Hydroxychloroquine attenuates double-stranded RNA-stimulated hyper-phosphorylation of tristetraprolin/ZFP36 and AU-rich mRNA stabilization
et al., Immunology, doi:10.1111/imm.13835, Jul 2024
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study showing that HCQ reduces inflammation by inhibiting the double-stranded RNA-stimulated phosphorylation of tristetraprolin (TTP) and decreasing the stability of AU-rich mRNAs. This suggests that HCQ could mitigate excessive inflammatory responses, such as cytokine storms, which are characteristic of severe COVID-19 and other viral infections.
39 preclinical studies support the efficacy of HCQ for COVID-19:
1.
Shang et al., Identification of Cathepsin L as the molecular target of hydroxychloroquine with chemical proteomics, Molecular & Cellular Proteomics, doi:10.1016/j.mcpro.2025.101314.
2.
González-Paz et al., Biophysical Analysis of Potential Inhibitors of SARS-CoV-2 Cell Recognition and Their Effect on Viral Dynamics in Different Cell Types: A Computational Prediction from In Vitro Experimental Data, ACS Omega, doi:10.1021/acsomega.3c06968.
3.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
4.
Guimarães Silva et al., Are Non-Structural Proteins From SARS-CoV-2 the Target of Hydroxychloroquine? An in Silico Study, ACTA MEDICA IRANICA, doi:10.18502/acta.v61i2.12533.
5.
Nguyen et al., The Potential of Ameliorating COVID-19 and Sequelae From Andrographis paniculata via Bioinformatics, Bioinformatics and Biology Insights, doi:10.1177/11779322221149622.
7.
Yadav et al., Repurposing the Combination Drug of Favipiravir, Hydroxychloroquine and Oseltamivir as a Potential Inhibitor Against SARS-CoV-2: A Computational Study, Research Square, doi:10.21203/rs.3.rs-628277/v1.
8.
Hussein et al., Molecular Docking Identification for the efficacy of Some Zinc Complexes with Chloroquine and Hydroxychloroquine against Main Protease of COVID-19, Journal of Molecular Structure, doi:10.1016/j.molstruc.2021.129979.
9.
Baildya et al., Inhibitory capacity of Chloroquine against SARS-COV-2 by effective binding with Angiotensin converting enzyme-2 receptor: An insight from molecular docking and MD-simulation studies, Journal of Molecular Structure, doi:10.1016/j.molstruc.2021.129891.
10.
Noureddine et al., Quantum chemical studies on molecular structure, AIM, ELF, RDG and antiviral activities of hybrid hydroxychloroquine in the treatment of COVID-19: molecular docking and DFT calculations, Journal of King Saud University - Science, doi:10.1016/j.jksus.2020.101334.
11.
Tarek et al., Pharmacokinetic Basis of the Hydroxychloroquine Response in COVID-19: Implications for Therapy and Prevention, European Journal of Drug Metabolism and Pharmacokinetics, doi:10.1007/s13318-020-00640-6.
12.
Rowland Yeo et al., Impact of Disease on Plasma and Lung Exposure of Chloroquine, Hydroxychloroquine and Azithromycin: Application of PBPK Modeling, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.1955.
13.
Hitti et al., Hydroxychloroquine attenuates double-stranded RNA-stimulated hyper-phosphorylation of tristetraprolin/ZFP36 and AU-rich mRNA stabilization, Immunology, doi:10.1111/imm.13835.
14.
Yan et al., Super-resolution imaging reveals the mechanism of endosomal acidification inhibitors against SARS-CoV-2 infection, ChemBioChem, doi:10.1002/cbic.202400404.
15.
Mohd Abd Razak et al., In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds, Natural Product Communications, doi:10.1177/1934578X231188861.
16.
Alsmadi et al., The In Vitro, In Vivo, and PBPK Evaluation of a Novel Lung-Targeted Cardiac-Safe Hydroxychloroquine Inhalation Aerogel, AAPS PharmSciTech, doi:10.1208/s12249-023-02627-3.
17.
Wen et al., Cholinergic α7 nAChR signaling suppresses SARS-CoV-2 infection and inflammation in lung epithelial cells, Journal of Molecular Cell Biology, doi:10.1093/jmcb/mjad048.
18.
Kamga Kapchoup et al., In vitro effect of hydroxychloroquine on pluripotent stem cells and their cardiomyocytes derivatives, Frontiers in Pharmacology, doi:10.3389/fphar.2023.1128382.
19.
Milan Bonotto et al., Cathepsin inhibitors nitroxoline and its derivatives inhibit SARS-CoV-2 infection, Antiviral Research, doi:10.1016/j.antiviral.2023.105655.
20.
Miao et al., SIM imaging resolves endocytosis of SARS-CoV-2 spike RBD in living cells, Cell Chemical Biology, doi:10.1016/j.chembiol.2023.02.001.
21.
Yuan et al., Hydroxychloroquine blocks SARS-CoV-2 entry into the endocytic pathway in mammalian cell culture, Communications Biology, doi:10.1038/s42003-022-03841-8.
22.
Faísca et al., Enhanced In Vitro Antiviral Activity of Hydroxychloroquine Ionic Liquids against SARS-CoV-2, Pharmaceutics, doi:10.3390/pharmaceutics14040877.
23.
Delandre et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445.
24.
Purwati et al., An in vitro study of dual drug combinations of anti-viral agents, antibiotics, and/or hydroxychloroquine against the SARS-CoV-2 virus isolated from hospitalized patients in Surabaya, Indonesia, PLOS One, doi:10.1371/journal.pone.0252302.
25.
Zhang et al., SARS-CoV-2 spike protein dictates syncytium-mediated lymphocyte elimination, Cell Death & Differentiation, doi:10.1038/s41418-021-00782-3.
26.
Dang et al., Structural basis of anti-SARS-CoV-2 activity of hydroxychloroquine: specific binding to NTD/CTD and disruption of LLPS of N protein, bioRxiv, doi:10.1101/2021.03.16.435741.
27.
Shang (B) et al., Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice, Virology Journal, doi:10.1186/s12985-021-01515-1.
28.
Wang et al., Chloroquine and hydroxychloroquine as ACE2 blockers to inhibit viropexis of 2019-nCoV Spike pseudotyped virus, Phytomedicine, doi:10.1016/j.phymed.2020.153333.
29.
Sheaff, R., A New Model of SARS-CoV-2 Infection Based on (Hydroxy)Chloroquine Activity, bioRxiv, doi:10.1101/2020.08.02.232892.
30.
Ou et al., Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2, PLOS Pathogens, doi:10.1371/journal.ppat.1009212.
31.
Andreani et al., In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect, Microbial Pathogenesis, doi:10.1016/j.micpath.2020.104228.
32.
Clementi et al., Combined Prophylactic and Therapeutic Use Maximizes Hydroxychloroquine Anti-SARS-CoV-2 Effects in vitro, Front. Microbiol., 10 July 2020, doi:10.3389/fmicb.2020.01704.
33.
Liu et al., Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discovery 6, 16 (2020), doi:10.1038/s41421-020-0156-0.
34.
Yao et al., In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin. Infect. Dis., 2020 Mar 9, doi:10.1093/cid/ciaa237.
Hitti et al., 24 Jul 2024, peer-reviewed, 5 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Hydroxychloroquine attenuates double‐stranded RNA‐stimulated hyper‐phosphorylation of tristetraprolin/ZFP36 and AU‐rich mRNA stabilization
Immunology, doi:10.1111/imm.13835
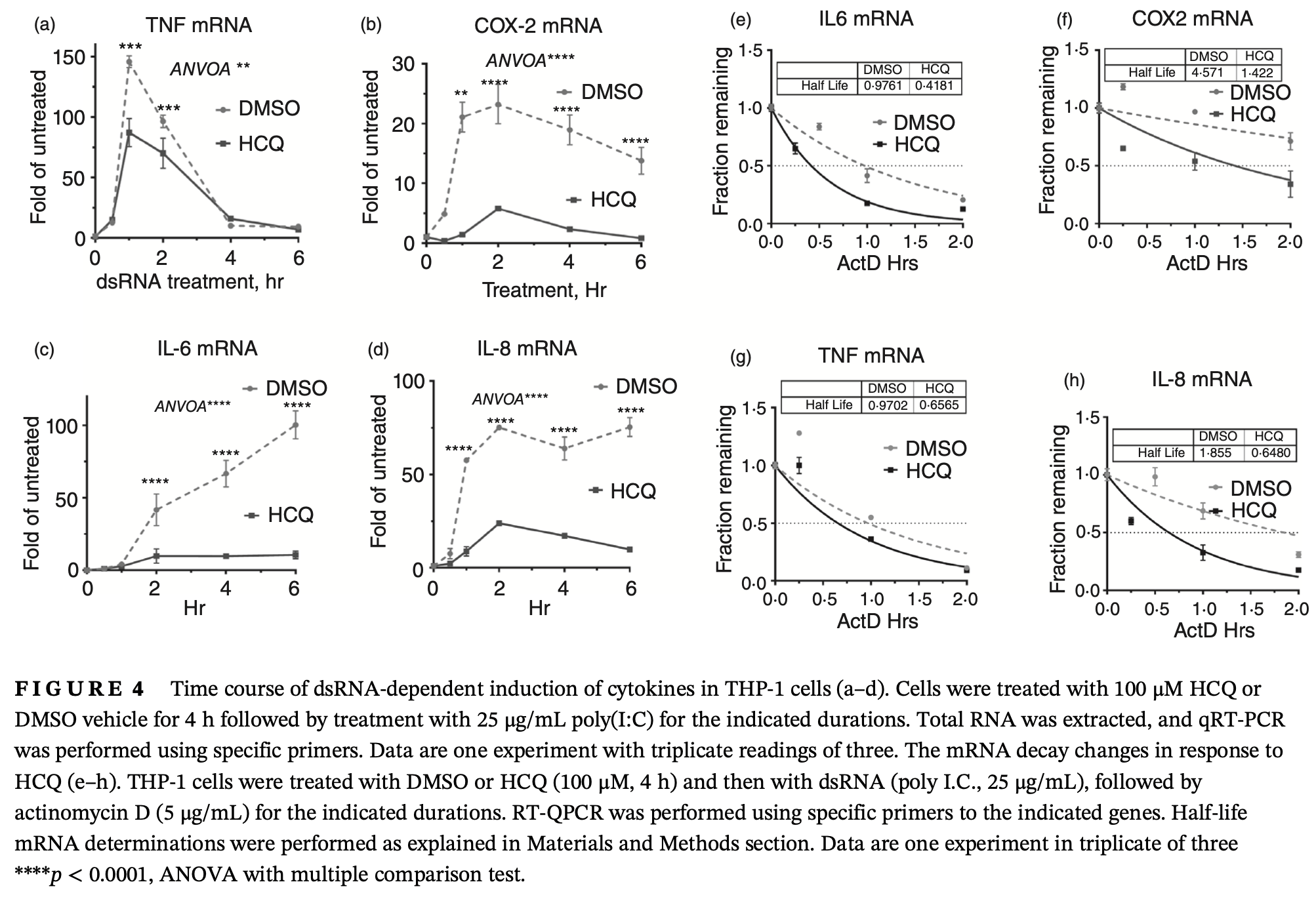
The human innate immune system recognizes dsRNA as a pathogen-associated molecular pattern that induces a potent inflammatory response. The primary source of pathogenic dsRNA is cells infected with replicating viruses, but can also be released from uninfected necrotic cells. Here, we show that the dsRNA poly(I:C) challenge in human macrophages activates the p38 MAPK-MK2 signalling pathway and subsequently the phosphorylation of tristetraprolin (TTP/ZFP36). The latter is an mRNA decaypromoting protein that controls the stability of AU-rich mRNAs (AREs) that code for many inflammatory mediators. Hydroxychloroquine (HCQ), a common anti-malaria drug, is used to treat inflammatory and autoimmune disorders and, controversially, during acute COVID-19 disease. We found that HCQ reduced the dsRNA-dependent phosphorylation of p38 MAPK and its downstream kinase MK2. Subsequently, HCQ reduced the abundance and protein stability of the inactive (phosphorylated) form of TTP. HCQ reduced the levels and the mRNA stability of poly (I:C)-induced cytokines and inflammatory mRNAs like TNF, IL-6, COX-2, and IL-8 in THP-1 and primary blood monocytes. Our results demonstrate a new mechanism of the anti-inflammatory role of HCQ at post-transcriptional level (TTP phosphorylation) in a model of dsRNA activation, which usually occurs in viral infections or RNA release from necrotic tissue.
AUTHOR CONTRIBUTIONS Concept, Design, Data analysis, and Writing: (E. Hitti, K. Khabar); Performance of experiments and data acquisition (Z. Muazzen, W. Moghrabi, S. Al-Yahya). All authors contributed to the article and approved the submitted version.
CONFLICT OF INTEREST STATEMENT The authors report no conflicts of interest.
References
Bakheet, Hitti, Khabar, ARED-plus: an updated and expanded database of AU-rich element-containing mRNAs and pre-mRNAs, Nucleic Acids Res
Brentano, Schorr, Gay, Gay, Kyburz et al., Hydroxychloroquine attenuates double-stranded RNA-stimulated hyper-phosphorylation of tristetraprolin/ZFP36 and AU-rich mRNA stabilization, Arthritis Rheum
Canovas, Nebreda, Diversity and versatility of p38 kinase signalling in health and disease, Nat Rev Mol Cell Biol
Cao, Deterding, Blackshear, Phosphorylation site analysis of the anti-inflammatory and mRNA-destabilizing protein tristetraprolin, Expert Rev Proteomics
Circu, Cardelli, Barr, Byrne, Mills et al., Modulating lysosomal function through lysosome membrane permeabilization or autophagy suppression restores sensitivity to cisplatin in refractory non-small-cell lung cancer cells, PLoS One
Clement, Scheckel, Stoecklin, Lykke-Andersen, Phosphorylation of tristetraprolin by MK2 impairs AU-rich element mRNA decay by preventing deadenylase recruitment, Mol Cell Biol
Gautret, Lagier, Parola, Hoang, Meddeb et al., Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label nonrandomized clinical trial, Int J Antimicrob Agents
Geleris, Sun, Platt, Zucker, Baldwin et al., Observational study of hydroxychloroquine in hospitalized patients with COVID-19, N Engl J Med
Gies, Bekaddour, Dieudonne, Guffroy, Frenger et al., Beyond anti-viral effects of chloroquine/hydroxychloroquine, Front Immunol
Hashem, Alghamdi, Algaissi, Alshehri, Bukhari et al., Therapeutic use of chloroquine and hydroxychloroquine in COVID-19 and other viral infections: a narrative review, Travel Med Infect Dis
Hitti, Iakovleva, Brook, Deppenmeier, Gruber et al., Mitogen-activated protein kinase-activated protein kinase 2 regulates tumor necrosis factor mRNA stability and translation mainly by altering tristetraprolin expression, stability, and binding to adenine/uridine-rich element, Mol Cell Biol
Hitti, Khabar, Sequence variations affecting AU-rich element function and disease, Front Biosci
Jang, Choi, Byun, Jue, Chloroquine inhibits production of TNF-alpha, IL-1beta and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes, Rheumatology
Johnsen, Nguyen, Bergstrom, Anthonsen, Toll-like receptor 3-elicited MAPK activation induces stabilization of interferon-beta mRNA, Cytokine
Khabar, Post-transcriptional control during chronic inflammation and cancer: a focus on AU-rich elements, Cell Mol Life Sci
Khabar, Post-transcriptional control of cytokine gene expression in health and disease, J Interferon Cytokine Res
Kuznik, Bencina, Svajger, Jeras, Rozman et al., Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines, J Immunol
Kyburz, Brentano, Gay, Mode of action of hydroxychloroquine in RA-evidence of an inhibitory effect on toll-like receptor signaling, Nat Clin Pract Rheumatol
Li, Wu, Pattern recognition receptors in health and diseases, Signal Transduct Target Ther
Liu, Cao, Xu, Wang, Zhang et al., Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discov
Mahmoud, Abdulkarim, Kutbi, Moghrabi, Altwijri et al., Post-transcriptional inflammatory response to intracellular bacterial c-di-AMP, Front Immunol
Mahmoud, Al-Enezi, Al-Saif, Warsy, Khabar et al., Sustained stabilization of Interleukin-8 mRNA in human macrophages, RNA Biol
Marchese, Aubareda, Tudor, Saklatvala, Clark et al., MAPKAP kinase 2 blocks tristetraprolin-directed mRNA decay by inhibiting CAF1 deadenylase recruitment, J Biol Chem
Mogensen, Pathogen recognition and inflammatory signaling in innate immune defenses, Clin Microbiol Rev
Moghrabi, Al-Yahya, Al-Haj, Al-Saif, Kinome inhibition reveals a role for polo-like kinase 1 in targeting post-transcriptional control in cancer, Mol Oncol
Nguyen, Smith, Tate, Belz, Barrios et al., SIDT2 transports extracellular dsRNA into the cytoplasm for innate immune recognition, Immunity
Olsen, Schleich, Karp, Multifaceted effects of hydroxychloroquine in human disease, Semin Arthritis Rheum
Pedersen, Ho, SARS-CoV-2: a storm is raging, J Clin Invest
Ponticelli, Moroni, Hydroxychloroquine in systemic lupus erythematosus (SLE), Expert Opin Drug Saf
Reddin, Fenton, Wass, Michaelis, Large inherent variability in data derived from highly standardised cell culture experiments, Pharmacol Res
Ronkina, Shushakova, Tiedje, Yakovleva, Tollenaere et al., The role of TTP phosphorylation in the regulation of inflammatory cytokine production by MK2/3, J Immunol
Sato, Imaizumi, Aizawa, Watanabe, Tsugawa et al., Inhibitory effect of anti-malarial agents on the expression of proinflammatory chemokines via toll-like receptor 3 signaling in human glomerular endothelial cells, Ren Fail
Schrezenmeier, Dorner, Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology, Nat Rev Rheumatol
Self, Semler, Leither, Casey, Angus et al., Network, effect of hydroxychloroquine on clinical status at 14 days in hospitalized patients with COVID-19: a randomized clinical trial, JAMA
Steer, Moran, Christmann, Lb, Corbett, Role of MAPK in the regulation of double-stranded RNA-and encephalomyocarditis virus-induced cyclooxygenase-2 expression by macrophages, J Immunol
Sun, Stoecklin, Van Way, Hinkovska-Galcheva, Guo et al., Tristetraprolin (TTP)-14-3-3 complex formation protects TTP from dephosphorylation by protein phosphatase 2a and stabilizes tumor necrosis factor-α mRNA, J Biol Chem
Tatematsu, Funami, Seya, Matsumoto, Extracellular RNA sensing by pattern recognition receptors, J Innate Immun
Thwaites, Chamberlain, Sacre, Emerging role of endosomal toll-like receptors in rheumatoid arthritis, Front Immunol
Tiedje, Diaz-Muñoz, Trulley, Ahlfors, Laaß et al., The RNA-binding protein TTP is a global post-transcriptional regulator of feedback control in inflammation, Nucleic Acids Res
Zhang, Zhao, Zhang, Wang, Li et al., The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China, Clin Immunol
