Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein
et al., International Journal of Molecular Sciences, doi:10.3390/ijms242115894, Nov 2023
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
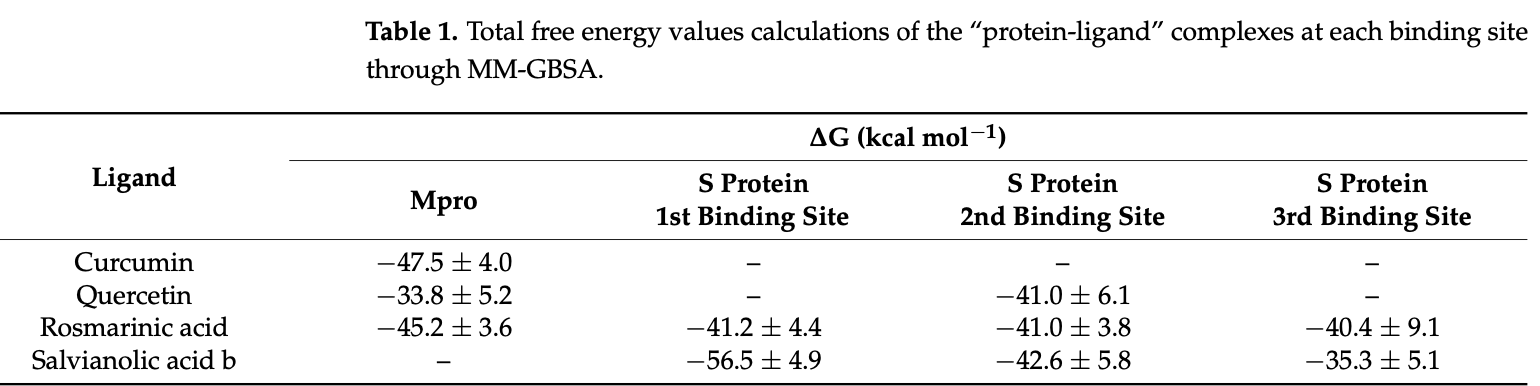
In silico molecular docking and molecular dynamics analysis identifying curcumin, quercetin, rosmarinic acid, and salvianolic acid B as having favorable binding to Mpro and three distinct sites on the S protein. Molecular dynamics simulations confirmed rosmarinic acid and quercetin's stable binding to Mpro. At the S protein sites, salvianolic acid B and rosmarinic acid formed robust complexes. A similarity search yielded compounds structurally related to the top binders, with two analogs of salvianolic acid emerging as promising multi-target inhibitors against both Mpro and S proteins.
62 preclinical studies support the efficacy of curcumin for COVID-19:
In silico studies predict inhibition of SARS-CoV-2 with curcumin or metabolites via binding to the spikeA,1,5,6,11,16,18,24,27 (and specifically the receptor binding domainB,2,4,14,17,20 ), MproC,4-6,11,13,15-17,19,20,22,25,27,28,30,48 , RNA-dependent RNA polymeraseD,4-6,17,26 , PLproE,6, ACE2F,2,18,19,21 , nucleocapsidG,12,29 , nsp10H,29, and helicaseI,36 proteins, and inhibition of spike-ACE2 interactionJ,3.
In vitro studies demonstrate inhibition of the spikeA,41 (and specifically the receptor binding domainB,51), MproC,23,41,48,50 , ACE2F,51, and TMPRSS2K,51 proteins, and inhibition of spike-ACE2 interactionJ,3,34 .
In vitro studies demonstrate efficacy in Calu-3L,49, A549M,41, A549-ATN,31, 293TO,7, HEK293-hACE2P,23,39 , 293T/hACE2/TMPRSS2Q,40, Vero E6R,1,13,17,27,39,41,43,45,47,49 , and SH-SY5YS,38 cells.
Curcumin decreases pro-inflammatory cytokines induced by SARS-CoV-2 in peripheral blood mononuclear cells47, alleviates SARS-CoV-2 spike protein-induced mitochondrial membrane damage and oxidative stress7, may limit COVID-19 induced cardiac damage by inhibiting the NF-κB signaling pathway which mediates the profibrotic effects of the SARS-CoV-2 spike protein on cardiac fibroblasts35, is predicted to inhibit the interaction between the SARS-CoV-2 spike protein receptor binding domain and the human ACE2 receptor for the delta and omicron variants14, lowers ACE2 and STAT3, curbing lung inflammation and ARDS in preclinical COVID-19 models32, inhibits SARS-CoV-2 ORF3a ion channel activity, which contributes to viral pathogenicity and cytotoxicity42, has direct virucidal action by disrupting viral envelope integrity44, may inhibit viral replication and modulate inflammatory pathways like NF-κB via SIRT1 activation52, and can function as a photosensitizer in photodynamic therapy to generate reactive oxygen species that damage the virus44.
Study covers quercetin and curcumin.
1.
Marzouk et al., Computational and Experimental Insights into the Antiviral Mechanism of Turmeric (Curcuma longa) against SARS-CoV-2 D614G, BIO Web of Conferences, doi:10.1051/bioconf/202519804002.
2.
Wu et al., Utilizing natural compounds as ligands to disrupt the binding of SARS-CoV-2 receptor-binding domain to angiotensin-converting enzyme 2, impeding viral infection, Phytochemistry Letters, doi:10.1016/j.phytol.2025.102999.
3.
Najimi et al., Phytochemical Inhibitors of SARS‐CoV‐2 Entry: Targeting the ACE2‐RBD Interaction with l‐Tartaric Acid, l‐Ascorbic Acid, and Curcuma longa Extract, ChemistrySelect, doi:10.1002/slct.202406035.
4.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
5.
Al balawi et al., Assessing multi-target antiviral and antioxidant activities of natural compounds against SARS-CoV-2: an integrated in vitro and in silico study, Bioresources and Bioprocessing, doi:10.1186/s40643-024-00822-z.
6.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
7.
Zhang et al., Computational Discovery of Mitochondrial Dysfunction Biomarkers in Severe SARS-CoV-2 Infection: Facilitating Pytomedicine Screening, Phytomedicine, doi:10.1016/j.phymed.2024.155784.
8.
Öztürkkan et al., In Silico investigation of the effects of curcuminoids on the spike protein of the omicron variant of SARS-CoV-2, Baku State University Journal of Chemistry and Material Sciences, 1:2, bsuj.bsu.edu.az/uploads/pdf/ec4204d62f7802de54e6092bf7860029.pdf.
9.
Yunze et al., Therapeutic effect and potential mechanism of curcumin, an active ingredient in Tongnao Decoction, on COVID-19 combined with stroke: a network pharmacology study and GEO database mining, Research Square, doi:10.21203/rs.3.rs-4329762/v1.
10.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
11.
Boseila et al., Throat spray formulated with virucidal Pharmaceutical excipients as an effective early prophylactic or treatment strategy against pharyngitis post-exposure to SARS CoV-2, European Journal of Pharmaceutics and Biopharmaceutics, doi:10.1016/j.ejpb.2024.114279.
12.
Hidayah et al., Bioinformatics study of curcumin, demethoxycurcumin, bisdemethoxycurcumin and cyclocurcumin compounds in Curcuma longa as an antiviral agent via nucleocapsid on SARS-CoV-2 inhibition, International Conference on Organic and Applied Chemistry, doi:10.1063/5.0197724.
13.
Singh et al., Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 M Pro protein - An in-silico and cell-based approach, Research Square, doi:10.21203/rs.3.rs-3888947/v1.
14.
Kant et al., Structure-based drug discovery to identify SARS-CoV2 spike protein–ACE2 interaction inhibitors, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2023.2300060.
15.
Naderi Beni et al., In silico studies of anti-oxidative and hot temperament-based phytochemicals as natural inhibitors of SARS-CoV-2 Mpro, PLOS ONE, doi:10.1371/journal.pone.0295014.
16.
Moschovou et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, International Journal of Molecular Sciences, doi:10.3390/ijms242115894.
17.
Eleraky et al., Curcumin Transferosome-Loaded Thermosensitive Intranasal in situ Gel as Prospective Antiviral Therapy for SARS-Cov-2, International Journal of Nanomedicine, doi:10.2147/IJN.S423251.
18.
Singh (B) et al., Computational studies to analyze effect of curcumin inhibition on coronavirus D614G mutated spike protein, The Seybold Report, doi:10.17605/OSF.IO/TKEXJ.
19.
Thapa et al., In-silico Approach for Predicting the Inhibitory Effect of Home Remedies on Severe Acute Respiratory Syndrome Coronavirus-2, Makara Journal of Science, doi:10.7454/mss.v27i3.1609.
20.
Srivastava et al., Paradigm of Well-Orchestrated Pharmacokinetic Properties of Curcuminoids Relative to Conventional Drugs for the Inactivation of SARS-CoV-2 Receptors: An In Silico Approach, Stresses, doi:10.3390/stresses3030043.
21.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
22.
Winih Kinasih et al., Analisis in silico interaksi senyawa kurkuminoid terhadap enzim main protease 6LU7 dari SARS-CoV-2, Duta Pharma Journal, doi:10.47701/djp.v3i1.2904.
23.
Wu (B) et al., Potential Mechanism of Curcumin and Resveratrol against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-2780614/v1.
24.
Nag et al., Curcumin inhibits spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in silico study, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2022.105552.
25.
Rampogu et al., Molecular Docking and Molecular Dynamics Simulations Discover Curcumin Analogue as a Plausible Dual Inhibitor for SARS-CoV-2, International Journal of Molecular Sciences, doi:10.3390/ijms23031771.
26.
Singh (C) et al., Potential of turmeric-derived compounds against RNA-dependent RNA polymerase of SARS-CoV-2: An in-silico approach, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2021.104965.
27.
Kandeil et al., Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2, Pathogens, doi:10.3390/pathogens10060758.
28.
Rajagopal et al., Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-020-00126-x.
29.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
30.
Sekiou et al., In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2, ChemRxiv, doi:10.26434/chemrxiv.12181404.v1.
31.
Grüneberg et al., Dose-dependent antiviral effects of glycyrrhizin, curcumin, and harmaline against clinical SARS-CoV-2 isolates, including D614G, Omicron BA.5, and Omicron XBB.1, BMC Complementary Medicine and Therapies, doi:10.1186/s12906-026-05253-1.
32.
Aktay et al., Oral Administration of Water-Soluble Curcumin Complex Prevents ARDS With the Potential for COVID-19 Treatment, Phytotherapy Research, doi:10.1002/ptr.70046.
33.
Olubiyi et al., Novel dietary herbal preparations with inhibitory activities against multiple SARS-CoV-2 targets: A multidisciplinary investigation into antiviral activities, Food Chemistry Advances, doi:10.1016/j.focha.2025.100969.
34.
Emam et al., Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors, AMB Express, doi:10.1186/s13568-024-01739-8.
35.
Van Tin et al., Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling, Cells, doi:10.3390/cells13161331.
36.
Li et al., Thermal shift assay (TSA)-based drug screening strategy for rapid discovery of inhibitors against the Nsp13 helicase of SARS-CoV-2, Animals and Zoonoses, doi:10.1016/j.azn.2024.06.001.
37.
Kamble et al., Nanoparticulate curcumin spray imparts prophylactic and therapeutic properties against SARS-CoV-2, Emergent Materials, doi:10.1007/s42247-024-00754-6.
38.
Nicoliche et al., Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2, Scientific Reports, doi:10.1038/s41598-024-61662-7.
39.
Nittayananta et al., A novel film spray containing curcumin inhibits SARS-CoV-2 and influenza virus infection and enhances mucosal immunity, Virology Journal, doi:10.1186/s12985-023-02282-x.
40.
Septisetyani et al., Curcumin and turmeric extract inhibited SARS-CoV-2 pseudovirus cell entry and Spike mediated cell fusion, bioRxiv, doi:10.1101/2023.09.28.560070.
41.
Mohd Abd Razak et al., In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds, Natural Product Communications, doi:10.1177/1934578X231188861.
42.
Fam et al., Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites, Scientific Reports, doi:10.1038/s41598-023-31764-9.
43.
Teshima et al., Antiviral activity of curcumin and its analogs selected by an artificial intelligence-supported activity prediction system in SARS-CoV-2-infected VeroE6 cells, Natural Product Research, doi:10.1080/14786419.2023.2194647.
44.
Zupin et al., Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer, Viruses, doi:10.3390/v14102132.
45.
Leka et al., In vitro antiviral activity against SARS-CoV-2 of common herbal medicinal extracts and their bioactive compounds, Phytotherapy Research, doi:10.1002/ptr.7463.
46.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
47.
Marín-Palma et al., Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms, Molecules, doi:10.3390/molecules26226900.
48.
Bahun et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594.
49.
Bormann et al., Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro, Viruses, doi:10.3390/v13101914.
50.
Guijarro-Real et al., Potential In Vitro Inhibition of Selected Plant Extracts against SARS-CoV-2 Chymotripsin-Like Protease (3CLPro) Activity, Foods, doi:10.3390/foods10071503.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The receptor binding domain is a specific region of the spike protein that binds ACE2 and is a major target of neutralizing antibodies. Focusing on the precise binding site allows highly specific disruption of viral attachment with reduced potential for off-target effects.
c.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
d.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
e.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
f.
The angiotensin converting enzyme 2 (ACE2) protein is a host cell transmembrane protein that serves as the cellular receptor for the SARS-CoV-2 spike protein. ACE2 is expressed on many cell types, including epithelial cells in the lungs, and allows the virus to enter and infect host cells. Inhibition may affect ACE2's physiological function in blood pressure control.
g.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
h.
Non-structural protein 10 (nsp10) serves as an RNA chaperone and stabilizes conformations of nsp12 and nsp14 in the replicase-transcriptase complex, which synthesizes new viral RNAs. Nsp10 disruption may destabilize replicase-transcriptase complex activity.
i.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
j.
The interaction between the SARS-CoV-2 spike protein and the human ACE2 receptor is a primary method of viral entry, inhibiting this interaction can prevent the virus from attaching to and entering host cells, halting infection at an early stage.
k.
Transmembrane protease serine 2 (TMPRSS2) is a host cell protease that primes the spike protein, facilitating cellular entry. TMPRSS2 activity helps enable cleavage of the spike protein required for membrane fusion and virus entry. Inhibition may especially protect respiratory epithelial cells, buy may have physiological effects.
l.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
m.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
n.
A549-AT is a human lung carcinoma cell line stably transfected with ACE2 and TMPRSS2 receptors. Unlike the parental line, this overexpression ensures stable infection and enhanced viral entry, allowing for the evaluation of antiviral efficacy against various SARS-CoV-2 variants.
o.
293T is a human embryonic kidney cell line that can be engineered for high ACE2 expression and SARS-CoV-2 susceptibility. 293T cells are easily transfected and support high protein expression.
p.
HEK293-hACE2 is a human embryonic kidney cell line with high ACE2 expression and SARS-CoV-2 susceptibility. Cells have been transfected with a plasmid to express the human ACE2 (hACE2) protein.
q.
293T/hACE2/TMPRSS2 is a human embryonic kidney cell line engineered for high ACE2 and TMPRSS2 expression, which mimics key aspects of human infection. 293T/hACE2/TMPRSS2 cells are very susceptible to SARS-CoV-2 infection.
r.
Vero E6 is an African green monkey kidney cell line with low/no ACE2 expression and high SARS-CoV-2 susceptibility. The cell line is easy to maintain and supports robust viral replication, however the monkey origin may not accurately represent human responses.
s.
SH-SY5Y is a human neuroblastoma cell line that exhibits neuronal phenotypes. It is commonly used as an in vitro model for studying neurotoxicity, neurodegenerative diseases, and neuronal differentiation.
Moschovou et al., 2 Nov 2023, peer-reviewed, 7 authors.
In silico studies are an important part of preclinical research, however results may be very different in vivo.
Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein
International Journal of Molecular Sciences, doi:10.3390/ijms242115894
In this in silico study, we conducted an in-depth exploration of the potential of natural products and antihypertensive molecules that could serve as inhibitors targeting the key proteins of the SARS-CoV-2 virus: the main protease (Mpro) and the spike (S) protein. By utilizing Induced Fit Docking (IFD), we assessed the binding affinities of the molecules under study to these crucial viral components. To further comprehend the stability and molecular interactions of the "proteinligand" complexes that derived from docking studies, we performed molecular dynamics (MD) simulations, shedding light on the molecular basis of potential drug candidates for COVID-19 treatment. Moreover, we employed Molecular Mechanics Generalized Born Surface Area (MM-GBSA) calculations on all "protein-ligand" complexes, underscoring the robust binding capabilities of rosmarinic acid, curcumin, and quercetin against Mpro, and salvianolic acid b, rosmarinic acid, and quercetin toward the S protein. Furthermore, in order to expand our search for potent inhibitors, we conducted a structure similarity analysis, using the Enalos Suite, based on the molecules that indicated the most favored results in the in silico studies. The Enalos Suite generated 115 structurally similar compounds to salvianolic acid, rosmarinic acid, and quercetin. These compounds underwent IFD calculations, leading to the identification of two salvianolic acid analogues that exhibited strong binding to all the examined binding sites in both proteins, showcasing their potential as multi-target inhibitors. These findings introduce exciting possibilities for the development of novel therapeutic agents aiming to effectively disrupt the SARS-CoV-2 virus lifecycle.
References
Adem, Eyupoglu, Sarfraz, Rasul, Ali, Identification of Potent COVID-19 Main Protease (Mpro) Inhibitors from Natural Polyphenols: An in Silico Strategy Unveils a Hope against CORONA, doi:10.20944/preprints202003.0333.v1
Adem, Eyupoglu, Sarfraz, Rasul, Zahoor et al., Caffeic acid derivatives (CAFDs) as inhibitors of SARS-CoV-2: CAFDs-based functional foods as a potential alternative approach to combat COVID-19, Phytomedicine, doi:10.1016/j.phymed.2020.153310
Afantitis, Tsoumanis, Melagraki, Enalos suite of tools: Enhance cheminformatics and nanoinformat-ics through knime, Curr. Med. Chem, doi:10.2174/0929867327666200727114410
Ali, Vijayan, Dynamics of the ACE2-SARS-CoV-2/SARS-CoV spike protein interface reveal unique mechanisms, Sci. Rep, doi:10.1038/s41598-020-71188-3
Alzaabi, Hamdy, Ashmawy, Hamoda, Alkhayat et al., Flavonoids are promising safe therapy against COVID-19, Phytochem. Rev, doi:10.1007/s11101-021-09759-z
Amin, Tabari, Iranpanah, Bahramsoltani, Rahimi, Flavonoids as Promising Antiviral Agents against SARS-CoV-2 Infection: A Mechanistic Review, Molecules, doi:10.3390/molecules26133900
Armstrong, Soltoff, Rieu-Werden, Metlay, Haas, Use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers associated with lower risk of COVID-19 in household contacts, PLoS ONE, doi:10.1371/journal.pone.0247548
Bahun, Jukić, Oblak, Kranjc, Bajc et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594
Behloul, Baha, Guo, Yang, Shi et al., In silico identification of strong binders of the SARS-CoV-2 receptorbinding domain, Eur. J. Pharmacol, doi:10.1016/j.ejphar.2020.173701
Bhati, Kaushik, Singh, Rational design of flavonoid based potential inhibitors targeting SARS-CoV 3CL protease for the treatment of COVID-19, J. Mol. Struct, doi:10.1016/j.molstruc.2021.130380
Bojadzic, Alcazar, Chen, Chuang, Condor Capcha et al., Small-Molecule Inhibitors of the Coronavirus Spike: ACE2 Protein-Protein Interaction as Blockers of Viral Attachment and Entry for SARS-CoV-2, ACS Infect. Dis, doi:10.1021/acsinfecdis.1c00070
Chakravarti, Singh, Ghosh, Dey, Sharma et al., A review on potential of natural products in the management of COVID-19, RSC Adv, doi:10.1039/D1RA00644D
Cherrak, Merzouk, Mokhtari-Soulimane, Potential bioactive glycosylated flavonoids as SARS-CoV-2 main protease inhibitors: A molecular docking and simulation studies, PLoS ONE, doi:10.1371/journal.pone.0240653
Dai, Zhang, Jiang, Su, Li et al., Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease, Science, doi:10.1126/science.abb4489
Dhar, Prasad, Tiwari, Pankaj, Bano et al., An In-Silico Study to Identify Hidden Features of Spike Protein and Main Protease of SARS-CoV-2, Preprints
Duarte, Pelorosso, Nicolosi, Victoria Salgado, Vetulli et al., Telmisartan for treatment of COVID-19 patients: An open multicenter randomized clinical trial, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100962
Durdagi, Avsar, Orhan, Serhatli, Balcioglu et al., The neutralization effect of montelukaston SARS-CoV-2 is shown by multiscale in silico simulations and combined in vitro studies, Mol. Ther, doi:10.1016/j.ymthe.2021.10.014
Elebeedy, Elkhatib, Kandeil, Ghanem, Kutkat et al., Anti-SARS-CoV-2 activities of tanshinone IIA, carnosic acid, rosmarinic acid, salvianolic acid, baicalein, and glycyrrhetinic acid between computational andin vitroinsights, RSC Adv, doi:10.1039/D1RA05268C
Forrester, Booz, Sigmund, Coffman, Kawai et al., Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology, Physiol. Rev, doi:10.1152/physrev.00038.2017
Han, Král, Computational Design of ACE2-Based Peptide Inhibitors of SARS-CoV-2, ACS Nano, doi:10.1021/acsnano.0c02857
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hu, Wang, Zhang, Bai, Wang et al., Three salvianolic acids inhibit 2019-nCoV spike pseudovirus viropexis by binding to both its RBD and receptor ACE2, J. Med. Virol, doi:10.1002/jmv.26874
Huang, Yang, Xu, Xu, Liu, Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19, Acta Pharmacol. Sin, doi:10.1038/s41401-020-0485-4
Jain, Mujwar, Repurposing metocurine as main protease inhibitor to develop novel antiviral therapy for COVID-19, Struct. Chem, doi:10.1007/s11224-020-01605-w
Jeon, Ko, Lee, Choi, Byun et al., Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs, Antimicrob. Agents Chemother, doi:10.1128/AAC.00819-20
Jin, Du, Xu, Deng, Liu et al., Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors, Nature, doi:10.1038/s41586-020-2223-y
Jorgensen, Chandrasekhar, Madura, Impey, Klein, Comparison of simple potential functions for simulating liquid water, J. Chem. Phys, doi:10.1063/1.445869
Jorgensen, Maxwell, Tirado-Rives, Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids, J. Am. Chem. Soc, doi:10.1021/ja9621760
Kaminski, Friesner, Tirado-Rives, Jorgensen, Evaluation and Reparametrization of the OPLS-AA Force Field for Proteins via Comparison with Accurate Quantum Chemical Calculations on Peptides, J. Phys. Chem, doi:10.1021/jp003919d
Khan, Heng, Wang, Qiu, Wei et al., In silico and in vitro evaluation of kaempferol as a potential inhibitor of the SARS-CoV-2 main protease (3CLpro), Phyther. Res, doi:10.1002/ptr.6998
Lan, Ge, Yu, Shan, Zhou et al., Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor, Nature, doi:10.1038/s41586-020-2180-5
Li, Abel, Zhu, Cao, Zhao et al., The VSGB 2.0 model: A next generation energy model for high resolution protein structure modeling, Proteins Struct. Funct. Bioinform, doi:10.1002/prot.23106
Li, Zhou, Guo, Xie, He et al., Potential inhibitors for blocking the interaction of the coronavirus SARS-CoV-2 spike protein and its host cell receptor ACE2, J. Transl. Med, doi:10.1186/s12967-022-03501-9
Lu, Stratton, Tang, Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle, J. Med. Virol, doi:10.1002/jmv.25678
Lyne, Lamb, Saeh, Accurate prediction of the relative potencies of members of a series of kinase inhibitors using molecular docking and MM-GBSA scoring, J. Med. Chem, doi:10.1021/jm060522a
Mavromoustakos, Agelis, Durdagi, AT1 antagonists: A patent review (2008-2012, Expert Opin. Ther. Pat, doi:10.1517/13543776.2013.830104
Merarchi, Dudha, Das, Garg, Natural products and phytochemicals as potential anti-SARS-CoV-2 drugs, Phytother. Res, doi:10.1002/ptr.7151
Onweni, Zhang, Caulfield, Hopkins, Fairweather et al., ACEI/ARB therapy in COVID-19: The double-edged sword of ACE2 and SARS-CoV-2 viral docking, Crit. Care, doi:10.1186/s13054-020-03195-9
Ou, Liu, Lei, Li, Mi et al., Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV, Nat. Commun, doi:10.1038/s41467-020-15562-9
Qian, Ou, Góes, Osborne, Castano et al., Identification of the Receptor-Binding Domain of the Spike Glycoprotein of Human Betacoronavirus HKU1, J. Virol, doi:10.1128/JVI.03737-14
Qiu, Shenkin, Hollinger, Still, The GB/SA continuum model for solvation. A fast analytical method for the calculation of approximate Born radii, J. Phys. Chem. A, doi:10.1021/jp961992r
Rashid, Xie, Suleman, Shah, Khan et al., Roles and functions of SARS-CoV-2 proteins in host immune evasion, Front. Immunol, doi:10.3389/fimmu.2022.940756
Roy, Sk, Tanwar, Kar, Computational studies indicated the effectiveness of human metabolites against SARS-CoV-2 main protease, Mol. Divers, doi:10.1007/s11030-022-10513-6
Rungruangmaitree, Phoochaijaroen, Chimprasit, Saparpakorn, Pootanakit et al., Structural analysis of the coronavirus main protease for the design of pan-variant inhibitors, Sci. Rep, doi:10.1038/s41598-023-34305-6
Russo, Tedesco, Spagnuolo, Russo, Antioxidant polyphenols in cancer treatment: Friend, foe or foil? Semin, Cancer Biol, doi:10.1016/j.semcancer.2017.05.005
Samy, Karunanithi, Sheshadhri, Rengarajan, Srinivasan et al., R)-(+)-Rosmarinic Acid as an Inhibitor of Herpes and Dengue Virus Replication: An In Silico Assessment, Rev. Bras. Farmacogn, doi:10.1007/s43450-023-00381-y
Schwantes, Pande, Bowers, Chow, Xu et al., Will Cannabis or Cannabinoids Protect You from SARS-CoV-2 Infection or Treat COVID-19?, J. Chem. Theory Comput, doi:10.1159/000522472
Shahhamzehei, Abdelfatah, Efferth, In Silico and In Vitro Identification of Pan-Coronaviral Main Protease Inhibitors from a Large Natural Product Library, Pharmaceuticals, doi:10.3390/ph15030308
Shinoda, Mikami, Rigid-body dynamics in the isothermal-isobaric ensemble: A test on the accuracy and computational efficiency, J. Comput. Chem, doi:10.1002/jcc.10249
Spagnuolo, Moccia, Russo, Anti-inflammatory effects of flavonoids in neurodegenerative disorders, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2017.09.001
Sriram, Insel, A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance, Br. J. Pharmacol, doi:10.1111/bph.15082
Still, Tempczyk, Hawley, Hendrickson, Semianalytical Treatment of Solvation for Molecular Mechanics and Dynamics, J. Am. Chem. Soc, doi:10.1021/ja00172a038
Suite, Protein Preparation Wizard; Epik Version 2.3
Van Breemen, Muchiri, Bates, Weinstein, Leier et al., Cannabinoids Block Cellular Entry of SARS-CoV-2 and the Emerging Variants, J. Nat. Prod, doi:10.1021/acs.jnatprod.1c00946
Varsou, Nikolakopoulos, Tsoumanis, Melagraki, Afantitis et al., Suite: New Cheminformatics Platform for Drug Discovery and Computational Toxicology
Xu, Gao, Liang, Chen, In silico screening of potential anti-COVID-19 bioactive natural constituents from food sources by molecular docking, Nutrition, doi:10.1016/j.nut.2020.111049
Yang, Pan, Xu, Cheng, Huang et al., Salvianolic acid C potently inhibits SARS-CoV-2 infection by blocking the formation of six-helix bundle core of spike protein, Signal Transduct. Target. Ther, doi:10.1038/s41392-020-00325-1
Zhang, Lin, Sun, Curth, Drosten et al., Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors, Science, doi:10.1126/science.abb3405
Zhu, Zhang, Wang, Li, Yang et al., A Novel Coronavirus from Patients with Pneumonia in China, N. Engl. J. Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.3390/ijms242115894",
"ISSN": [
"1422-0067"
],
"URL": "http://dx.doi.org/10.3390/ijms242115894",
"abstract": "<jats:p>In this in silico study, we conducted an in-depth exploration of the potential of natural products and antihypertensive molecules that could serve as inhibitors targeting the key proteins of the SARS-CoV-2 virus: the main protease (Mpro) and the spike (S) protein. By utilizing Induced Fit Docking (IFD), we assessed the binding affinities of the molecules under study to these crucial viral components. To further comprehend the stability and molecular interactions of the “protein-ligand” complexes that derived from docking studies, we performed molecular dynamics (MD) simulations, shedding light on the molecular basis of potential drug candidates for COVID-19 treatment. Moreover, we employed Molecular Mechanics Generalized Born Surface Area (MM-GBSA) calculations on all “protein-ligand” complexes, underscoring the robust binding capabilities of rosmarinic acid, curcumin, and quercetin against Mpro, and salvianolic acid b, rosmarinic acid, and quercetin toward the S protein. Furthermore, in order to expand our search for potent inhibitors, we conducted a structure similarity analysis, using the Enalos Suite, based on the molecules that indicated the most favored results in the in silico studies. The Enalos Suite generated 115 structurally similar compounds to salvianolic acid, rosmarinic acid, and quercetin. These compounds underwent IFD calculations, leading to the identification of two salvianolic acid analogues that exhibited strong binding to all the examined binding sites in both proteins, showcasing their potential as multi-target inhibitors. These findings introduce exciting possibilities for the development of novel therapeutic agents aiming to effectively disrupt the SARS-CoV-2 virus lifecycle.</jats:p>",
"alternative-id": [
"ijms242115894"
],
"author": [
{
"affiliation": [
{
"name": "Department of Chemistry, National and Kapodistrian University of Athens, 15771 Athens, Greece"
}
],
"family": "Moschovou",
"given": "Kalliopi",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of ChemoInformatics, NovaMechanics Ltd., 1046 Nicosia, Cyprus"
},
{
"name": "Department of Chemoinformatics, NovaMechanics MIKE, 18536 Piraeus, Greece"
}
],
"family": "Antoniou",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Chemistry, National and Kapodistrian University of Athens, 15771 Athens, Greece"
}
],
"family": "Chontzopoulou",
"given": "Eleni",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-2322-7422",
"affiliation": [
{
"name": "Department of ChemoInformatics, NovaMechanics Ltd., 1046 Nicosia, Cyprus"
},
{
"name": "Department of Chemoinformatics, NovaMechanics MIKE, 18536 Piraeus, Greece"
}
],
"authenticated-orcid": false,
"family": "Papavasileiou",
"given": "Konstantinos D.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Division of Physical Sciences & Applications, Hellenic Military Academy, 16672 Vari, Greece"
}
],
"family": "Melagraki",
"given": "Georgia",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0977-8180",
"affiliation": [
{
"name": "Department of ChemoInformatics, NovaMechanics Ltd., 1046 Nicosia, Cyprus"
},
{
"name": "Department of Chemoinformatics, NovaMechanics MIKE, 18536 Piraeus, Greece"
}
],
"authenticated-orcid": false,
"family": "Afantitis",
"given": "Antreas",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5309-992X",
"affiliation": [
{
"name": "Department of Chemistry, National and Kapodistrian University of Athens, 15771 Athens, Greece"
}
],
"authenticated-orcid": false,
"family": "Mavromoustakos",
"given": "Thomas",
"sequence": "additional"
}
],
"container-title": "International Journal of Molecular Sciences",
"container-title-short": "IJMS",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
11,
2
]
],
"date-time": "2023-11-02T13:16:21Z",
"timestamp": 1698930981000
},
"deposited": {
"date-parts": [
[
2023,
11,
2
]
],
"date-time": "2023-11-02T13:20:26Z",
"timestamp": 1698931226000
},
"funder": [
{
"name": "Greece"
}
],
"indexed": {
"date-parts": [
[
2023,
11,
3
]
],
"date-time": "2023-11-03T01:03:47Z",
"timestamp": 1698973427065
},
"is-referenced-by-count": 0,
"issue": "21",
"issued": {
"date-parts": [
[
2023,
11,
2
]
]
},
"journal-issue": {
"issue": "21",
"published-online": {
"date-parts": [
[
2023,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
11,
2
]
],
"date-time": "2023-11-02T00:00:00Z",
"timestamp": 1698883200000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1422-0067/24/21/15894/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "15894",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
11,
2
]
]
},
"published-online": {
"date-parts": [
[
2023,
11,
2
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A Novel Coronavirus from Patients with Pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"journal-title": "N. Engl. J. Med.",
"key": "ref_1",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25678",
"article-title": "Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle",
"author": "Lu",
"doi-asserted-by": "crossref",
"first-page": "401",
"journal-title": "J. Med. Virol.",
"key": "ref_2",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1128/JVI.03737-14",
"article-title": "Identification of the Receptor-Binding Domain of the Spike Glycoprotein of Human Betacoronavirus HKU1",
"author": "Qian",
"doi-asserted-by": "crossref",
"first-page": "8816",
"journal-title": "J. Virol.",
"key": "ref_3",
"volume": "89",
"year": "2015"
},
{
"DOI": "10.1038/s41467-020-15562-9",
"article-title": "Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV",
"author": "Ou",
"doi-asserted-by": "crossref",
"first-page": "1620",
"journal-title": "Nat. Commun.",
"key": "ref_4",
"volume": "11",
"year": "2020"
},
{
"key": "ref_5",
"unstructured": "Dhar, Y.V., Prasad, P., Tiwari, N., Pankaj, V., Bano, N., Bag, S.K., and Asif, M.H. (2020). An In-Silico Study to Identify Hidden Features of Spike Protein and Main Protease of SARS-CoV-2. Preprints, 2020060191."
},
{
"DOI": "10.1126/science.abb3405",
"article-title": "Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "409",
"journal-title": "Science",
"key": "ref_6",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.3389/fimmu.2022.940756",
"article-title": "Roles and functions of SARS-CoV-2 proteins in host immune evasion",
"author": "Rashid",
"doi-asserted-by": "crossref",
"first-page": "940756",
"journal-title": "Front. Immunol.",
"key": "ref_7",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.20944/preprints202003.0333.v1",
"doi-asserted-by": "crossref",
"key": "ref_8",
"unstructured": "Adem, S., Eyupoglu, V., Sarfraz, I., Rasul, A., and Ali, M. (2020). Identification of Potent COVID-19 Main Protease (Mpro) Inhibitors from Natural Polyphenols: An in Silico Strategy Unveils a Hope against CORONA. Preprints."
},
{
"DOI": "10.1002/ptr.6998",
"article-title": "In silico and in vitro evaluation of kaempferol as a potential inhibitor of the SARS-CoV-2 main protease (3CLpro)",
"author": "Khan",
"doi-asserted-by": "crossref",
"first-page": "2841",
"journal-title": "Phyther. Res.",
"key": "ref_9",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "ref_10",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1038/s41401-020-0485-4",
"article-title": "Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19",
"author": "Huang",
"doi-asserted-by": "crossref",
"first-page": "1141",
"journal-title": "Acta Pharmacol. Sin.",
"key": "ref_11",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2223-y",
"article-title": "Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors",
"author": "Jin",
"doi-asserted-by": "crossref",
"first-page": "289",
"journal-title": "Nature",
"key": "ref_12",
"volume": "582",
"year": "2020"
},
{
"DOI": "10.1126/science.abb4489",
"article-title": "Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease",
"author": "Dai",
"doi-asserted-by": "crossref",
"first-page": "1331",
"journal-title": "Science",
"key": "ref_13",
"volume": "368",
"year": "2020"
},
{
"DOI": "10.1007/s11224-020-01605-w",
"article-title": "Repurposing metocurine as main protease inhibitor to develop novel antiviral therapy for COVID-19",
"author": "Jain",
"doi-asserted-by": "crossref",
"first-page": "2487",
"journal-title": "Struct. Chem.",
"key": "ref_14",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1007/s11030-022-10513-6",
"article-title": "Computational studies indicated the effectiveness of human metabolites against SARS-CoV-2 main protease",
"author": "Roy",
"doi-asserted-by": "crossref",
"first-page": "1587",
"journal-title": "Mol. Divers.",
"key": "ref_15",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1038/s41598-020-71188-3",
"article-title": "Dynamics of the ACE2–SARS-CoV-2/SARS-CoV spike protein interface reveal unique mechanisms",
"author": "Ali",
"doi-asserted-by": "crossref",
"first-page": "14214",
"journal-title": "Sci. Rep.",
"key": "ref_16",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1038/s41598-023-34305-6",
"article-title": "Structural analysis of the coronavirus main protease for the design of pan-variant inhibitors",
"author": "Rungruangmaitree",
"doi-asserted-by": "crossref",
"first-page": "7055",
"journal-title": "Sci. Rep.",
"key": "ref_17",
"volume": "13",
"year": "2023"
},
{
"DOI": "10.1021/acsinfecdis.1c00070",
"article-title": "Small-Molecule Inhibitors of the Coronavirus Spike: ACE2 Protein-Protein Interaction as Blockers of Viral Attachment and Entry for SARS-CoV-2",
"author": "Bojadzic",
"doi-asserted-by": "crossref",
"first-page": "1519",
"journal-title": "ACS Infect. Dis.",
"key": "ref_18",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1186/s12967-022-03501-9",
"article-title": "Potential inhibitors for blocking the interaction of the coronavirus SARS-CoV-2 spike protein and its host cell receptor ACE2",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "314",
"journal-title": "J. Transl. Med.",
"key": "ref_19",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1021/acsnano.0c02857",
"article-title": "Computational Design of ACE2-Based Peptide Inhibitors of SARS-CoV-2",
"author": "Han",
"doi-asserted-by": "crossref",
"first-page": "5143",
"journal-title": "ACS Nano",
"key": "ref_20",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.ymthe.2021.10.014",
"article-title": "The neutralization effect of montelukaston SARS-CoV-2 is shown by multiscale in silico simulations and combined in vitro studies",
"author": "Durdagi",
"doi-asserted-by": "crossref",
"first-page": "963",
"journal-title": "Mol. Ther.",
"key": "ref_21",
"volume": "30",
"year": "2021"
},
{
"DOI": "10.3390/ph15030308",
"doi-asserted-by": "crossref",
"key": "ref_22",
"unstructured": "Shahhamzehei, N., Abdelfatah, S., and Efferth, T. (2022). In Silico and In Vitro Identification of Pan-Coronaviral Main Protease Inhibitors from a Large Natural Product Library. Pharmaceuticals, 15."
},
{
"DOI": "10.1016/j.foodchem.2021.131594",
"article-title": "Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols",
"author": "Bahun",
"doi-asserted-by": "crossref",
"first-page": "131594",
"journal-title": "Food Chemistry",
"key": "ref_23",
"volume": "373",
"year": "2022"
},
{
"DOI": "10.1016/j.molstruc.2021.130380",
"article-title": "Rational design of flavonoid based potential inhibitors targeting SARS-CoV 3CL protease for the treatment of COVID-19",
"author": "Bhati",
"doi-asserted-by": "crossref",
"first-page": "130380",
"journal-title": "J. Mol. Struct.",
"key": "ref_24",
"volume": "1237",
"year": "2021"
},
{
"DOI": "10.1016/j.semcancer.2017.05.005",
"article-title": "Antioxidant polyphenols in cancer treatment: Friend, foe or foil?",
"author": "Russo",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Semin. Cancer Biol.",
"key": "ref_25",
"volume": "46",
"year": "2017"
},
{
"DOI": "10.1016/j.ejmech.2017.09.001",
"article-title": "Anti-inflammatory effects of flavonoids in neurodegenerative disorders",
"author": "Spagnuolo",
"doi-asserted-by": "crossref",
"first-page": "105",
"journal-title": "Eur. J. Med. Chem.",
"key": "ref_26",
"volume": "153",
"year": "2018"
},
{
"DOI": "10.31219/osf.io/k4h5f",
"doi-asserted-by": "crossref",
"key": "ref_27",
"unstructured": "Cherrak, S.A., Merzouk, H., and Mokhtari-Soulimane, N. (2020). Potential bioactive glycosylated flavonoids as SARS-CoV-2 main protease inhibitors: A molecular docking and simulation studies. PLoS ONE, 15."
},
{
"DOI": "10.1002/ptr.7151",
"article-title": "Natural products and phytochemicals as potential anti-SARS-CoV-2 drugs",
"author": "Merarchi",
"doi-asserted-by": "crossref",
"first-page": "5384",
"journal-title": "Phytother. Res.",
"key": "ref_28",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1039/D1RA00644D",
"article-title": "A review on potential of natural products in the management of COVID-19",
"author": "Chakravarti",
"doi-asserted-by": "crossref",
"first-page": "16711",
"journal-title": "RSC Adv.",
"key": "ref_29",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.3390/molecules26133900",
"doi-asserted-by": "crossref",
"key": "ref_30",
"unstructured": "Amin, M., Tabari, K., Iranpanah, A., Bahramsoltani, R., and Rahimi, R. (2021). Flavonoids as Promising Antiviral Agents against SARS-CoV-2 Infection: A Mechanistic Review. Molecules, 26."
},
{
"DOI": "10.1007/s11101-021-09759-z",
"article-title": "Flavonoids are promising safe therapy against COVID-19",
"author": "Alzaabi",
"doi-asserted-by": "crossref",
"first-page": "291",
"journal-title": "Phytochem. Rev.",
"key": "ref_31",
"volume": "21",
"year": "2022"
},
{
"DOI": "10.1016/j.nut.2020.111049",
"article-title": "In silico screening of potential anti–COVID-19 bioactive natural constituents from food sources by molecular docking",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "111049",
"journal-title": "Nutrition",
"key": "ref_32",
"volume": "82",
"year": "2021"
},
{
"DOI": "10.1007/s43450-023-00381-y",
"article-title": "(R)-(+)-Rosmarinic Acid as an Inhibitor of Herpes and Dengue Virus Replication: An In Silico Assessment",
"author": "Samy",
"doi-asserted-by": "crossref",
"first-page": "543",
"journal-title": "Rev. Bras. Farmacogn.",
"key": "ref_33",
"volume": "33",
"year": "2023"
},
{
"DOI": "10.1039/D1RA05268C",
"article-title": "Anti-SARS-CoV-2 activities of tanshinone IIA, carnosic acid, rosmarinic acid, salvianolic acid, baicalein, and glycyrrhetinic acid between computational andin vitroinsights",
"author": "Elebeedy",
"doi-asserted-by": "crossref",
"first-page": "29267",
"journal-title": "RSC Adv.",
"key": "ref_34",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1016/j.phymed.2020.153310",
"article-title": "Caffeic acid derivatives (CAFDs) as inhibitors of SARS-CoV-2: CAFDs-based functional foods as a potential alternative approach to combat COVID-19",
"author": "Adem",
"doi-asserted-by": "crossref",
"first-page": "153310",
"journal-title": "Phytomedicine",
"key": "ref_35",
"volume": "85",
"year": "2021"
},
{
"article-title": "Salvianolic acid C potently inhibits SARS-CoV-2 infection by blocking the formation of six-helix bundle core of spike protein",
"author": "Yang",
"first-page": "2",
"journal-title": "Signal Transduct. Target. Ther.",
"key": "ref_36",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26874",
"article-title": "Three salvianolic acids inhibit 2019-nCoV spike pseudovirus viropexis by binding to both its RBD and receptor ACE2",
"author": "Hu",
"doi-asserted-by": "crossref",
"first-page": "3143",
"journal-title": "J. Med. Virol.",
"key": "ref_37",
"volume": "93",
"year": "2021"
},
{
"article-title": "Will Cannabis or Cannabinoids Protect You from SARS-CoV-2 Infection or Treat COVID-19?",
"author": "Schwantes",
"first-page": "101554",
"journal-title": "J. Chem. Theory Comput.",
"key": "ref_38",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.1021/acs.jnatprod.1c00946",
"article-title": "Cannabinoids Block Cellular Entry of SARS-CoV-2 and the Emerging Variants",
"author": "Muchiri",
"doi-asserted-by": "crossref",
"first-page": "176",
"journal-title": "J. Nat. Prod.",
"key": "ref_39",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0247548",
"doi-asserted-by": "crossref",
"key": "ref_40",
"unstructured": "Armstrong, K.A., Soltoff, A., Rieu-Werden, M., Metlay, J., and Haas, J. (2021). Use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers associated with lower risk of COVID-19 in household contacts. PLoS ONE, 16."
},
{
"DOI": "10.1111/bph.15082",
"article-title": "A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance",
"author": "Sriram",
"doi-asserted-by": "crossref",
"first-page": "4825",
"journal-title": "Br. J. Pharmacol.",
"key": "ref_41",
"volume": "177",
"year": "2020"
},
{
"DOI": "10.1128/AAC.00819-20",
"article-title": "Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs",
"author": "Jeon",
"doi-asserted-by": "crossref",
"first-page": "e00819-20",
"journal-title": "Antimicrob. Agents Chemother.",
"key": "ref_42",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.1152/physrev.00038.2017",
"article-title": "Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology",
"author": "Forrester",
"doi-asserted-by": "crossref",
"first-page": "1627",
"journal-title": "Physiol. Rev.",
"key": "ref_43",
"volume": "98",
"year": "2018"
},
{
"DOI": "10.1517/13543776.2013.830104",
"article-title": "AT1 antagonists: A patent review (2008–2012)",
"author": "Mavromoustakos",
"doi-asserted-by": "crossref",
"first-page": "1483",
"journal-title": "Expert Opin. Ther. Pat.",
"key": "ref_44",
"volume": "23",
"year": "2013"
},
{
"article-title": "ACEI/ARB therapy in COVID-19: The double-edged sword of ACE2 and SARS-CoV-2 viral docking",
"author": "Onweni",
"first-page": "2020",
"journal-title": "Crit. Care",
"key": "ref_45",
"volume": "475",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2021.100962",
"article-title": "Telmisartan for treatment of COVID-19 patients: An open multicenter randomized clinical trial",
"author": "Duarte",
"doi-asserted-by": "crossref",
"first-page": "100962",
"journal-title": "EClinicalMedicine",
"key": "ref_46",
"volume": "37",
"year": "2021"
},
{
"key": "ref_47",
"unstructured": "Schrödinger Suite (2012). Protein Preparation Wizard, Schrödinger, LLC.. Epik Version 2.3; Impact Version 5.8; Prime Version 3.1."
},
{
"DOI": "10.1038/s41586-020-2180-5",
"article-title": "Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor",
"author": "Lan",
"doi-asserted-by": "crossref",
"first-page": "215",
"journal-title": "Nature",
"key": "ref_48",
"volume": "581",
"year": "2020"
},
{
"key": "ref_49",
"unstructured": "(Prime, 2015). Prime, version 4.0."
},
{
"DOI": "10.1021/jp003919d",
"article-title": "Evaluation and Reparametrization of the OPLS-AA Force Field for Proteins via Comparison with Accurate Quantum Chemical Calculations on Peptides",
"author": "Kaminski",
"doi-asserted-by": "crossref",
"first-page": "6474",
"journal-title": "J. Phys. Chem.",
"key": "ref_50",
"volume": "105",
"year": "2001"
},
{
"key": "ref_51",
"unstructured": "(LigPrep, 2017). LigPrep, version 3.4."
},
{
"key": "ref_52",
"unstructured": "(Glide, 2012). Glide, version 5.8."
},
{
"key": "ref_53",
"unstructured": "(Induced Fit Docking, Schrödinger Software Release 2017-1, 2017). Induced Fit Docking, Schrödinger Software Release 2017-1."
},
{
"DOI": "10.1016/j.ejphar.2020.173701",
"article-title": "In silico identification of strong binders of the SARS-CoV-2 receptor-binding domain",
"author": "Behloul",
"doi-asserted-by": "crossref",
"first-page": "173701",
"journal-title": "Eur. J. Pharmacol.",
"key": "ref_54",
"volume": "890",
"year": "2021"
},
{
"DOI": "10.1021/ja9621760",
"article-title": "Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids",
"author": "Jorgensen",
"doi-asserted-by": "crossref",
"first-page": "11225",
"journal-title": "J. Am. Chem. Soc.",
"key": "ref_55",
"volume": "118",
"year": "1996"
},
{
"DOI": "10.1063/1.445869",
"article-title": "Comparison of simple potential functions for simulating liquid water",
"author": "Jorgensen",
"doi-asserted-by": "crossref",
"first-page": "926",
"journal-title": "J. Chem. Phys.",
"key": "ref_56",
"volume": "79",
"year": "1983"
},
{
"key": "ref_57",
"unstructured": "(Schrödinger Release 2017-1: Desmond Molecular Dynamics System, 2017). Schrödinger Release 2017-1: Desmond Molecular Dynamics System, Maestro-Desmond Interoperability Tools."
},
{
"DOI": "10.1002/jcc.10249",
"article-title": "Rigid-body dynamics in the isothermal-isobaric ensemble: A test on the accuracy and computational efficiency",
"author": "Shinoda",
"doi-asserted-by": "crossref",
"first-page": "920",
"journal-title": "J. Comput. Chem.",
"key": "ref_58",
"volume": "24",
"year": "2003"
},
{
"DOI": "10.1021/jm060522a",
"article-title": "Accurate prediction of the relative potencies of members of a series of kinase inhibitors using molecular docking and MM-GBSA scoring",
"author": "Lyne",
"doi-asserted-by": "crossref",
"first-page": "4805",
"journal-title": "J. Med. Chem.",
"key": "ref_59",
"volume": "49",
"year": "2006"
},
{
"DOI": "10.1002/prot.23106",
"article-title": "The VSGB 2.0 model: A next generation energy model for high resolution protein structure modeling",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "2794",
"journal-title": "Proteins Struct. Funct. Bioinform.",
"key": "ref_60",
"volume": "79",
"year": "2011"
},
{
"DOI": "10.1021/ja00172a038",
"article-title": "Semianalytical Treatment of Solvation for Molecular Mechanics and Dynamics",
"author": "Tempczyk",
"doi-asserted-by": "crossref",
"first-page": "6127",
"journal-title": "J. Am. Chem. Soc.",
"key": "ref_61",
"volume": "112",
"year": "1990"
},
{
"DOI": "10.1021/jp961992r",
"article-title": "The GB/SA continuum model for solvation. A fast analytical method for the calculation of approximate Born radii",
"author": "Qiu",
"doi-asserted-by": "crossref",
"first-page": "3005",
"journal-title": "J. Phys. Chem. A",
"key": "ref_62",
"volume": "101",
"year": "1997"
},
{
"key": "ref_63",
"unstructured": "Varsou, D.-D., Nikolakopoulos, S., Tsoumanis, A., Melagraki, G., and Afantitis, A. (2018). Methods in Molecular Biology, Springer."
},
{
"DOI": "10.2174/0929867327666200727114410",
"article-title": "Enalos suite of tools: Enhance cheminformatics and nanoinformat-ics through knime",
"author": "Afantitis",
"doi-asserted-by": "crossref",
"first-page": "6523",
"journal-title": "Curr. Med. Chem.",
"key": "ref_64",
"volume": "27",
"year": "2020"
}
],
"reference-count": 64,
"references-count": 64,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1422-0067/24/21/15894"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Inorganic Chemistry",
"Organic Chemistry",
"Physical and Theoretical Chemistry",
"Computer Science Applications",
"Spectroscopy",
"Molecular Biology",
"General Medicine",
"Catalysis"
],
"subtitle": [],
"title": "Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein",
"type": "journal-article",
"volume": "24"
}
moschovou
