Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms
et al., Molecules, doi:10.3390/molecules26226900, Nov 2021
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study showing antiviral and anti-inflammatory effects of curcumin during SARS-CoV-2 infection. Inhibition was seen with Vero E6 cells pre-infection and post-infection, and with D614G and the delta variant. The anti-inflammatory effect was shown with peripheral blood mononuclear cells.
62 preclinical studies support the efficacy of curcumin for COVID-19:
In silico studies predict inhibition of SARS-CoV-2 with curcumin or metabolites via binding to the spikeA,1,5,6,11,16,18,24,27 (and specifically the receptor binding domainB,2,4,14,17,20 ), MproC,4-6,11,13,15-17,19,20,22,25,27,28,30,48 , RNA-dependent RNA polymeraseD,4-6,17,26 , PLproE,6, ACE2F,2,18,19,21 , nucleocapsidG,12,29 , nsp10H,29, and helicaseI,36 proteins, and inhibition of spike-ACE2 interactionJ,3.
In vitro studies demonstrate inhibition of the spikeA,41 (and specifically the receptor binding domainB,51), MproC,23,41,48,50 , ACE2F,51, and TMPRSS2K,51 proteins, and inhibition of spike-ACE2 interactionJ,3,34 .
In vitro studies demonstrate efficacy in Calu-3L,49, A549M,41, A549-ATN,31, 293TO,7, HEK293-hACE2P,23,39 , 293T/hACE2/TMPRSS2Q,40, Vero E6R,1,13,17,27,39,41,43,45,47,49 , and SH-SY5YS,38 cells.
Curcumin decreases pro-inflammatory cytokines induced by SARS-CoV-2 in peripheral blood mononuclear cells47, alleviates SARS-CoV-2 spike protein-induced mitochondrial membrane damage and oxidative stress7, may limit COVID-19 induced cardiac damage by inhibiting the NF-κB signaling pathway which mediates the profibrotic effects of the SARS-CoV-2 spike protein on cardiac fibroblasts35, is predicted to inhibit the interaction between the SARS-CoV-2 spike protein receptor binding domain and the human ACE2 receptor for the delta and omicron variants14, lowers ACE2 and STAT3, curbing lung inflammation and ARDS in preclinical COVID-19 models32, inhibits SARS-CoV-2 ORF3a ion channel activity, which contributes to viral pathogenicity and cytotoxicity42, has direct virucidal action by disrupting viral envelope integrity44, may inhibit viral replication and modulate inflammatory pathways like NF-κB via SIRT1 activation52, and can function as a photosensitizer in photodynamic therapy to generate reactive oxygen species that damage the virus44.
1.
Marzouk et al., Computational and Experimental Insights into the Antiviral Mechanism of Turmeric (Curcuma longa) against SARS-CoV-2 D614G, BIO Web of Conferences, doi:10.1051/bioconf/202519804002.
2.
Wu et al., Utilizing natural compounds as ligands to disrupt the binding of SARS-CoV-2 receptor-binding domain to angiotensin-converting enzyme 2, impeding viral infection, Phytochemistry Letters, doi:10.1016/j.phytol.2025.102999.
3.
Najimi et al., Phytochemical Inhibitors of SARS‐CoV‐2 Entry: Targeting the ACE2‐RBD Interaction with l‐Tartaric Acid, l‐Ascorbic Acid, and Curcuma longa Extract, ChemistrySelect, doi:10.1002/slct.202406035.
4.
Rajamanickam et al., Exploring the Potential of Siddha Formulation MilagaiKudineer-Derived Phytotherapeutics Against SARS-CoV-2: An In-Silico Investigation for Antiviral Intervention, Journal of Pharmacy and Pharmacology Research, doi:10.26502/fjppr.0105.
5.
Al balawi et al., Assessing multi-target antiviral and antioxidant activities of natural compounds against SARS-CoV-2: an integrated in vitro and in silico study, Bioresources and Bioprocessing, doi:10.1186/s40643-024-00822-z.
6.
Haque et al., Exploring potential therapeutic candidates against COVID-19: a molecular docking study, Discover Molecules, doi:10.1007/s44345-024-00005-5.
7.
Zhang et al., Computational Discovery of Mitochondrial Dysfunction Biomarkers in Severe SARS-CoV-2 Infection: Facilitating Pytomedicine Screening, Phytomedicine, doi:10.1016/j.phymed.2024.155784.
8.
Öztürkkan et al., In Silico investigation of the effects of curcuminoids on the spike protein of the omicron variant of SARS-CoV-2, Baku State University Journal of Chemistry and Material Sciences, 1:2, bsuj.bsu.edu.az/uploads/pdf/ec4204d62f7802de54e6092bf7860029.pdf.
9.
Yunze et al., Therapeutic effect and potential mechanism of curcumin, an active ingredient in Tongnao Decoction, on COVID-19 combined with stroke: a network pharmacology study and GEO database mining, Research Square, doi:10.21203/rs.3.rs-4329762/v1.
10.
Agamah et al., Network-based multi-omics-disease-drug associations reveal drug repurposing candidates for COVID-19 disease phases, ScienceOpen, doi:10.58647/DRUGARXIV.PR000010.v1.
11.
Boseila et al., Throat spray formulated with virucidal Pharmaceutical excipients as an effective early prophylactic or treatment strategy against pharyngitis post-exposure to SARS CoV-2, European Journal of Pharmaceutics and Biopharmaceutics, doi:10.1016/j.ejpb.2024.114279.
12.
Hidayah et al., Bioinformatics study of curcumin, demethoxycurcumin, bisdemethoxycurcumin and cyclocurcumin compounds in Curcuma longa as an antiviral agent via nucleocapsid on SARS-CoV-2 inhibition, International Conference on Organic and Applied Chemistry, doi:10.1063/5.0197724.
13.
Singh et al., Unlocking the potential of phytochemicals in inhibiting SARS-CoV-2 M Pro protein - An in-silico and cell-based approach, Research Square, doi:10.21203/rs.3.rs-3888947/v1.
14.
Kant et al., Structure-based drug discovery to identify SARS-CoV2 spike protein–ACE2 interaction inhibitors, Journal of Biomolecular Structure and Dynamics, doi:10.1080/07391102.2023.2300060.
15.
Naderi Beni et al., In silico studies of anti-oxidative and hot temperament-based phytochemicals as natural inhibitors of SARS-CoV-2 Mpro, PLOS ONE, doi:10.1371/journal.pone.0295014.
16.
Moschovou et al., Exploring the Binding Effects of Natural Products and Antihypertensive Drugs on SARS-CoV-2: An In Silico Investigation of Main Protease and Spike Protein, International Journal of Molecular Sciences, doi:10.3390/ijms242115894.
17.
Eleraky et al., Curcumin Transferosome-Loaded Thermosensitive Intranasal in situ Gel as Prospective Antiviral Therapy for SARS-Cov-2, International Journal of Nanomedicine, doi:10.2147/IJN.S423251.
18.
Singh (B) et al., Computational studies to analyze effect of curcumin inhibition on coronavirus D614G mutated spike protein, The Seybold Report, doi:10.17605/OSF.IO/TKEXJ.
19.
Thapa et al., In-silico Approach for Predicting the Inhibitory Effect of Home Remedies on Severe Acute Respiratory Syndrome Coronavirus-2, Makara Journal of Science, doi:10.7454/mss.v27i3.1609.
20.
Srivastava et al., Paradigm of Well-Orchestrated Pharmacokinetic Properties of Curcuminoids Relative to Conventional Drugs for the Inactivation of SARS-CoV-2 Receptors: An In Silico Approach, Stresses, doi:10.3390/stresses3030043.
21.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
22.
Winih Kinasih et al., Analisis in silico interaksi senyawa kurkuminoid terhadap enzim main protease 6LU7 dari SARS-CoV-2, Duta Pharma Journal, doi:10.47701/djp.v3i1.2904.
23.
Wu (B) et al., Potential Mechanism of Curcumin and Resveratrol against SARS-CoV-2, Research Square, doi:10.21203/rs.3.rs-2780614/v1.
24.
Nag et al., Curcumin inhibits spike protein of new SARS-CoV-2 variant of concern (VOC) Omicron, an in silico study, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2022.105552.
25.
Rampogu et al., Molecular Docking and Molecular Dynamics Simulations Discover Curcumin Analogue as a Plausible Dual Inhibitor for SARS-CoV-2, International Journal of Molecular Sciences, doi:10.3390/ijms23031771.
26.
Singh (C) et al., Potential of turmeric-derived compounds against RNA-dependent RNA polymerase of SARS-CoV-2: An in-silico approach, Computers in Biology and Medicine, doi:10.1016/j.compbiomed.2021.104965.
27.
Kandeil et al., Bioactive Polyphenolic Compounds Showing Strong Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus 2, Pathogens, doi:10.3390/pathogens10060758.
28.
Rajagopal et al., Activity of phytochemical constituents of Curcuma longa (turmeric) and Andrographis paniculata against coronavirus (COVID-19): an in silico approach, Future Journal of Pharmaceutical Sciences, doi:10.1186/s43094-020-00126-x.
29.
Suravajhala et al., Comparative Docking Studies on Curcumin with COVID-19 Proteins, Preprints, doi:10.20944/preprints202005.0439.v3.
30.
Sekiou et al., In-Silico Identification of Potent Inhibitors of COVID-19 Main Protease (Mpro) and Angiotensin Converting Enzyme 2 (ACE2) from Natural Products: Quercetin, Hispidulin, and Cirsimaritin Exhibited Better Potential Inhibition than Hydroxy-Chloroquine Against COVID-19 Main Protease Active Site and ACE2, ChemRxiv, doi:10.26434/chemrxiv.12181404.v1.
31.
Grüneberg et al., Dose-dependent antiviral effects of glycyrrhizin, curcumin, and harmaline against clinical SARS-CoV-2 isolates, including D614G, Omicron BA.5, and Omicron XBB.1, BMC Complementary Medicine and Therapies, doi:10.1186/s12906-026-05253-1.
32.
Aktay et al., Oral Administration of Water-Soluble Curcumin Complex Prevents ARDS With the Potential for COVID-19 Treatment, Phytotherapy Research, doi:10.1002/ptr.70046.
33.
Olubiyi et al., Novel dietary herbal preparations with inhibitory activities against multiple SARS-CoV-2 targets: A multidisciplinary investigation into antiviral activities, Food Chemistry Advances, doi:10.1016/j.focha.2025.100969.
34.
Emam et al., Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors, AMB Express, doi:10.1186/s13568-024-01739-8.
35.
Van Tin et al., Spike Protein of SARS-CoV-2 Activates Cardiac Fibrogenesis through NLRP3 Inflammasomes and NF-κB Signaling, Cells, doi:10.3390/cells13161331.
36.
Li et al., Thermal shift assay (TSA)-based drug screening strategy for rapid discovery of inhibitors against the Nsp13 helicase of SARS-CoV-2, Animals and Zoonoses, doi:10.1016/j.azn.2024.06.001.
37.
Kamble et al., Nanoparticulate curcumin spray imparts prophylactic and therapeutic properties against SARS-CoV-2, Emergent Materials, doi:10.1007/s42247-024-00754-6.
38.
Nicoliche et al., Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2, Scientific Reports, doi:10.1038/s41598-024-61662-7.
39.
Nittayananta et al., A novel film spray containing curcumin inhibits SARS-CoV-2 and influenza virus infection and enhances mucosal immunity, Virology Journal, doi:10.1186/s12985-023-02282-x.
40.
Septisetyani et al., Curcumin and turmeric extract inhibited SARS-CoV-2 pseudovirus cell entry and Spike mediated cell fusion, bioRxiv, doi:10.1101/2023.09.28.560070.
41.
Mohd Abd Razak et al., In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds, Natural Product Communications, doi:10.1177/1934578X231188861.
42.
Fam et al., Channel activity of SARS-CoV-2 viroporin ORF3a inhibited by adamantanes and phenolic plant metabolites, Scientific Reports, doi:10.1038/s41598-023-31764-9.
43.
Teshima et al., Antiviral activity of curcumin and its analogs selected by an artificial intelligence-supported activity prediction system in SARS-CoV-2-infected VeroE6 cells, Natural Product Research, doi:10.1080/14786419.2023.2194647.
44.
Zupin et al., Optimization of Anti-SARS-CoV-2 Treatments Based on Curcumin, Used Alone or Employed as a Photosensitizer, Viruses, doi:10.3390/v14102132.
45.
Leka et al., In vitro antiviral activity against SARS-CoV-2 of common herbal medicinal extracts and their bioactive compounds, Phytotherapy Research, doi:10.1002/ptr.7463.
46.
Goc et al., Inhibitory effects of specific combination of natural compounds against SARS-CoV-2 and its Alpha, Beta, Gamma, Delta, Kappa, and Mu variants, European Journal of Microbiology and Immunology, doi:10.1556/1886.2021.00022.
47.
Marín-Palma et al., Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms, Molecules, doi:10.3390/molecules26226900.
48.
Bahun et al., Inhibition of the SARS-CoV-2 3CLpro main protease by plant polyphenols, Food Chemistry, doi:10.1016/j.foodchem.2021.131594.
49.
Bormann et al., Turmeric Root and Its Bioactive Ingredient Curcumin Effectively Neutralize SARS-CoV-2 In Vitro, Viruses, doi:10.3390/v13101914.
50.
Guijarro-Real et al., Potential In Vitro Inhibition of Selected Plant Extracts against SARS-CoV-2 Chymotripsin-Like Protease (3CLPro) Activity, Foods, doi:10.3390/foods10071503.
a.
The trimeric spike (S) protein is a glycoprotein that mediates viral entry by binding to the host ACE2 receptor, is critical for SARS-CoV-2's ability to infect host cells, and is a target of neutralizing antibodies. Inhibition of the spike protein prevents viral attachment, halting infection at the earliest stage.
b.
The receptor binding domain is a specific region of the spike protein that binds ACE2 and is a major target of neutralizing antibodies. Focusing on the precise binding site allows highly specific disruption of viral attachment with reduced potential for off-target effects.
c.
The main protease or Mpro, also known as 3CLpro or nsp5, is a cysteine protease that cleaves viral polyproteins into functional units needed for replication. Inhibiting Mpro disrupts the SARS-CoV-2 lifecycle within the host cell, preventing the creation of new copies.
d.
RNA-dependent RNA polymerase (RdRp), also called nsp12, is the core enzyme of the viral replicase-transcriptase complex that copies the positive-sense viral RNA genome into negative-sense templates for progeny RNA synthesis. Inhibiting RdRp blocks viral genome replication and transcription.
e.
The papain-like protease (PLpro) has multiple functions including cleaving viral polyproteins and suppressing the host immune response by deubiquitination and deISGylation of host proteins. Inhibiting PLpro may block viral replication and help restore normal immune responses.
f.
The angiotensin converting enzyme 2 (ACE2) protein is a host cell transmembrane protein that serves as the cellular receptor for the SARS-CoV-2 spike protein. ACE2 is expressed on many cell types, including epithelial cells in the lungs, and allows the virus to enter and infect host cells. Inhibition may affect ACE2's physiological function in blood pressure control.
g.
The nucleocapsid (N) protein binds and encapsulates the viral genome by coating the viral RNA. N enables formation and release of infectious virions and plays additional roles in viral replication and pathogenesis. N is also an immunodominant antigen used in diagnostic assays.
h.
Non-structural protein 10 (nsp10) serves as an RNA chaperone and stabilizes conformations of nsp12 and nsp14 in the replicase-transcriptase complex, which synthesizes new viral RNAs. Nsp10 disruption may destabilize replicase-transcriptase complex activity.
i.
The helicase, or nsp13, protein unwinds the double-stranded viral RNA, a crucial step in replication and transcription. Inhibition may prevent viral genome replication and the creation of new virus components.
j.
The interaction between the SARS-CoV-2 spike protein and the human ACE2 receptor is a primary method of viral entry, inhibiting this interaction can prevent the virus from attaching to and entering host cells, halting infection at an early stage.
k.
Transmembrane protease serine 2 (TMPRSS2) is a host cell protease that primes the spike protein, facilitating cellular entry. TMPRSS2 activity helps enable cleavage of the spike protein required for membrane fusion and virus entry. Inhibition may especially protect respiratory epithelial cells, buy may have physiological effects.
l.
Calu-3 is a human lung adenocarcinoma cell line with moderate ACE2 and TMPRSS2 expression and SARS-CoV-2 susceptibility. It provides a model of the human respiratory epithelium, but many not be ideal for modeling early stages of infection due to the moderate expression levels of ACE2 and TMPRSS2.
m.
A549 is a human lung carcinoma cell line with low ACE2 expression and SARS-CoV-2 susceptibility. Viral entry/replication can be studied but the cells may not replicate all aspects of lung infection.
n.
A549-AT is a human lung carcinoma cell line stably transfected with ACE2 and TMPRSS2 receptors. Unlike the parental line, this overexpression ensures stable infection and enhanced viral entry, allowing for the evaluation of antiviral efficacy against various SARS-CoV-2 variants.
o.
293T is a human embryonic kidney cell line that can be engineered for high ACE2 expression and SARS-CoV-2 susceptibility. 293T cells are easily transfected and support high protein expression.
p.
HEK293-hACE2 is a human embryonic kidney cell line with high ACE2 expression and SARS-CoV-2 susceptibility. Cells have been transfected with a plasmid to express the human ACE2 (hACE2) protein.
q.
293T/hACE2/TMPRSS2 is a human embryonic kidney cell line engineered for high ACE2 and TMPRSS2 expression, which mimics key aspects of human infection. 293T/hACE2/TMPRSS2 cells are very susceptible to SARS-CoV-2 infection.
r.
Vero E6 is an African green monkey kidney cell line with low/no ACE2 expression and high SARS-CoV-2 susceptibility. The cell line is easy to maintain and supports robust viral replication, however the monkey origin may not accurately represent human responses.
s.
SH-SY5Y is a human neuroblastoma cell line that exhibits neuronal phenotypes. It is commonly used as an in vitro model for studying neurotoxicity, neurodegenerative diseases, and neuronal differentiation.
Marín-Palma et al., 16 Nov 2021, peer-reviewed, 9 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms
Molecules, doi:10.3390/molecules26226900
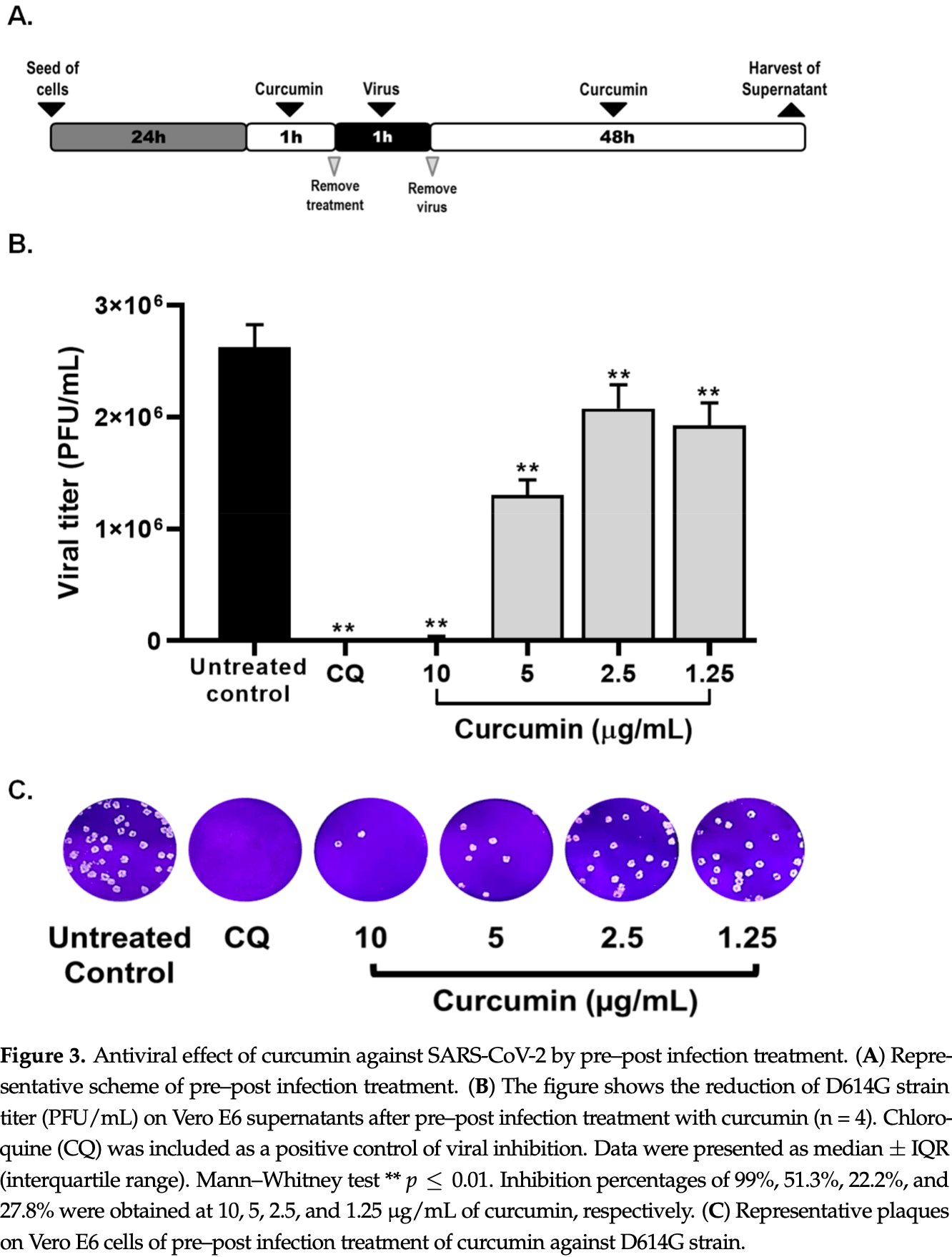
Due to the scarcity of therapeutic approaches for COVID-19, we investigated the antiviral and anti-inflammatory properties of curcumin against SARS-CoV-2 using in vitro models. The cytotoxicity of curcumin was evaluated using MTT assay in Vero E6 cells. The antiviral activity of this compound against SARS-CoV-2 was evaluated using four treatment strategies (i. pre-post infection treatment, ii. co-treatment, iii. pre-infection, and iv. post-infection). The D614G strain and Delta variant of SARS-CoV-2 were used, and the viral titer was quantified by plaque assay. The anti-inflammatory effect was evaluated in peripheral blood mononuclear cells (PBMCs) using qPCR and ELISA. By pre-post infection treatment, Curcumin (10 µg/mL) exhibited antiviral effect of 99% and 99.8% against DG614 strain and Delta variant, respectively. Curcumin also inhibited D614G strain by pre-infection and post-infection treatment. In addition, curcumin showed a virucidal effect against D614G strain and Delta variant. Finally, the pro-inflammatory cytokines (IL-1β, IL-6, and IL-8) released by PBMCs triggered by SARS-CoV-2 were decreased after treatment with curcumin. Our results suggest that curcumin affects the SARS-CoV-2 replicative cycle and exhibits virucidal effect with a variant/strain independent antiviral effect and immune-modulatory properties. This is the first study that showed a combined (antiviral/anti-inflammatory) effect of curcumin during SARS-CoV-2 infection. However, additional studies are required to define its use as a treatment for the COVID-19.
Institutional Review Board Statement: This study was approved by the ethics committee of the Universidad Cooperativa de Colombia (Acta BIO106). It was carried out keeping good records, practicing good data collection and management, transparency of data-sharing, and realistic representation of study results. The data were analyzed anonymously. All donors were adults, read, and signed an informed consent. All research protocols were made according to the principles of the Declaration of Helsinki. Informed Consent Statement: Informed consent was obtained from all subjects involved in the study.
Data Availability Statement: All data generated or analyzed during this study are included in this published article (and its supporting information files).
Conflicts of Interest: The authors declare no conflict of interest. Sample Availability: Samples of the compounds are available from the authors.
References
Aguilar-Jiménez, Flórez-Álvarez, Rincón, Marín-Palma, Sánchez-Martínez et al., Caracterización inmunológica de un grupo familiar colombiano con infección por SARS-CoV-2, Biomedica, doi:10.7705/biomedica.5976
Ali, Banerjea, Curcumin inhibits HIV-1 by promoting Tat protein degradation, Sci. Rep, doi:10.1038/srep27539
Balasubramanian, Pilankatta, Teramoto, Sajith, Nwulia et al., Inhibition of dengue virus by curcuminoids, Antivir. Res, doi:10.1016/j.antiviral.2018.12.002
Blanco-Melo, Nilsson-Payant, Liu, Uhl, Hoagland et al., Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19, Cell, doi:10.1016/j.cell.2020.04.026
Chen, Chen, Wen, Ou, Chiou et al., Inhibition of enveloped viruses infectivity by curcumin, PLoS ONE, doi:10.1371/journal.pone.0062482
Chen, Shien, Tiley, Chiou, Wang et al., Curcumin inhibits influenza virus infection and haemagglutination activity, Food Chem, doi:10.1016/j.foodchem.2009.09.011
Cheng, Hsu, Lin, Hsu, Ho et al., Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions, Anticancer Res
Dai, Zhou, Xu, Song, Curcumin alleviates rheumatoid arthritis-induced inflammation and synovial hyperplasia by targeting mTOR pathway in rats, Drug Des. Devel, doi:10.2147/DDDT.S175763
Di Nunzio, Valli, Tomas-Cobos, Tomas-Chisbert, Murgui-Bosch et al., Is cytotoxicity a determinant of the different in vitro and in vivo effects of bioactives? BMC Complement, Altern. Med, doi:10.1186/s12906-017-1962-2
Du, He, Zhou, Liu, Zheng et al., The spike protein of SARS-CoV-A target for vaccine and therapeutic development, Nat. Rev. Microbiol, doi:10.1038/nrmicro2090
Du, Nan, Xiao, Zhao, Zhou, Antiviral Strategies against PRRSV Infection, Trends Microbiol, doi:10.1016/j.tim.2017.06.001
Díaz, Aguilar-Jiménez, Flórez-Álvarez, Valencia, Laiton-Donato et al., Isolation and characterization of an early SARS-CoV-2 isolate from the 2020 epidemic in Medellín, Colombia, Biomédica, doi:10.7705/biomedica.5834
Feria-Garzon, Rugeles, Hernandez, Lujan, Taborda, Sulfasalazine as an Immunomodulator of the Inflammatory Process during HIV-1 Infection, Int. J. Mol. Sci, doi:10.3390/ijms20184476
Forni, Cagliani, Clerici, Sironi, Molecular Evolution of Human Coronavirus Genomes, Trends Microbiol, doi:10.1016/j.tim.2016.09.001
Ghanbari, Teimoori, Sadeghi, Mohamadkhani, Rezasoltani et al., Existing antiviral options against SARS-CoV-2 replication in COVID-19 patients, Future Microbiol, doi:10.2217/fmb-2020-0120
Gong, Zhou, Li, Gao, Xu et al., Curcumin suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock, Mol. Nutr. Food Res, doi:10.1002/mnfr.201500316
Gupta, Gupta, Bhargava, Potential use of turmeric in COVID-19, Clin. Exp. Derm, doi:10.1111/ced.14357
Haneklaus, O'neill, NLRP3 at the interface of metabolism and inflammation, Immunol. Rev, doi:10.1111/imr.12285
Hasan, Zingg, Kwan, Noble, Smith et al., Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice, Atherosclerosis, doi:10.1016/j.atherosclerosis.2013.10.016
Hasanzadeh, Read, Bland, Majeed, Jamialahmadi et al., Curcumin: An inflammasome silencer, Pharm. Res, doi:10.1016/j.phrs.2020.104921
Hirano, Murakami, COVID-19: A New but a Familiar Receptor and Cytokine Release Syndrome, Immunity, doi:10.1016/j.immuni.2020.04.003
Hoffmann, Kleine-Weber, Schroeder, Kruger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Jena, Kanungo, Nayak, Chainy, Dandapat, Catechin and curcumin interact with S protein of SARS-CoV2 and ACE2 of human cell membrane:Insights from computational studies, Sci. Rep, doi:10.1038/s41598-021-81462-7
Kandeel, Al-Nazawi, Virtual screening and repurposing of FDA approved drugs against COVID-19 main protease, Life Sci, doi:10.1016/j.lfs.2020.117627
Katta, Srivastava, Thangapazham, Rosner, Cullen et al., Curcumin-Gene Expression Response in Hormone Dependent and Independent Metastatic Prostate Cancer Cells, Int. J. Mol. Sci, doi:10.3390/ijms20194891
Khaerunnisa, Awaluddin, Suhartati, Soetjipto, Potential Inhibitor of COVID-19 Main Protease (Mpro) From Several Medicinal Plant Compounds by Molecular Docking Study, Preprints, doi:10.20944/preprints202003.0226.v1
Khateeb, Li, Zhang, Emerging SARS-CoV-2 variants of concern and potential intervention approaches, Crit. Care, doi:10.1186/s13054-021-03662-x
Koch, Uckeley, Doldan, Stanifer, Boulant et al., Host Cell Proteases Drive Early or Late SARS-CoV-2 Penetration, bioRxiv, doi:10.1101/2020.12.22.423906
Korber, Fischer, Gnanakaran, Yoon, Theiler et al., Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus, Cell, doi:10.1016/j.cell.2020.06.043
Kumar, Sodhi, Singh, Addressing the potential role of curcumin in the prevention of COVID-19 by targeting the Nsp9 replicase protein through molecular docking, Arch. Microbiol, doi:10.1007/s00203-020-02163-9
Lao, Ruffin, Normolle, Heath, Murray et al., Dose escalation of a curcuminoid formulation, BMC Complement. Altern. Med, doi:10.1186/1472-6882-6-10
Lim, Chu, Yang, Beech, Frautschy et al., The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse, J. Neurosci, doi:10.1523/JNEUROSCI.21-21-08370.2001
Lopez Bernal, Andrews, Gower, Gallagher, Simmons et al., Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant, N. Engl. J. Med, doi:10.1056/NEJMoa2108891
Lu, Zhao, Li, Niu, Yang et al., Genomic characterisation and epidemiology of 2019 novel coronavirus:Implications for virus origins and receptor binding, Lancet, doi:10.1016/S0140-6736(20)30251-8
Malik, Properties of Coronavirus and SARS-CoV-2, Malays. J. Pathol
Mani, Johnson, Steel, Broszczak, Neilsen et al., Natural product-derived phytochemicals as potential agents against coronaviruses: A review, Virus Res, doi:10.1016/j.virusres.2020.197989
Marin-Palma, Castro, Cardona-Arias, Urcuqui-Inchima, Hernandez, Lower High-Density Lipoproteins Levels During Human Immunodeficiency Virus Type 1 Infection Are Associated with Increased Inflammatory Markers and Disease Progression, Front Immunol, doi:10.3389/fimmu.2018.01350
Marin-Palma, Sirois, Urcuqui-Inchima, Hernandez, Inflammatory status and severity of disease in dengue patients are associated with lipoprotein alterations, PLoS ONE, doi:10.1371/journal.pone.0214245
Mathew, Hsu, Antiviral potential of curcumin, J. Funct. Foods, doi:10.1016/j.jff.2017.12.017
Maurya, Kumar, Prasad, Bhatt, Saxena, Structure-based drug designing for potential antiviral activity of selected natural products from Ayurveda against SARS-CoV-2 spike glycoprotein and its cellular receptor, Virusdisease, doi:10.1007/s13337-020-00598-8
Mazumder, Raghavan, Weinstein, Kohn, Pommier, Inhibition of human immunodeficiency virus type-1 integrase by curcumin, Biochem. Pharm, doi:10.1016/0006-2952(95)98514-A
Moghadamtousi, Kadir, Hassandarvish, Tajik, Abubakar et al., A review on antibacterial, antiviral, and antifungal activity of curcumin, Biomed. Res. Int, doi:10.1155/2014/186864
Mohajeri, Sadeghizadeh, Najafi, Javan, Polymerized nano-curcumin attenuates neurological symptoms in EAE model of multiple sclerosis through down regulation of inflammatory and oxidative processes and enhancing neuroprotection and myelin repair, Neuropharmacology, doi:10.1016/j.neuropharm.2015.07.013
Mounce, Cesaro, Carrau, Vallet, Vignuzzi, Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding, Antivir. Res, doi:10.1016/j.antiviral.2017.03.014
Nagahama, Utsumi, Kumano, Maekawa, Oyama et al., Discovery of a new function of curcumin which enhances its anticancer therapeutic potency, Sci. Rep, doi:10.1038/srep30962
Obata, Kojima, Masaki, Okabayashi, Yokota et al., Curcumin prevents replication of respiratory syncytial virus and the epithelial responses to it in human nasal epithelial cells, PLoS ONE, doi:10.1371/journal.pone.0070225
Paces, Strizova, Smrz, Cerny, COVID-19 and the immune system, Physiol. Res, doi:10.33549/physiolres.934492
Padmanabhan, Desikan, Dixit, Targeting TMPRSS2 and Cathepsin B/L together may be synergistic against SARS-CoV-2 infection, PLoS Comput. Biol, doi:10.1371/journal.pcbi.1008461
Pgk, Housekeeping gene) Fw: GTTGACCGAATCACCGACC
Planas, Veyer, Baidaliuk, Staropoli, Guivel-Benhassine et al., Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization, Nature, doi:10.1038/s41586-021-03777-9
Praditya, Kirchhoff, Bruning, Rachmawati, Steinmann et al., Anti-infective Properties of the Golden Spice Curcumin, Front Microbiol, doi:10.3389/fmicb.2019.00912
Ranjan, Chen, Johnston, Jeon, Nagabhushan, Curcumin inhibits mitogen stimulated lymphocyte proliferation, NFkappaB activation, and IL-2 signaling, J. Surg. Res, doi:10.1016/j.jss.2004.04.004
Richart, Li, Mizushina, Chang, Chung et al., Synergic effect of curcumin and its structural analogue (Monoacetylcurcumin) on anti-influenza virus infection, J. Food Drug Anal, doi:10.1016/j.jfda.2017.12.006
Sahebkar, Are curcuminoids effective C-reactive protein-lowering agents in clinical practice? Evidence from a meta-analysis, Phytother Res, doi:10.1002/ptr.5045
Su, Wong, Shi, Liu, Lai et al., Genetic Recombination, and Pathogenesis of Coronaviruses, Trends Microbiol, doi:10.1016/j.tim.2016.03.003
Sun, He, Wang, Lai, Ji et al., COVID-19: Epidemiology, Evolution, and Cross-Disciplinary Perspectives, Trends Mol. Med, doi:10.1016/j.molmed.2020.02.008
Tabares-Guevara, Jaramillo, Ospina-Quintero, Piedrahíta-Ochoa, García-Valencia et al., IL-10-Dependent Amelioration of Chronic Inflammatory Disease by Microdose Subcutaneous Delivery of a Prototypic Immunoregulatory Small Molecule, Front Immunol, doi:10.3389/fimmu.2021.708955
Tandon, Sharp, Zhang, Pomin, Ashpole et al., Effective Inhibition of SARS-CoV-2 Entry by Heparin and Enoxaparin Derivatives, J. Virol, doi:10.1128/JVI.01987-20
Tang, Bidon, Jaimes, Whittaker, Daniel, Coronavirus membrane fusion mechanism offers a potential target for antiviral development, Antivir. Res, doi:10.1016/j.antiviral.2020.104792
Trujillo-Correa, Quintero-Gil, Diaz-Castillo, Quiñones, Robledo et al., In vitro and in silico anti-dengue activity of compounds obtained from Psidium guajava through bioprospecting, BMC Complement. Altern. Med, doi:10.1186/s12906-019-2695-1
Um, Hwang, Choi, Ahn, Jung et al., Curcumin attenuates adhesion molecules and matrix metalloproteinase expression in hypercholesterolemic rabbits, Nutr. Res, doi:10.1016/j.nutres.2014.09.001
Uzunova, Filipova, Pavlova, Vekov, Insights into antiviral mechanisms of remdesivir, lopinavir/ritonavir and chloroquine/hydroxychloroquine affecting the new SARS-CoV-2, Biomed. Pharm, doi:10.1016/j.biopha.2020.110668
Valizadeh, Abdolmohammadi-Vahid, Danshina, Ziya Gencer, Ammari et al., Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients, Int. Immunol, doi:10.1016/j.intimp.2020.107088
Vardhana, Wolchok, The many faces of the anti-COVID immune response, J. Exp. Med, doi:10.1084/jem.20200678
Walls, Park, Tortorici, Wall, Mcguire et al., Structure, Function, and Antigenicity of the SARS-CoV-2
Wen, Kuo, Jan, Liang, Wang et al., Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus, J. Med. Chem, doi:10.1021/jm070295s
Woo, Lau, Huang, Yuen, Coronavirus diversity, phylogeny and interspecies jumping, Exp. Biol. Med. (Maywood), doi:10.3181/0903-MR-94
Wu, Hou, Cao, Zuo, Xue et al., Virucidal efficacy of treatment with photodynamically activated curcumin on murine norovirus bio-accumulated in oysters, Photodiagnosis. Photodyn, doi:10.1016/j.pdpdt.2015.06.005
Xiong, Liu, Cao, Wang, Guo et al., Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients, Emerg. Microbes Infect, doi:10.1080/22221751.2020.1747363
Xu, Liu, Curcumin alleviates macrophage activation and lung inflammation induced by influenza virus infection through inhibiting the NF-kappaB signaling pathway. Influenza Other, Respir. Viruses, doi:10.1111/irv.12459
Yang, Li, Huang, Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection, Nanoscale, doi:10.1039/C5NR07918G
Yepes-Perez, Herrera-Calderon, Oliveros, Florez-Alvarez, Zapata-Cardona et al., The Hydroalcoholic Extract of Uncaria tomentosa (Cat's Claw) Inhibits the Infection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) In Vitro, Evid. Based Complement. Altern. Med, doi:10.1155/2021/6679761
Yin, Guo, Li, Tang, Li et al., Curcumin Suppresses IL-1beta Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome, J. Immunol, doi:10.4049/jimmunol.1701495
Zandi, Teoh, Sam, Wong, Mustafa et al., Antiviral activity of four types of bioflavonoid against dengue virus type-2, Virol. J, doi:10.1186/1743-422X-8-560
Zapata-Cardona, Flórez-Álvarez, Zapata-Builes, Guerra-Sandoval, Guerra-Almonacid et al., Atorvastatin effectively inhibits late replicative cycle steps of SARS-CoV-2 in vitro, bioRxiv
Zhang, Zou, Li, Zheng, Feng, Curcumin Protects against Atherosclerosis in Apolipoprotein E-Knockout Mice by Inhibiting Toll-like Receptor 4 Expression, J. Agric. Food Chem, doi:10.1021/acs.jafc.7b04260
Zhao, Ching, Huang, Chen, Chiang et al., Molecular mechanism of curcumin on the suppression of cholesterol accumulation in macrophage foam cells and atherosclerosis, Mol. Nutr. Food Res, doi:10.1002/mnfr.201100735
Zhou, Yang, Wang, Hu, Zhang et al., Addendum: A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature, doi:10.1038/s41586-020-2951-z
Zouharova, Lipenska, Fojtikova, Kulich, Neca et al., Antiviral activities of 2,6-diaminopurine-based acyclic nucleoside phosphonates against herpesviruses: In vitro study results with pseudorabies virus (PrV, SuHV-1), Vet. Microbiol, doi:10.1016/j.vetmic.2016.01.010
DOI record:
{
"DOI": "10.3390/molecules26226900",
"ISSN": [
"1420-3049"
],
"URL": "http://dx.doi.org/10.3390/molecules26226900",
"abstract": "<jats:p>Due to the scarcity of therapeutic approaches for COVID-19, we investigated the antiviral and anti-inflammatory properties of curcumin against SARS-CoV-2 using in vitro models. The cytotoxicity of curcumin was evaluated using MTT assay in Vero E6 cells. The antiviral activity of this compound against SARS-CoV-2 was evaluated using four treatment strategies (i. pre–post infection treatment, ii. co-treatment, iii. pre-infection, and iv. post-infection). The D614G strain and Delta variant of SARS-CoV-2 were used, and the viral titer was quantified by plaque assay. The anti-inflammatory effect was evaluated in peripheral blood mononuclear cells (PBMCs) using qPCR and ELISA. By pre–post infection treatment, Curcumin (10 µg/mL) exhibited antiviral effect of 99% and 99.8% against DG614 strain and Delta variant, respectively. Curcumin also inhibited D614G strain by pre-infection and post-infection treatment. In addition, curcumin showed a virucidal effect against D614G strain and Delta variant. Finally, the pro-inflammatory cytokines (IL-1β, IL-6, and IL-8) released by PBMCs triggered by SARS-CoV-2 were decreased after treatment with curcumin. Our results suggest that curcumin affects the SARS-CoV-2 replicative cycle and exhibits virucidal effect with a variant/strain independent antiviral effect and immune-modulatory properties. This is the first study that showed a combined (antiviral/anti-inflammatory) effect of curcumin during SARS-CoV-2 infection. However, additional studies are required to define its use as a treatment for the COVID-19.</jats:p>",
"alternative-id": [
"molecules26226900"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-2074-8983",
"affiliation": [],
"authenticated-orcid": false,
"family": "Marín-Palma",
"given": "Damariz",
"sequence": "first"
},
{
"affiliation": [],
"family": "Tabares-Guevara",
"given": "Jorge H.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2714-9190",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zapata-Cardona",
"given": "María I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flórez-Álvarez",
"given": "Lizdany",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7043-5868",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yepes",
"given": "Lina M.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rugeles",
"given": "Maria T.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7351-8738",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zapata-Builes",
"given": "Wildeman",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-9200-5698",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hernandez",
"given": "Juan C.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Taborda",
"given": "Natalia A.",
"sequence": "additional"
}
],
"container-title": [
"Molecules"
],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
11,
17
]
],
"date-time": "2021-11-17T07:42:28Z",
"timestamp": 1637134948000
},
"deposited": {
"date-parts": [
[
2021,
11,
17
]
],
"date-time": "2021-11-17T08:53:26Z",
"timestamp": 1637139206000
},
"funder": [
{
"DOI": "10.13039/501100005278",
"award": [
"BPIN 2020000100131-SGR"
],
"doi-asserted-by": "publisher",
"name": "Universidad de Antioquia"
},
{
"award": [
"NAT",
"JCH"
],
"name": "Corporación Universitaria Remington"
}
],
"indexed": {
"date-parts": [
[
2021,
11,
17
]
],
"date-time": "2021-11-17T09:17:05Z",
"timestamp": 1637140625011
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "electronic",
"value": "1420-3049"
}
],
"issue": "22",
"issued": {
"date-parts": [
[
2021,
11,
16
]
]
},
"journal-issue": {
"issue": "22",
"published-online": {
"date-parts": [
[
2021,
11
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
16
]
],
"date-time": "2021-11-16T00:00:00Z",
"timestamp": 1637020800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1420-3049/26/22/6900/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "6900",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2021,
11,
16
]
]
},
"published-online": {
"date-parts": [
[
2021,
11,
16
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"DOI": "10.1038/s41586-020-2951-z",
"doi-asserted-by": "publisher",
"key": "ref1"
},
{
"DOI": "10.3181/0903-MR-94",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1016/j.tim.2016.03.003",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"DOI": "10.1016/j.tim.2016.09.001",
"doi-asserted-by": "publisher",
"key": "ref4"
},
{
"article-title": "Properties of Coronavirus and SARS-CoV-2",
"author": "Malik",
"first-page": "3",
"journal-title": "Malays. J. Pathol.",
"key": "ref5",
"volume": "42",
"year": "2020"
},
{
"DOI": "10.1016/j.molmed.2020.02.008",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1038/nrmicro2090",
"doi-asserted-by": "publisher",
"key": "ref7"
},
{
"DOI": "10.1016/j.cell.2020.11.032",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1016/S0140-6736(20)30251-8",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.2217/fmb-2020-0120",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.33549/physiolres.934492",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.7705/biomedica.5976",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1080/22221751.2020.1747363",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1016/j.virusres.2020.197989",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1155/2021/6679761",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1155/2014/186864",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1111/ced.14357",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1002/ptr.5045",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1016/j.jss.2004.04.004",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1002/mnfr.201500316",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.4049/jimmunol.1701495",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1016/j.antiviral.2018.12.002",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1016/j.antiviral.2017.03.014",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1016/j.jfda.2017.12.006",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1016/0006-2952(95)98514-A",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1038/srep27539",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1038/s41598-021-81462-7",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1371/journal.pcbi.1008461",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.3389/fmicb.2019.00912",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1016/j.cell.2020.06.043",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.1186/s13054-021-03662-x",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.1186/s12906-019-2695-1",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1016/j.foodchem.2009.09.011",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1371/journal.pone.0062482",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.1186/1743-422X-8-560",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1016/j.lfs.2020.117627",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.3390/ijms20194891",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1039/C5NR07918G",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1016/j.pdpdt.2015.06.005",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1016/j.antiviral.2020.104792",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1101/2020.12.22.423906",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1038/srep30962",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.1021/jm070295s",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1371/journal.pone.0070225",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.1016/j.jff.2017.12.017",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.20944/preprints202003.0226.v1",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1007/s00203-020-02163-9",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1038/s41586-021-03777-9",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.1016/j.tim.2017.06.001",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.1056/NEJMoa2108891",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.1016/j.cell.2020.04.026",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.1016/j.immuni.2020.04.003",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"DOI": "10.1084/jem.20200678",
"doi-asserted-by": "publisher",
"key": "ref55"
},
{
"DOI": "10.1016/j.intimp.2020.107088",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"DOI": "10.1111/imr.12285",
"doi-asserted-by": "publisher",
"key": "ref57"
},
{
"DOI": "10.1021/acs.jafc.7b04260",
"doi-asserted-by": "publisher",
"key": "ref58"
},
{
"DOI": "10.1111/irv.12459",
"doi-asserted-by": "publisher",
"key": "ref59"
},
{
"DOI": "10.1016/j.phrs.2020.104921",
"doi-asserted-by": "publisher",
"key": "ref60"
},
{
"DOI": "10.2147/DDDT.S175763",
"doi-asserted-by": "publisher",
"key": "ref61"
},
{
"DOI": "10.1016/j.atherosclerosis.2013.10.016",
"doi-asserted-by": "publisher",
"key": "ref62"
},
{
"DOI": "10.1523/JNEUROSCI.21-21-08370.2001",
"doi-asserted-by": "publisher",
"key": "ref63"
},
{
"DOI": "10.1016/j.neuropharm.2015.07.013",
"doi-asserted-by": "publisher",
"key": "ref64"
},
{
"DOI": "10.1002/mnfr.201100735",
"doi-asserted-by": "publisher",
"key": "ref65"
},
{
"DOI": "10.1007/s13337-020-00598-8",
"doi-asserted-by": "publisher",
"key": "ref66"
},
{
"DOI": "10.1016/j.nutres.2014.09.001",
"doi-asserted-by": "publisher",
"key": "ref67"
},
{
"DOI": "10.3389/fimmu.2021.708955",
"doi-asserted-by": "publisher",
"key": "ref68"
},
{
"DOI": "10.1186/1472-6882-6-10",
"doi-asserted-by": "publisher",
"key": "ref69"
},
{
"DOI": "10.1186/s12906-017-1962-2",
"doi-asserted-by": "publisher",
"key": "ref70"
},
{
"article-title": "Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions",
"author": "Cheng",
"first-page": "2895",
"journal-title": "Anticancer Res.",
"key": "ref71",
"volume": "21",
"year": "2001"
},
{
"DOI": "10.7705/biomedica.5834",
"doi-asserted-by": "publisher",
"key": "ref72"
},
{
"DOI": "10.1016/j.vetmic.2016.01.010",
"doi-asserted-by": "publisher",
"key": "ref73"
},
{
"DOI": "10.1101/2021.03.01.433498",
"doi-asserted-by": "publisher",
"key": "ref74"
},
{
"DOI": "10.1016/j.biopha.2020.110668",
"doi-asserted-by": "publisher",
"key": "ref75"
},
{
"DOI": "10.1128/JVI.01987-20",
"doi-asserted-by": "publisher",
"key": "ref76"
},
{
"DOI": "10.3389/fimmu.2018.01350",
"doi-asserted-by": "publisher",
"key": "ref77"
},
{
"DOI": "10.1371/journal.pone.0214245",
"doi-asserted-by": "publisher",
"key": "ref78"
},
{
"DOI": "10.3390/ijms20184476",
"doi-asserted-by": "publisher",
"key": "ref79"
}
],
"reference-count": 79,
"references-count": 79,
"relation": {},
"score": 1,
"short-container-title": [
"Molecules"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Chemistry (miscellaneous)",
"Analytical Chemistry",
"Organic Chemistry",
"Physical and Theoretical Chemistry",
"Molecular Medicine",
"Drug Discovery",
"Pharmaceutical Science"
],
"subtitle": [],
"title": [
"Curcumin Inhibits In Vitro SARS-CoV-2 Infection In Vero E6 Cells through Multiple Antiviral Mechanisms"
],
"type": "journal-article",
"volume": "26"
}
