In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds
et al., Natural Product Communications, doi:10.1177/1934578X231188861, Sep 2023
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
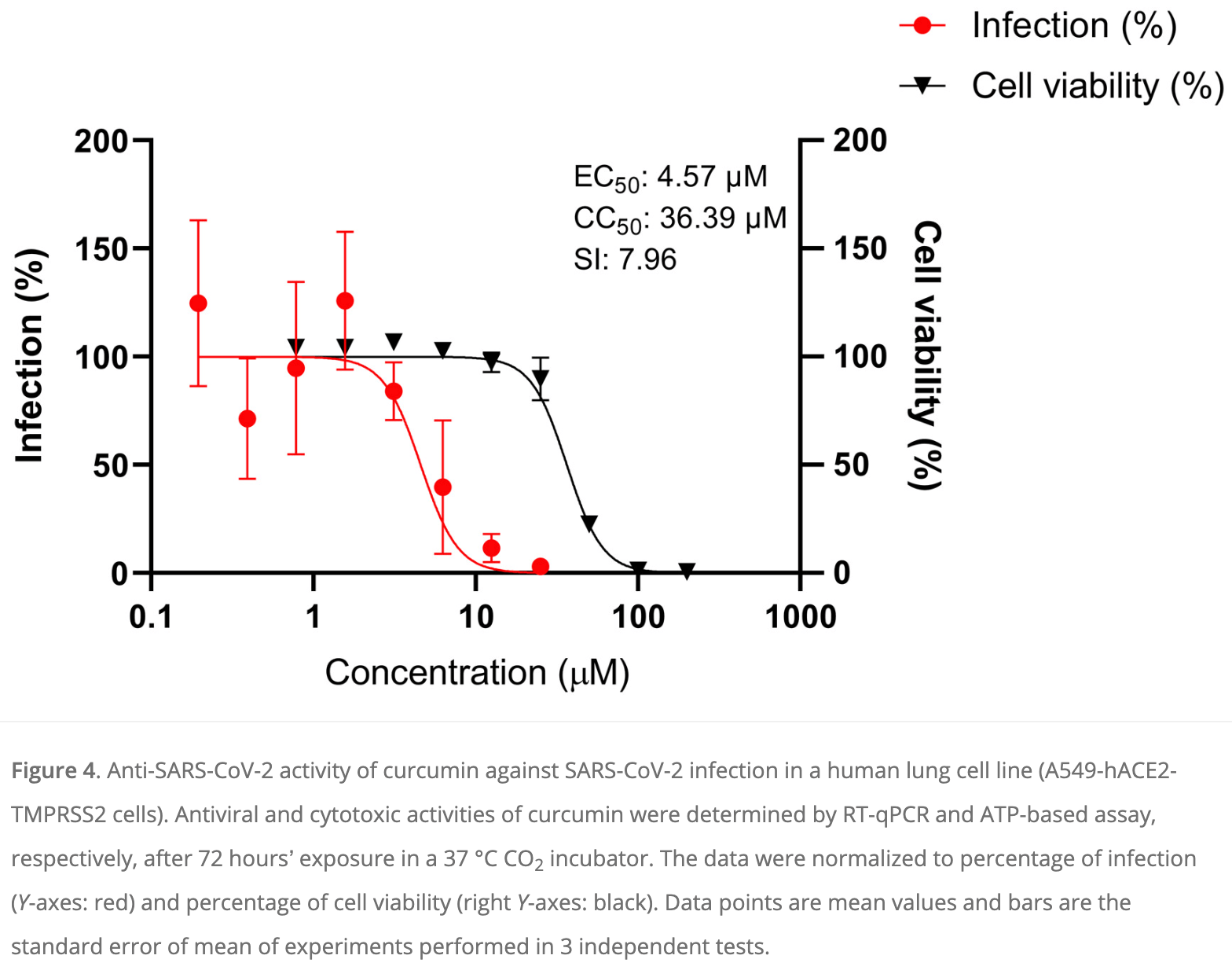
In vitro study showing that of 9 phenolic compounds tested, only curcumin inhibited SARS-CoV-2 cytopathic effects in infected monkey kidney Vero E6 cells. Curcumin showed antiviral activity against wildtype, alpha, delta, and omicron variants, with EC50 values around 25µM for the variants and 13.63µM for the wildtype, but with a low selectivity index (SI < 5). Curcumin was more effective against SARS-CoV-2 infection in human lung A549 cells expressing ACE2 and TMPRSS2 receptors, with an EC50 of 4.57μM and a higher selectivity index of 7.96. Curcumin also inhibited SARS-CoV-2 spike protein-ACE2 interaction and 3CL protease activity at 10-20μM concentrations. The results suggest curcumin has moderate antiviral activity against SARS-CoV-2 through multiple targets, although bioavailability may limit efficacy, requiring formulations for improved bioavailability.
Positive controls andrographolide, chloroquine, and remdesivir inhibited the viral-induced CPE with EC50 values of less than 10μM with a high selectivity index (SI ≥ 10).
39 preclinical studies support the efficacy of HCQ for COVID-19:
1.
Shang et al., Identification of Cathepsin L as the molecular target of hydroxychloroquine with chemical proteomics, Molecular & Cellular Proteomics, doi:10.1016/j.mcpro.2025.101314.
2.
González-Paz et al., Biophysical Analysis of Potential Inhibitors of SARS-CoV-2 Cell Recognition and Their Effect on Viral Dynamics in Different Cell Types: A Computational Prediction from In Vitro Experimental Data, ACS Omega, doi:10.1021/acsomega.3c06968.
3.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
4.
Guimarães Silva et al., Are Non-Structural Proteins From SARS-CoV-2 the Target of Hydroxychloroquine? An in Silico Study, ACTA MEDICA IRANICA, doi:10.18502/acta.v61i2.12533.
5.
Nguyen et al., The Potential of Ameliorating COVID-19 and Sequelae From Andrographis paniculata via Bioinformatics, Bioinformatics and Biology Insights, doi:10.1177/11779322221149622.
7.
Yadav et al., Repurposing the Combination Drug of Favipiravir, Hydroxychloroquine and Oseltamivir as a Potential Inhibitor Against SARS-CoV-2: A Computational Study, Research Square, doi:10.21203/rs.3.rs-628277/v1.
8.
Hussein et al., Molecular Docking Identification for the efficacy of Some Zinc Complexes with Chloroquine and Hydroxychloroquine against Main Protease of COVID-19, Journal of Molecular Structure, doi:10.1016/j.molstruc.2021.129979.
9.
Baildya et al., Inhibitory capacity of Chloroquine against SARS-COV-2 by effective binding with Angiotensin converting enzyme-2 receptor: An insight from molecular docking and MD-simulation studies, Journal of Molecular Structure, doi:10.1016/j.molstruc.2021.129891.
10.
Noureddine et al., Quantum chemical studies on molecular structure, AIM, ELF, RDG and antiviral activities of hybrid hydroxychloroquine in the treatment of COVID-19: molecular docking and DFT calculations, Journal of King Saud University - Science, doi:10.1016/j.jksus.2020.101334.
11.
Tarek et al., Pharmacokinetic Basis of the Hydroxychloroquine Response in COVID-19: Implications for Therapy and Prevention, European Journal of Drug Metabolism and Pharmacokinetics, doi:10.1007/s13318-020-00640-6.
12.
Rowland Yeo et al., Impact of Disease on Plasma and Lung Exposure of Chloroquine, Hydroxychloroquine and Azithromycin: Application of PBPK Modeling, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.1955.
13.
Hitti et al., Hydroxychloroquine attenuates double-stranded RNA-stimulated hyper-phosphorylation of tristetraprolin/ZFP36 and AU-rich mRNA stabilization, Immunology, doi:10.1111/imm.13835.
14.
Yan et al., Super-resolution imaging reveals the mechanism of endosomal acidification inhibitors against SARS-CoV-2 infection, ChemBioChem, doi:10.1002/cbic.202400404.
15.
Mohd Abd Razak et al., In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds, Natural Product Communications, doi:10.1177/1934578X231188861.
16.
Alsmadi et al., The In Vitro, In Vivo, and PBPK Evaluation of a Novel Lung-Targeted Cardiac-Safe Hydroxychloroquine Inhalation Aerogel, AAPS PharmSciTech, doi:10.1208/s12249-023-02627-3.
17.
Wen et al., Cholinergic α7 nAChR signaling suppresses SARS-CoV-2 infection and inflammation in lung epithelial cells, Journal of Molecular Cell Biology, doi:10.1093/jmcb/mjad048.
18.
Kamga Kapchoup et al., In vitro effect of hydroxychloroquine on pluripotent stem cells and their cardiomyocytes derivatives, Frontiers in Pharmacology, doi:10.3389/fphar.2023.1128382.
19.
Milan Bonotto et al., Cathepsin inhibitors nitroxoline and its derivatives inhibit SARS-CoV-2 infection, Antiviral Research, doi:10.1016/j.antiviral.2023.105655.
20.
Miao et al., SIM imaging resolves endocytosis of SARS-CoV-2 spike RBD in living cells, Cell Chemical Biology, doi:10.1016/j.chembiol.2023.02.001.
21.
Yuan et al., Hydroxychloroquine blocks SARS-CoV-2 entry into the endocytic pathway in mammalian cell culture, Communications Biology, doi:10.1038/s42003-022-03841-8.
22.
Faísca et al., Enhanced In Vitro Antiviral Activity of Hydroxychloroquine Ionic Liquids against SARS-CoV-2, Pharmaceutics, doi:10.3390/pharmaceutics14040877.
23.
Delandre et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445.
24.
Purwati et al., An in vitro study of dual drug combinations of anti-viral agents, antibiotics, and/or hydroxychloroquine against the SARS-CoV-2 virus isolated from hospitalized patients in Surabaya, Indonesia, PLOS One, doi:10.1371/journal.pone.0252302.
25.
Zhang et al., SARS-CoV-2 spike protein dictates syncytium-mediated lymphocyte elimination, Cell Death & Differentiation, doi:10.1038/s41418-021-00782-3.
26.
Dang et al., Structural basis of anti-SARS-CoV-2 activity of hydroxychloroquine: specific binding to NTD/CTD and disruption of LLPS of N protein, bioRxiv, doi:10.1101/2021.03.16.435741.
27.
Shang (B) et al., Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice, Virology Journal, doi:10.1186/s12985-021-01515-1.
28.
Wang et al., Chloroquine and hydroxychloroquine as ACE2 blockers to inhibit viropexis of 2019-nCoV Spike pseudotyped virus, Phytomedicine, doi:10.1016/j.phymed.2020.153333.
29.
Sheaff, R., A New Model of SARS-CoV-2 Infection Based on (Hydroxy)Chloroquine Activity, bioRxiv, doi:10.1101/2020.08.02.232892.
30.
Ou et al., Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2, PLOS Pathogens, doi:10.1371/journal.ppat.1009212.
31.
Andreani et al., In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect, Microbial Pathogenesis, doi:10.1016/j.micpath.2020.104228.
32.
Clementi et al., Combined Prophylactic and Therapeutic Use Maximizes Hydroxychloroquine Anti-SARS-CoV-2 Effects in vitro, Front. Microbiol., 10 July 2020, doi:10.3389/fmicb.2020.01704.
33.
Liu et al., Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discovery 6, 16 (2020), doi:10.1038/s41421-020-0156-0.
34.
Yao et al., In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin. Infect. Dis., 2020 Mar 9, doi:10.1093/cid/ciaa237.
Mohd Abd Razak et al., 16 Sep 2023, peer-reviewed, 11 authors.
Contact: ridzuan.ar@moh.gov.my.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds
Natural Product Communications, doi:10.1177/1934578x231188861
Since the COVID-19 pandemic in 2020, many reports have highlighted several potential anti-SARS-CoV-2 drug candidates, including phenolic compounds. Therefore, this study aimed to evaluate the anti-SARS-CoV-2 activity of nine common phenolic compounds found in plants using the in vitro cellular infection model. The anti-SARS-CoV-2 activity of curcumin, quercetin, gallic acid, catechin, rutin, kaempferol, naringenin, coumaric acid and caffeic acid were evaluated on SARS-CoV-2-infected Vero E6 cells by using a cytopathic effect (CPE)-based assay. The anti-SARS-CoV-2 activity in human lung cells, A549 expressing human ACE2 and TMPRSS2, was evaluated by the RT-qPCR technique. S1-ACE2 interaction and 3CL protease activity assays were also performed for the potent compound. Of the nine phenolic compounds, only curcumin inhibited the SARS-CoV-2 induced CPE activity (EC 50 of 13.63 µM) in Vero E6 cells, but with a low selective index (SI) value. Interestingly, curcumin exhibited potent anti-SARS-CoV-2 activity in A549 cells with an EC 50 of 4.57 µM and an SI value of 7.96. S1-ACE2 interaction and 3CL protease inhibitory activities of curcumin were also observed. In conclusion, curcumin showed a moderate in vitro anti-SARS-CoV-2 activity. The true potential of curcumin as an anti-SARS-CoV-2 candidate could be further evaluated in a COVID-19 animal model.
Additional Information The data presented in this study are available on request from the corresponding author.
Declaration of Conflicting Interests The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval Ethical Approval is not applicable for this article.
ORCID iD Mohd Ridzuan Mohd Abd Razak https://orcid.org/0000-0002-9589-5892
Statement of Human and Animal Rights This article does not contain any studies with human or animal subjects.
Informed Consent There are no human subjects in this article and informed consent is not applicable.
References
Abdallah, El-Halawany, Sirwi, Repurposing of some natural product isolates as SARS-COV-2 main protease inhibitors via in vitro cell free and cell-based antiviral assessments and molecular modeling approaches, Pharmaceuticals
Abu-Raddad, Chemaitelly, Butt, National Study Group for C-V. Effectiveness of the BNT162b2 COVID-19 vaccine against the B.1.1.7 and B.1.351 variants, N Engl J Med
Accorsi, Britton, Ke, Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and Delta variants, JAMA
Agrawal, Agrawal, Blunden, Naringenin as a possible candidate against SARS-CoV-2 infection and in the pathogenesis of COVID-19, Nat Prod Commun, doi:10.1177/1934578X211066723
Agrawal, Agrawal, Blunden, Quercetin: antiviral significance and possible COVID-19 integrative considerations, Nat Prod Commun, doi:10.1177/1934578X20976293
Agrawal, Agrawal, Blunden, Rutin: a potential antiviral for repurposing as a SARS-CoV-2 main protease (mpro) inhibitor, Nat Prod Commun, doi:10.1177/1934578X21991723
Alzaabi, Hamdy, Ashmawy, Flavonoids are promising safe therapy against COVID-19, Phytochem Rev
Bahun, Jukic, Oblak, Inhibition of the SARS-CoV-2 3CL(pro) main protease by plant polyphenols, Food Chem
Bormann, Alt, Schipper, Turmeric root and its bioactive ingredient curcumin effectively neutralize SARS-CoV-2 in vitro, Viruses
Chemaitelly, Tang, Hasan, Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar, N Engl J Med
Chen, Shinn, Itkin, Drug repurposing screen for compounds inhibiting the cytopathic effect of SARS-CoV-2, Front Pharmacol, doi:10.3389/fphar.2020.592737
Choy, Wong, Kaewpreedee, Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro, Antiviral Res
Couzin-Frankel, Antiviral pills could change pandemic's course, Science
Drozdzal, Rosik, Lechowicz, An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment, Drug Resist Updat
El Gizawy, Boshra, Mostafa, bioactive constituents exert anti-SARS-CoV-2 and antiinflammatory activities: molecular docking and dynamics, in vitro, and in vivo studies, Molecules
Elfiky, Natural products may interfere with SARS-CoV-2 attachment to the host cell, J Biomol Struct Dyn
Fischer Wa 2nd, Eron, Jr, Holman, A phase 2a clinical trial of molnupiravir in patients with COVID-19 shows accelerated SARS-CoV-2 RNA clearance and elimination of infectious virus, Sci Transl Med
Goc, Sumera, Rath, Niedzwiecki, Phenolic compounds disrupt spike-mediated receptor-binding and entry of SARS-CoV-2 pseudo-virions, PLoS One
Gordon, Jang, Bouhaddou, A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature
Harvey, Carabelli, Jackson, SARS-CoV-2 variants, spike mutations and immune escape, Nat Rev Microbiol
Hoffmann, Mosbauer, Hofmann-Winkler, Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2, Nature
Jena, Kanungo, Nayak, Chainy, Dandapat, Catechin and curcumin interact with S protein of SARS-CoV2 and ACE2 of human cell membrane: insights from computational studies, Sci Rep
Kanjanasirirat, Suksatu, Manopwisedjaroen, High-content screening of Thai medicinal plants reveals Boesenbergia rotunda extract and its component Panduratin A as anti-SARS-CoV-2 agents, Sci Rep
Mangiavacchi, Botwina, Menichetti, Selenofunctionalization of quercetin improves the non-covalent inhibition of M(pro) and its antiviral activity in cells against SARS-CoV-2, Int J Mol Sci
Marin-Palma, Tabares-Guevara, Zapata-Cardona, Curcumin inhibits in vitro SARS-CoV-2 infection in Vero E6 cells through multiple antiviral mechanisms, Molecules
Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J Immunol Methods
Nguyen, Jung, Kim, The inhibitory effects of plant derivate polyphenols on the main protease of SARS coronavirus 2 and their structure-activity relationship, Molecules
Polack, Thomas, Kitchin, Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine, N Engl J Med
Rebello, Beyl, Lertora, Safety and pharmacokinetics of naringenin: a randomized, controlled, single-ascending-dose clinical trial, Diabetes Obes Metab
Sa-Ngiamsuntorn, Suksatu, Pewkliang, Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives, J Nat Prod
Sancineto, Ostacolo, Ortega-Alarcon, L-Arginine improves solubility and Anti-SARS-CoV-2 Mpro activity of rutin but not the antiviral activity in cells, Molecules
Stohs, Ji, Bucci, Preuss, A comparative pharmacokinetic assessment of a novel highly bioavailable curcumin formulation with 95% curcumin: a randomized, double-blind, crossover study, J Am Coll Nutr
Teli, Shah, Chhabria, In silico screening of natural compounds as potential inhibitors of SARS-CoV-2 main protease and spike RBD: targets for COVID-19, Front Mol Biosci, doi:10.3389/fmolb.2020.599079
Vahedian-Azimi, Abbasifard, Rahimi-Bashar, Effectiveness of curcumin on outcomes of hospitalized COVID-19 patients: a systematic review of clinical trials, Nutrients
Vangeel, Chiu, Jonghe, Molnupiravir and Nirmatrelvir remain active against SARS-CoV-2 Omicron and other variants of concern, Antiviral Res
Voysey, Clemens, Madhi, Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK, Lancet
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Zhang, Zeng, Pan, Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial, Lancet Infect Dis
Zhang, Zhang, Li, A cell-based large-scale screening of natural compounds for inhibitors of SARS-CoV-2, Signal Transduct Target Ther
DOI record:
{
"DOI": "10.1177/1934578x231188861",
"ISSN": [
"1934-578X",
"1555-9475"
],
"URL": "http://dx.doi.org/10.1177/1934578X231188861",
"abstract": "<jats:p> Since the COVID-19 pandemic in 2020, many reports have highlighted several potential anti-SARS-CoV-2 drug candidates, including phenolic compounds. Therefore, this study aimed to evaluate the anti-SARS-CoV-2 activity of nine common phenolic compounds found in plants using the in vitro cellular infection model. The anti-SARS-CoV-2 activity of curcumin, quercetin, gallic acid, catechin, rutin, kaempferol, naringenin, coumaric acid and caffeic acid were evaluated on SARS-CoV-2-infected Vero E6 cells by using a cytopathic effect (CPE)-based assay. The anti-SARS-CoV-2 activity in human lung cells, A549 expressing human ACE2 and TMPRSS2, was evaluated by the RT-qPCR technique. S1-ACE2 interaction and 3CL protease activity assays were also performed for the potent compound. Of the nine phenolic compounds, only curcumin inhibited the SARS-CoV-2 induced CPE activity (EC<jats:sub>50</jats:sub> of 13.63 µM) in Vero E6 cells, but with a low selective index (SI) value. Interestingly, curcumin exhibited potent anti-SARS-CoV-2 activity in A549 cells with an EC<jats:sub>50</jats:sub> of 4.57 µM and an SI value of 7.96. S1-ACE2 interaction and 3CL protease inhibitory activities of curcumin were also observed. In conclusion, curcumin showed a moderate in vitro anti-SARS-CoV-2 activity. The true potential of curcumin as an anti-SARS-CoV-2 candidate could be further evaluated in a COVID-19 animal model. </jats:p>",
"alternative-id": [
"10.1177/1934578X231188861"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-9589-5892",
"affiliation": [
{
"name": "Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"authenticated-orcid": false,
"family": "Mohd Abd Razak",
"given": "Mohd Ridzuan",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Md Jelas",
"given": "Nur Hana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Muhammad",
"given": "Amirrudin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Padlan",
"given": "Noorsofiana",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Sa’at",
"given": "Muhammad Nor Farhan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Azizan",
"given": "Muhammad Afif",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Rosli",
"given": "Siti Nur Zawani",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Ellan",
"given": "E. Kavithambigai",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Zainol",
"given": "Murizal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Thayan",
"given": "Ravindran",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Herbal Medicine Research Centre, Institute for Medical Research, National Institutes of Health, Ministry of Health Malaysia, Selangor, Malaysia"
}
],
"family": "Syed Mohamed",
"given": "Ami Fazlin",
"sequence": "additional"
}
],
"container-title": "Natural Product Communications",
"container-title-short": "Natural Product Communications",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2023,
9,
16
]
],
"date-time": "2023-09-16T10:05:15Z",
"timestamp": 1694858715000
},
"deposited": {
"date-parts": [
[
2023,
9,
16
]
],
"date-time": "2023-09-16T10:05:20Z",
"timestamp": 1694858720000
},
"funder": [
{
"DOI": "10.13039/501100013885",
"award": [
"NMRR-20-874-54715 (20-035)"
],
"doi-asserted-by": "publisher",
"name": "Kementerian Kesihatan Malaysia"
}
],
"indexed": {
"date-parts": [
[
2023,
9,
17
]
],
"date-time": "2023-09-17T14:42:47Z",
"timestamp": 1694961767447
},
"is-referenced-by-count": 0,
"issue": "9",
"issued": {
"date-parts": [
[
2023,
9
]
]
},
"journal-issue": {
"issue": "9",
"published-print": {
"date-parts": [
[
2023,
9
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
9,
1
]
],
"date-time": "2023-09-01T00:00:00Z",
"timestamp": 1693526400000
}
}
],
"link": [
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/1934578X231188861",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/full-xml/10.1177/1934578X231188861",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://journals.sagepub.com/doi/pdf/10.1177/1934578X231188861",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"prefix": "10.1177",
"published": {
"date-parts": [
[
2023,
9
]
]
},
"published-online": {
"date-parts": [
[
2023,
9,
16
]
]
},
"published-print": {
"date-parts": [
[
2023,
9
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1016/S1473-3099(20)30843-4",
"doi-asserted-by": "publisher",
"key": "bibr1-1934578X231188861"
},
{
"DOI": "10.1016/S0140-6736(20)32661-1",
"doi-asserted-by": "publisher",
"key": "bibr2-1934578X231188861"
},
{
"DOI": "10.1056/NEJMoa2034577",
"doi-asserted-by": "publisher",
"key": "bibr3-1934578X231188861"
},
{
"DOI": "10.1056/NEJMc2104974",
"doi-asserted-by": "publisher",
"key": "bibr4-1934578X231188861"
},
{
"DOI": "10.1038/s41579-021-00573-0",
"doi-asserted-by": "publisher",
"key": "bibr5-1934578X231188861"
},
{
"DOI": "10.1056/NEJMoa2114114",
"doi-asserted-by": "publisher",
"key": "bibr6-1934578X231188861"
},
{
"DOI": "10.1001/jama.2022.0470",
"doi-asserted-by": "publisher",
"key": "bibr7-1934578X231188861"
},
{
"DOI": "10.1016/j.drup.2021.100794",
"doi-asserted-by": "publisher",
"key": "bibr8-1934578X231188861"
},
{
"DOI": "10.1126/scitranslmed.abl7430",
"doi-asserted-by": "publisher",
"key": "bibr9-1934578X231188861"
},
{
"DOI": "10.1126/science.acx9605",
"doi-asserted-by": "publisher",
"key": "bibr10-1934578X231188861"
},
{
"DOI": "10.1016/j.antiviral.2022.105252",
"doi-asserted-by": "publisher",
"key": "bibr11-1934578X231188861"
},
{
"DOI": "10.3390/ph14030213",
"doi-asserted-by": "publisher",
"key": "bibr12-1934578X231188861"
},
{
"DOI": "10.3389/fphar.2020.592737",
"doi-asserted-by": "publisher",
"key": "bibr13-1934578X231188861"
},
{
"DOI": "10.1021/acs.jnatprod.0c01324",
"doi-asserted-by": "publisher",
"key": "bibr14-1934578X231188861"
},
{
"DOI": "10.1038/s41392-020-00343-z",
"doi-asserted-by": "publisher",
"key": "bibr15-1934578X231188861"
},
{
"author": "Agrawal PK",
"first-page": "1934578X",
"issue": "12",
"journal-title": "Nat Prod Commun.",
"key": "bibr16-1934578X231188861",
"volume": "16",
"year": "2021"
},
{
"author": "Agrawal PK",
"first-page": "1934578X",
"issue": "4",
"journal-title": "Nat Prod Commun.",
"key": "bibr17-1934578X231188861",
"volume": "16",
"year": "2021"
},
{
"author": "Agrawal PK",
"first-page": "1934578X",
"issue": "12",
"journal-title": "Nat Prod Commun.",
"key": "bibr18-1934578X231188861",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.3390/molecules26226900",
"doi-asserted-by": "publisher",
"key": "bibr19-1934578X231188861"
},
{
"DOI": "10.1016/j.foodchem.2021.131594",
"doi-asserted-by": "publisher",
"key": "bibr20-1934578X231188861"
},
{
"DOI": "10.3390/molecules26071924",
"doi-asserted-by": "publisher",
"key": "bibr21-1934578X231188861"
},
{
"DOI": "10.3390/ijms22137048",
"doi-asserted-by": "publisher",
"key": "bibr22-1934578X231188861"
},
{
"DOI": "10.3390/molecules26196062",
"doi-asserted-by": "publisher",
"key": "bibr23-1934578X231188861"
},
{
"DOI": "10.1007/s11101-021-09759-z",
"doi-asserted-by": "publisher",
"key": "bibr24-1934578X231188861"
},
{
"DOI": "10.1038/s41598-021-81462-7",
"doi-asserted-by": "publisher",
"key": "bibr25-1934578X231188861"
},
{
"DOI": "10.3390/v13101914",
"doi-asserted-by": "publisher",
"key": "bibr26-1934578X231188861"
},
{
"author": "Elfiky AA",
"first-page": "3194",
"issue": "9",
"journal-title": "J Biomol Struct Dyn",
"key": "bibr27-1934578X231188861",
"volume": "39",
"year": "2021"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "bibr28-1934578X231188861"
},
{
"DOI": "10.1016/0022-1759(83)90303-4",
"doi-asserted-by": "publisher",
"key": "bibr29-1934578X231188861"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"doi-asserted-by": "publisher",
"key": "bibr30-1934578X231188861"
},
{
"DOI": "10.1371/journal.pone.0253489",
"doi-asserted-by": "publisher",
"key": "bibr31-1934578X231188861"
},
{
"DOI": "10.3389/fmolb.2020.599079",
"doi-asserted-by": "publisher",
"key": "bibr32-1934578X231188861"
},
{
"DOI": "10.1080/07315724.2017.1358118",
"doi-asserted-by": "publisher",
"key": "bibr33-1934578X231188861"
},
{
"DOI": "10.3390/nu14020256",
"doi-asserted-by": "publisher",
"key": "bibr34-1934578X231188861"
},
{
"DOI": "10.3390/molecules26195844",
"doi-asserted-by": "publisher",
"key": "bibr35-1934578X231188861"
},
{
"DOI": "10.1111/dom.13868",
"doi-asserted-by": "publisher",
"key": "bibr36-1934578X231188861"
},
{
"DOI": "10.1016/j.antiviral.2020.104786",
"doi-asserted-by": "publisher",
"key": "bibr37-1934578X231188861"
},
{
"DOI": "10.1038/s41598-020-77003-3",
"doi-asserted-by": "publisher",
"key": "bibr38-1934578X231188861"
},
{
"DOI": "10.1038/s41586-020-2575-3",
"doi-asserted-by": "publisher",
"key": "bibr39-1934578X231188861"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "http://journals.sagepub.com/doi/10.1177/1934578X231188861"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Complementary and alternative medicine",
"Plant Science",
"Drug Discovery",
"Pharmacology",
"General Medicine"
],
"subtitle": [],
"title": "In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1177/sage-journals-update-policy",
"volume": "18"
}
mohdabdrazak
