020.1766572
Chan, Yuan, Kok, To, Chu et al., A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster, Lancet, doi:10.1016/S0140-6736(20)30154-9
Chen, Zhou, Dong, Qu, Gong et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study, Lancet, doi:10.1016/S0140-6736(20
Darden, York, Pedersen, Particle mesh Ewald: An N•log(N) method for Ewald sums in large systems, J. Chem. Phys, doi:10.1063/1.464397
Ebrahimi, Ansari, Hosseyni Moghaddam, Ebrahimi, Salehi et al., In silico investigation on the inhibitory effect of fungal secondary metabolites on RNA dependent RNA polymerase of SARS-CoV-II: A docking and molecular dynamic simulation study, Comput. Biol. Med, doi:10.1016/J.COMPBIOMED.2021.104613
Gao, Yan, Huang, Liu, Zhao et al., Structure of the RNAdependent RNA polymerase from COVID-19 virus, Science, doi:10.1126/science.abb7498
Gong, Peersen, Structural basis for active site closure by the poliovirus RNAdependent RNA polymerase, Proc. Natl. Acad. Sci. U. S. A, doi:10.1073/pnas.1007626107
Gordon, Tchesnokov, Woolner, Perry, Feng et al., Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency, J. Biol. Chem, doi:10.1074/jbc.RA120.013679
Hess, Bekker, Berendsen, Fraaije, LINCS: A Linear Constraint Solver for molecular simulations, J. Comput. Chem
Hillen, Kokic, Farnung, Dienemann, Tegunov et al., Structure of replicating SARS-CoV-2 polymerase, Nature, doi:10.1038/s41586-020-2368-8
Holshue, Debolt, Lindquist, Lofy, Wiesman et al., First Case of 2019 Novel Coronavirus in the United States, N. Engl. J. Med, doi:10.1056/nejmoa2001191
Hosseini, Chen, Xiao, Wang, Computational molecular docking and virtual screening revealed promising SARS-CoV-2 drugs, Precis, Clin. Med, doi:10.1093/pcmedi/pbab001
Kirchdoerfer, Ward, Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors, Nat. Commun, doi:10.1038/s41467-019-10280-3.JournalPre-proof
Koulgi, Jani, Mallikarjunachari Uppuladinne, Sonavane, Joshi, Natural plant products as potential inhibitors of RNA dependent RNA polymerase of Severe Acute Respiratory Syndrome Coronavirus-2, PLoS One, doi:10.1371/journal.pone.0251801
Kumar, Bhardwaj, Singh, Purohit, Explicit-solvent molecular dynamics simulations revealed conformational regain and aggregation inhibition of I113T SOD1 by Himalayan bioactive molecules, J. Mol. Liq, doi:10.1016/j.molliq.2021.116798
Kumari, Kumar, Lynn, G-mmpbsa -A GROMACS tool for high-throughput MM-PBSA calculations, J. Chem. Inf. Model, doi:10.1021/ci500020m
Lehmann, Gulyaeva, Zevenhoven-Dobbe, Janssen, Ruben et al., Discovery of an essential nucleotidylating activity associated with a newly delineated conserved domain in the RNA polymerase-containing protein of all nidoviruses, Nucleic Acids Res, doi:10.1093/nar/gkv838
Mcdonald, RNA synthetic mechanisms employed by diverse families of RNA viruses, Wiley Interdiscip. Rev. RNA, doi:10.1002/wrna.1164
Moghadamtousi, Abdul Kadir, Hassandarvish, Tajik, Abubakar et al., A review on antibacterial, antiviral, and antifungal activity of curcumin, Biomed Res. Int, doi:10.1155/2014/186864
Mounce, Cesaro, Carrau, Vallet, Vignuzzi, Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding, Antiviral Res, doi:10.1016/j.antiviral.2017.03.014
Niranjan, Singh, Dhiman, Tewari, Biochemical Composition of Curcuma longa L. Accessions, Anal. Lett, doi:10.1080/00032719.2012.751541
Parrinello, Rahman, Polymorphic transitions in single crystals: A new molecular dynamics method, J. Appl. Phys, doi:10.1063/1.328693
Priyadarsini, The chemistry of curcumin: From extraction to therapeutic agent, Molecules, doi:10.3390/molecules191220091
Rajagopal, Varakumar, Baliwada, Byran, Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach, Futur. J. Pharm. Sci, doi:10.1186/s43094-020-00126-x
Rattis, Ramos, Celes, Curcumin as a Potential Treatment for COVID-19, Front. Pharmacol, doi:10.3389/fphar.2021.675287
Shannon, Le, Selisko, Eydoux, Alvarez et al., Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites, Antiviral Res, doi:10.1016/j.antiviral.2020.104793
Sharma, Bhardwaj, Singh, Rajendran, Purohit et al., An in-silico evaluation of different bioactive molecules of Tea for their inhibition potency against non structural protein-15 of SARS-CoV-2, Food Chem, doi:10.1016/j.foodchem.2020.128933
Singh, Bhardwaj, Das, Purohit, A computational approach for rational discovery of inhibitors for non-structural protein 1 of SARS-CoV-2, Comput. Biol. Med, doi:10.1016/j.compbiomed.2021.104555
Singh, Bhardwaj, Sharma, Kumar, Purohit, Identification of potential plant bioactive as SARS-CoV-2 Spike protein and human ACE2 fusion inhibitors, Comput. Biol. Med, doi:10.1016/J.COMPBIOMED.2021
Singh, Bhardwaj, Sharma, Purohit, Kumar, In-silico evaluation of bioactive compounds from tea as potential SARS-CoV-2 nonstructural protein 16 inhibitors, J. Tradit. Complement. Med, doi:10.1016/j.jtcme.2021.05.005
Skariyachan, Gopal, Muddebihalkar, Uttarkar, Niranjan, Structural insights on the interaction potential of natural leads against major protein targets of SARS-CoV-2: Molecular modelling, docking and dynamic simulation studies, Comput. Biol. Med, doi:10.1016/J.COMPBIOMED.2021.104325
Studio, Dassault Systemes BIOVIA
Subissi, Posthuma, Collet, Zevenhoven-Dobbe, Gorbalenya et al., One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities, Proc. Natl. Acad. Sci. U. S. A, doi:10.1073/pnas.1323705111
Umashankar, Deshpande, Hegde, Singh, Chattopadhyay, Phytochemical Moieties From Indian Traditional Medicine for Targeting Dual Hotspots on SARS-CoV-2
Van Der, Spoel, Lindahl, Hess, Groenhof et al., GROMACS: Fast, flexible, and free, J. Comput. Chem, doi:10.1002/jcc.20291
Vanommeslaeghe, Hatcher, Acharya, Kundu, Zhong et al., CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields, J. Comput. Chem, doi:10.1002/jcc.21367.JournalPre-proof
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Yin, Mao, Luan, Shen, Shen et al., Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir, Science, doi:10.1126/science.abc1560
Zheng, Frisch, Efficient Geometry Minimization and Transition Structure Optimization Using Interpolated Potential Energy Surfaces and Iteratively Updated Hessians, J. Chem. Theory Comput, doi:10.1021/acs.jctc.7b00719
DOI record:
{
"DOI": "10.1016/j.compbiomed.2021.104965",
"ISSN": [
"0010-4825"
],
"URL": "http://dx.doi.org/10.1016/j.compbiomed.2021.104965",
"alternative-id": [
"S0010482521007599"
],
"article-number": "104965",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Potential of turmeric-derived compounds against RNA‐dependent RNA polymerase of SARS‐CoV‐2: An in-silico approach"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Computers in Biology and Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.compbiomed.2021.104965"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Singh",
"given": "Rahul",
"sequence": "first"
},
{
"affiliation": [],
"family": "Bhardwaj",
"given": "Vijay Kumar",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2101-9398",
"affiliation": [],
"authenticated-orcid": false,
"family": "Purohit",
"given": "Rituraj",
"sequence": "additional"
}
],
"container-title": "Computers in Biology and Medicine",
"container-title-short": "Computers in Biology and Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
10,
22
]
],
"date-time": "2021-10-22T21:32:51Z",
"timestamp": 1634938371000
},
"deposited": {
"date-parts": [
[
2022,
12,
22
]
],
"date-time": "2022-12-22T18:01:32Z",
"timestamp": 1671732092000
},
"indexed": {
"date-parts": [
[
2023,
3,
26
]
],
"date-time": "2023-03-26T22:59:28Z",
"timestamp": 1679871568424
},
"is-referenced-by-count": 36,
"issued": {
"date-parts": [
[
2021,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0010482521007599?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0010482521007599?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "104965",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
12
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)30154-9",
"article-title": "A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster",
"author": "Chan",
"doi-asserted-by": "crossref",
"first-page": "514",
"journal-title": "Lancet",
"key": "10.1016/j.compbiomed.2021.104965_bib1",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30211-7",
"article-title": "Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study",
"author": "Chen",
"doi-asserted-by": "crossref",
"first-page": "507",
"journal-title": "Lancet",
"key": "10.1016/j.compbiomed.2021.104965_bib2",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1074/jbc.RA120.013679",
"article-title": "Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "6785",
"journal-title": "J. Biol. Chem.",
"key": "10.1016/j.compbiomed.2021.104965_bib3",
"volume": "295",
"year": "2020"
},
{
"DOI": "10.1126/science.abc1560",
"article-title": "Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir",
"author": "Yin",
"doi-asserted-by": "crossref",
"first-page": "1499",
"journal-title": "Science",
"key": "10.1016/j.compbiomed.2021.104965_bib4",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1073/pnas.1323705111",
"article-title": "One severe acute respiratory syndrome coronavirus protein complex integrates processive RNA polymerase and exonuclease activities",
"author": "Subissi",
"doi-asserted-by": "crossref",
"first-page": "E3900",
"journal-title": "Proc. Natl. Acad. Sci. U.S.A.",
"key": "10.1016/j.compbiomed.2021.104965_bib5",
"volume": "111",
"year": "2014"
},
{
"DOI": "10.1002/wrna.1164",
"article-title": "RNA synthetic mechanisms employed by diverse families of RNA viruses",
"author": "Mcdonald",
"doi-asserted-by": "crossref",
"first-page": "351",
"journal-title": "Wiley Interdiscip. Rev. RNA.",
"key": "10.1016/j.compbiomed.2021.104965_bib6",
"volume": "4",
"year": "2013"
},
{
"DOI": "10.1093/nar/gkv838",
"article-title": "Discovery of an essential nucleotidylating activity associated with a newly delineated conserved domain in the RNA polymerase-containing protein of all nidoviruses",
"author": "Lehmann",
"doi-asserted-by": "crossref",
"first-page": "8416",
"journal-title": "Nucleic Acids Res.",
"key": "10.1016/j.compbiomed.2021.104965_bib7",
"volume": "43",
"year": "2015"
},
{
"DOI": "10.1038/s41467-019-10280-3",
"article-title": "Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors",
"author": "Kirchdoerfer",
"doi-asserted-by": "crossref",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.compbiomed.2021.104965_bib8",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1073/pnas.1007626107",
"article-title": "Structural basis for active site closure by the poliovirus RNA-dependent RNA polymerase",
"author": "Gong",
"doi-asserted-by": "crossref",
"first-page": "22505",
"journal-title": "Proc. Natl. Acad. Sci. U.S.A.",
"key": "10.1016/j.compbiomed.2021.104965_bib9",
"volume": "107",
"year": "2010"
},
{
"DOI": "10.1016/j.antiviral.2020.104793",
"article-title": "Remdesivir and SARS-CoV-2: structural requirements at both nsp12 RdRp and nsp14 Exonuclease active-sites",
"author": "Shannon",
"doi-asserted-by": "crossref",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.compbiomed.2021.104965_bib10",
"volume": "178",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res.",
"key": "10.1016/j.compbiomed.2021.104965_bib11",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001191",
"article-title": "First case of 2019 novel coronavirus in the United States, N",
"author": "Holshue",
"doi-asserted-by": "crossref",
"first-page": "929",
"journal-title": "Engl. J. Med.",
"key": "10.1016/j.compbiomed.2021.104965_bib12",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1155/2014/186864",
"article-title": "A review on antibacterial, antiviral, and antifungal activity of curcumin",
"author": "Zorofchian Moghadamtousi",
"doi-asserted-by": "crossref",
"journal-title": "BioMed Res. Int.",
"key": "10.1016/j.compbiomed.2021.104965_bib13",
"volume": "2014",
"year": "2014"
},
{
"DOI": "10.1016/j.antiviral.2017.03.014",
"article-title": "Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding",
"author": "Mounce",
"doi-asserted-by": "crossref",
"first-page": "148",
"journal-title": "Antivir. Res.",
"key": "10.1016/j.compbiomed.2021.104965_bib14",
"volume": "142",
"year": "2017"
},
{
"DOI": "10.1080/00032719.2012.751541",
"article-title": "Biochemical composition of curcuma longa L. Accessions",
"author": "Niranjan",
"doi-asserted-by": "crossref",
"first-page": "1069",
"journal-title": "Anal. Lett.",
"key": "10.1016/j.compbiomed.2021.104965_bib15",
"volume": "46",
"year": "2013"
},
{
"DOI": "10.3390/molecules191220091",
"article-title": "The chemistry of curcumin: from extraction to therapeutic agent",
"author": "Priyadarsini",
"doi-asserted-by": "crossref",
"first-page": "20091",
"journal-title": "Molecules",
"key": "10.1016/j.compbiomed.2021.104965_bib16",
"volume": "19",
"year": "2014"
},
{
"DOI": "10.1021/acs.jctc.7b00719",
"article-title": "Efficient geometry minimization and transition structure optimization using interpolated potential energy surfaces and iteratively updated hessians",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "6424",
"journal-title": "J. Chem. Theor. Comput.",
"key": "10.1016/j.compbiomed.2021.104965_bib17",
"volume": "13",
"year": "2017"
},
{
"article-title": "Dassault systemes BIOVIA, discovery studio modelling environment, release 4.5",
"author": "Studio",
"first-page": "98",
"journal-title": "Accelrys Softw. Inc.",
"key": "10.1016/j.compbiomed.2021.104965_bib18",
"year": "2015"
},
{
"article-title": "CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields",
"author": "Vanommeslaeghe",
"first-page": "671",
"journal-title": "J. Comput. Chem.",
"key": "10.1016/j.compbiomed.2021.104965_bib19",
"volume": "31",
"year": "2010"
},
{
"DOI": "10.1002/jcc.20291",
"article-title": "GROMACS: fast, flexible, and free",
"author": "Van Der Spoel",
"doi-asserted-by": "crossref",
"first-page": "1701",
"journal-title": "J. Comput. Chem.",
"key": "10.1016/j.compbiomed.2021.104965_bib20",
"volume": "26",
"year": "2005"
},
{
"DOI": "10.1063/1.328693",
"article-title": "Polymorphic transitions in single crystals: a new molecular dynamics method",
"author": "Parrinello",
"doi-asserted-by": "crossref",
"first-page": "7182",
"journal-title": "J. Appl. Phys.",
"key": "10.1016/j.compbiomed.2021.104965_bib21",
"volume": "52",
"year": "1981"
},
{
"DOI": "10.1002/(SICI)1096-987X(199709)18:12<1463::AID-JCC4>3.0.CO;2-H",
"article-title": "LINCS: a linear constraint solver for molecular simulations",
"author": "Hess",
"doi-asserted-by": "crossref",
"first-page": "1463",
"journal-title": "J. Comput. Chem.",
"key": "10.1016/j.compbiomed.2021.104965_bib22",
"volume": "18",
"year": "1997"
},
{
"DOI": "10.1063/1.464397",
"article-title": "Particle mesh Ewald: an N·log(N) method for Ewald sums in large systems",
"author": "Darden",
"doi-asserted-by": "crossref",
"first-page": "10089",
"journal-title": "J. Chem. Phys.",
"key": "10.1016/j.compbiomed.2021.104965_bib23",
"volume": "98",
"year": "1993"
},
{
"DOI": "10.1021/ci500020m",
"article-title": "G-mmpbsa -A GROMACS tool for high-throughput MM-PBSA calculations",
"author": "Kumari",
"doi-asserted-by": "crossref",
"first-page": "1951",
"journal-title": "J. Chem. Inf. Model.",
"key": "10.1016/j.compbiomed.2021.104965_bib24",
"volume": "54",
"year": "2014"
},
{
"DOI": "10.3389/fphar.2021.675287",
"article-title": "Curcumin as a potential treatment for COVID-19",
"author": "Rattis",
"doi-asserted-by": "crossref",
"first-page": "1068",
"journal-title": "Front. Pharmacol.",
"key": "10.1016/j.compbiomed.2021.104965_bib25",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1186/s43094-020-00126-x",
"article-title": "Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach",
"author": "Rajagopal",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Futur. J. Pharm. Sci.",
"key": "10.1016/j.compbiomed.2021.104965_bib26",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2021.672629",
"article-title": "Phytochemical moieties from Indian traditional medicine for targeting dual hotspots on SARS-CoV-2 spike protein: an integrative in-silico approach",
"author": "Umashankar",
"doi-asserted-by": "crossref",
"journal-title": "Front. Med.",
"key": "10.1016/j.compbiomed.2021.104965_bib27",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/j.compbiomed.2021.104631",
"article-title": "Identification of potential plant bioactive as SARS-CoV-2 Spike protein and human ACE2 fusion inhibitors",
"author": "Singh",
"doi-asserted-by": "crossref",
"journal-title": "Comput. Biol. Med.",
"key": "10.1016/j.compbiomed.2021.104965_bib28",
"volume": "136",
"year": "2021"
},
{
"DOI": "10.1038/s41586-020-2368-8",
"article-title": "Structure of replicating SARS-CoV-2 polymerase",
"author": "Hillen",
"doi-asserted-by": "crossref",
"first-page": "154",
"journal-title": "Nature",
"key": "10.1016/j.compbiomed.2021.104965_bib29",
"volume": "584",
"year": "2020"
},
{
"DOI": "10.1126/science.abb7498",
"article-title": "Structure of the RNA-dependent RNA polymerase from COVID-19 virus",
"author": "Gao",
"doi-asserted-by": "crossref",
"first-page": "779",
"journal-title": "Science",
"key": "10.1016/j.compbiomed.2021.104965_bib30",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2021.684020",
"article-title": "Bioactive molecules of Tea as potential inhibitors for RNA-dependent RNA polymerase of SARS-CoV-2",
"author": "Bhardwaj",
"doi-asserted-by": "crossref",
"first-page": "645",
"journal-title": "Front. Med.",
"key": "10.1016/j.compbiomed.2021.104965_bib31",
"volume": "8",
"year": "2021"
},
{
"DOI": "10.1016/j.molliq.2021.116798",
"article-title": "Explicit-solvent molecular dynamics simulations revealed conformational regain and aggregation inhibition of I113T SOD1 by Himalayan bioactive molecules",
"author": "Kumar",
"doi-asserted-by": "crossref",
"journal-title": "J. Mol. Liq.",
"key": "10.1016/j.compbiomed.2021.104965_bib32",
"year": "2021"
},
{
"DOI": "10.1016/j.compbiomed.2020.104117",
"article-title": "Evaluation of acridinedione analogs as potential SARS-CoV-2 main protease inhibitors and their comparison with repurposed anti-viral drugs",
"author": "Bhardwaj",
"doi-asserted-by": "crossref",
"journal-title": "Comput. Biol. Med.",
"key": "10.1016/j.compbiomed.2021.104965_bib33",
"volume": "128",
"year": "2021"
},
{
"article-title": "A computational approach for rational discovery of inhibitors for non-structural protein 1 of SARS-CoV-2, Comput",
"author": "Singh",
"journal-title": "Biol. Med.",
"key": "10.1016/j.compbiomed.2021.104965_bib34",
"year": "2021"
},
{
"article-title": "An in-silico evaluation of different bioactive molecules of Tea for their inhibition potency against non structural protein-15 of SARS-CoV-2",
"author": "Sharma",
"journal-title": "Food Chem.",
"key": "10.1016/j.compbiomed.2021.104965_bib35",
"year": "2020"
},
{
"article-title": "In-silico evaluation of bioactive compounds from tea as potential SARS-CoV-2 nonstructural protein 16 inhibitors",
"author": "Singh",
"journal-title": "J. Tradit. Complement. Med",
"key": "10.1016/j.compbiomed.2021.104965_bib36",
"year": "2021"
},
{
"article-title": "Identification of bioactive molecules from tea plant as SARS-CoV-2 main protease inhibitors",
"author": "Bhardwaj",
"first-page": "1",
"journal-title": "J. Biomol. Struct. Dyn.",
"key": "10.1016/j.compbiomed.2021.104965_bib37",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0251801",
"article-title": "Natural plant products as potential inhibitors of RNA dependent RNA polymerase of Severe Acute Respiratory Syndrome Coronavirus-2",
"author": "Koulgi",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/j.compbiomed.2021.104965_bib38",
"volume": "16",
"year": "2021"
},
{
"article-title": "Computational molecular docking and virtual screening revealed promising SARS-CoV-2 drugs, Precis",
"author": "Hosseini",
"first-page": "1",
"journal-title": "Clin. Med.",
"key": "10.1016/j.compbiomed.2021.104965_bib39",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1016/j.compbiomed.2021.104613",
"article-title": "In silico investigation on the inhibitory effect of fungal secondary metabolites on RNA dependent RNA polymerase of SARS-CoV-II: a docking and molecular dynamic simulation study",
"author": "Ebrahimi",
"doi-asserted-by": "crossref",
"journal-title": "Comput. Biol. Med.",
"key": "10.1016/j.compbiomed.2021.104965_bib40",
"volume": "135",
"year": "2021"
},
{
"DOI": "10.1016/j.compbiomed.2021.104325",
"article-title": "Structural insights on the interaction potential of natural leads against major protein targets of SARS-CoV-2: molecular modelling, docking and dynamic simulation studies",
"author": "Skariyachan",
"doi-asserted-by": "crossref",
"journal-title": "Comput. Biol. Med.",
"key": "10.1016/j.compbiomed.2021.104965_bib41",
"volume": "132",
"year": "2021"
}
],
"reference-count": 41,
"references-count": 41,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0010482521007599"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Health Informatics",
"Computer Science Applications"
],
"subtitle": [],
"title": "Potential of turmeric-derived compounds against RNA‐dependent RNA polymerase of SARS‐CoV‐2: An in-silico approach",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "139"
}
