Enhanced In Vitro Antiviral Activity of Hydroxychloroquine Ionic Liquids against SARS-CoV-2
et al., Pharmaceutics, doi:10.3390/pharmaceutics14040877, Apr 2022
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 423 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
In vitro study showing improved antiviral activity with ionic formulations of HCQ.
39 preclinical studies support the efficacy of HCQ for COVID-19:
1.
Shang et al., Identification of Cathepsin L as the molecular target of hydroxychloroquine with chemical proteomics, Molecular & Cellular Proteomics, doi:10.1016/j.mcpro.2025.101314.
2.
González-Paz et al., Biophysical Analysis of Potential Inhibitors of SARS-CoV-2 Cell Recognition and Their Effect on Viral Dynamics in Different Cell Types: A Computational Prediction from In Vitro Experimental Data, ACS Omega, doi:10.1021/acsomega.3c06968.
3.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
4.
Guimarães Silva et al., Are Non-Structural Proteins From SARS-CoV-2 the Target of Hydroxychloroquine? An in Silico Study, ACTA MEDICA IRANICA, doi:10.18502/acta.v61i2.12533.
5.
Nguyen et al., The Potential of Ameliorating COVID-19 and Sequelae From Andrographis paniculata via Bioinformatics, Bioinformatics and Biology Insights, doi:10.1177/11779322221149622.
7.
Yadav et al., Repurposing the Combination Drug of Favipiravir, Hydroxychloroquine and Oseltamivir as a Potential Inhibitor Against SARS-CoV-2: A Computational Study, Research Square, doi:10.21203/rs.3.rs-628277/v1.
8.
Hussein et al., Molecular Docking Identification for the efficacy of Some Zinc Complexes with Chloroquine and Hydroxychloroquine against Main Protease of COVID-19, Journal of Molecular Structure, doi:10.1016/j.molstruc.2021.129979.
9.
Baildya et al., Inhibitory capacity of Chloroquine against SARS-COV-2 by effective binding with Angiotensin converting enzyme-2 receptor: An insight from molecular docking and MD-simulation studies, Journal of Molecular Structure, doi:10.1016/j.molstruc.2021.129891.
10.
Noureddine et al., Quantum chemical studies on molecular structure, AIM, ELF, RDG and antiviral activities of hybrid hydroxychloroquine in the treatment of COVID-19: molecular docking and DFT calculations, Journal of King Saud University - Science, doi:10.1016/j.jksus.2020.101334.
11.
Tarek et al., Pharmacokinetic Basis of the Hydroxychloroquine Response in COVID-19: Implications for Therapy and Prevention, European Journal of Drug Metabolism and Pharmacokinetics, doi:10.1007/s13318-020-00640-6.
12.
Rowland Yeo et al., Impact of Disease on Plasma and Lung Exposure of Chloroquine, Hydroxychloroquine and Azithromycin: Application of PBPK Modeling, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.1955.
13.
Hitti et al., Hydroxychloroquine attenuates double-stranded RNA-stimulated hyper-phosphorylation of tristetraprolin/ZFP36 and AU-rich mRNA stabilization, Immunology, doi:10.1111/imm.13835.
14.
Yan et al., Super-resolution imaging reveals the mechanism of endosomal acidification inhibitors against SARS-CoV-2 infection, ChemBioChem, doi:10.1002/cbic.202400404.
15.
Mohd Abd Razak et al., In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds, Natural Product Communications, doi:10.1177/1934578X231188861.
16.
Alsmadi et al., The In Vitro, In Vivo, and PBPK Evaluation of a Novel Lung-Targeted Cardiac-Safe Hydroxychloroquine Inhalation Aerogel, AAPS PharmSciTech, doi:10.1208/s12249-023-02627-3.
17.
Wen et al., Cholinergic α7 nAChR signaling suppresses SARS-CoV-2 infection and inflammation in lung epithelial cells, Journal of Molecular Cell Biology, doi:10.1093/jmcb/mjad048.
18.
Kamga Kapchoup et al., In vitro effect of hydroxychloroquine on pluripotent stem cells and their cardiomyocytes derivatives, Frontiers in Pharmacology, doi:10.3389/fphar.2023.1128382.
19.
Milan Bonotto et al., Cathepsin inhibitors nitroxoline and its derivatives inhibit SARS-CoV-2 infection, Antiviral Research, doi:10.1016/j.antiviral.2023.105655.
20.
Miao et al., SIM imaging resolves endocytosis of SARS-CoV-2 spike RBD in living cells, Cell Chemical Biology, doi:10.1016/j.chembiol.2023.02.001.
21.
Yuan et al., Hydroxychloroquine blocks SARS-CoV-2 entry into the endocytic pathway in mammalian cell culture, Communications Biology, doi:10.1038/s42003-022-03841-8.
22.
Faísca et al., Enhanced In Vitro Antiviral Activity of Hydroxychloroquine Ionic Liquids against SARS-CoV-2, Pharmaceutics, doi:10.3390/pharmaceutics14040877.
23.
Delandre et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445.
24.
Purwati et al., An in vitro study of dual drug combinations of anti-viral agents, antibiotics, and/or hydroxychloroquine against the SARS-CoV-2 virus isolated from hospitalized patients in Surabaya, Indonesia, PLOS One, doi:10.1371/journal.pone.0252302.
25.
Zhang et al., SARS-CoV-2 spike protein dictates syncytium-mediated lymphocyte elimination, Cell Death & Differentiation, doi:10.1038/s41418-021-00782-3.
26.
Dang et al., Structural basis of anti-SARS-CoV-2 activity of hydroxychloroquine: specific binding to NTD/CTD and disruption of LLPS of N protein, bioRxiv, doi:10.1101/2021.03.16.435741.
27.
Shang (B) et al., Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice, Virology Journal, doi:10.1186/s12985-021-01515-1.
28.
Wang et al., Chloroquine and hydroxychloroquine as ACE2 blockers to inhibit viropexis of 2019-nCoV Spike pseudotyped virus, Phytomedicine, doi:10.1016/j.phymed.2020.153333.
29.
Sheaff, R., A New Model of SARS-CoV-2 Infection Based on (Hydroxy)Chloroquine Activity, bioRxiv, doi:10.1101/2020.08.02.232892.
30.
Ou et al., Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2, PLOS Pathogens, doi:10.1371/journal.ppat.1009212.
31.
Andreani et al., In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect, Microbial Pathogenesis, doi:10.1016/j.micpath.2020.104228.
32.
Clementi et al., Combined Prophylactic and Therapeutic Use Maximizes Hydroxychloroquine Anti-SARS-CoV-2 Effects in vitro, Front. Microbiol., 10 July 2020, doi:10.3389/fmicb.2020.01704.
33.
Liu et al., Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discovery 6, 16 (2020), doi:10.1038/s41421-020-0156-0.
34.
Yao et al., In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin. Infect. Dis., 2020 Mar 9, doi:10.1093/cid/ciaa237.
35.
Wang (B) et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res. 30, 269–271, doi:10.1038/s41422-020-0282-0.
36.
Shu-Han Lin et al., Inhalable Chitosan-Based Hydrogel as a Mucosal Adjuvant for Hydroxychloroquine in the Treatment for SARS-CoV-2 Infection in a Hamster Model, Journal of Microbiology, Immunology and Infection, doi:10.1016/j.jmii.2023.08.001.
37.
Zelenko, Z., Nebulized Hydroxychloroquine for COVID-19 Treatment: 80x Improvement in Breathing, Preprint, faculty.utrgv.edu/eleftherios.gkioulekas/zelenko/Zelenko-nebulized-hcq.pdf.
Faísca et al., 17 Apr 2022, peer-reviewed, 6 authors.
In vitro studies are an important part of preclinical research, however results may be very different in vivo.
Enhanced In Vitro Antiviral Activity of Hydroxychloroquine Ionic Liquids against SARS-CoV-2
Pharmaceutics, doi:10.3390/pharmaceutics14040877
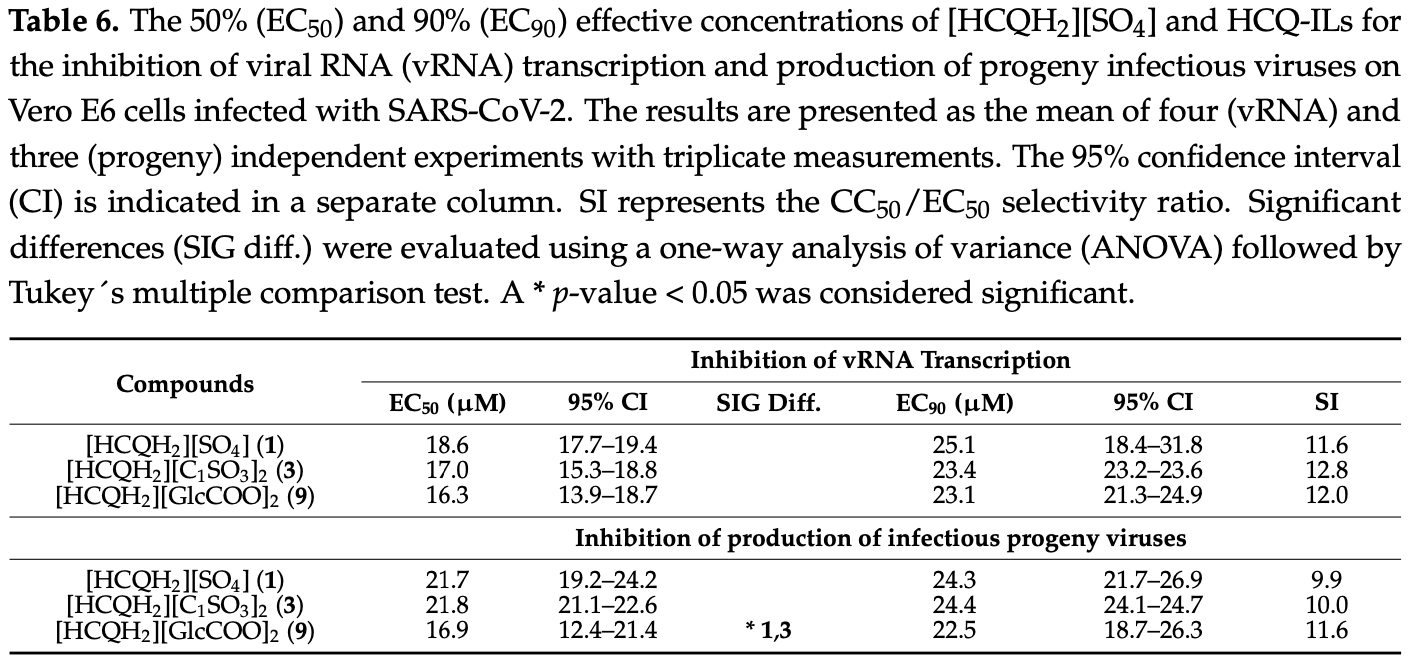
The development of effective antiviral drugs against SARS-CoV-2 is urgently needed and a global health priority. In light of the initial data regarding the repurposing of hydroxychloroquine (HCQ) to tackle this coronavirus, herein we present a quantitative synthesis and spectroscopic and thermal characterization of seven HCQ room temperature ionic liquids (HCQ-ILs) obtained by direct protonation of the base with two equivalents of organic sulfonic, sulfuric and carboxylic acids of different polarities. Two non-toxic and hydrophilic HCQ-ILs, in particular, [HCQH 2 ][C 1 SO 3 ] 2 and [HCQH 2 ][GlcCOO] 2 , decreased the virus-induced cytopathic effect by two-fold in comparison with the original drug, [HCQH 2 ][SO 4 ]. Despite there being no significant differences in viral RNA production between the three compounds, progeny virus production was significantly affected (p < 0.05) by [HCQH 2 ][GlcCOO] 2 . Overall, the data suggest that the in vitro antiviral activities of the HCQ-ILs are most likely the result of specific intra-and intermolecular interactions and not so much related with their hydrophilic or lipophilic character. This work paves the way for the development of future novel ionic formulations of hydroxychloroquine with enhanced physicochemical properties.
(9) resulted in more than 60% inhibition of CPE at 10 µ M (70.5%, 60.8% and 62.5%, respectively), which contrasted with the remaining compounds, including 1 (lower than 20%; see Figure 3A ). The EC50 values of these three novel formulations (8.1, 8.9 and 8.5 µ M, respectively) were significantly lower than those of all other HCQ-ILs and differed by ca. two-fold from the EC50 of 1 (16.5 µ M) (Table 5 ). No significant differences in EC90 were observed between the seven novel HCQ-ILs and 1, with all values being registered beyond 20 µM. Table 5 . The 50% (EC50) and 90% (EC90) effective concentrations of [HCQH2][SO4] (1) and HCQ-ILs 3-9 for the inhibition of the virus-induced cytopathic effect (CPE) on Vero E6 cells infected with SARS-CoV-2. The results are presented as the mean of three independent experiments with triplicate measurements. The 95% confidence interval (CI) is indicated in a separate column. SI represents the CC50/EC50 selectivity ratio. No inhibitory effects were found for the anions. Significant differences (SIG diff.) were evaluated using a one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test. A p-value < 0.05 was considered significant, with * p < 0.05, ** p < 0.01, *** p < 0.001. The enhanced antiviral activity of 3 and 9 in this initial screening doubled their SI ratio (26.9 and 23.1, respectively) in comparison with 1 (13.0) (Table 5 ), leading them to be identified as the most promising HCQ-ILs. Hence, these..
References
Al-Sabagh, Azzam, Mahmoud, Saleh, Synthesis of Ethoxylated Alkyl Sulfosuccinate Surfactants and the Investigation of Mixed Solutions, J. Surfactants Deterg, doi:10.1007/s11743-006-1000-8
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of Covid-19-Final Report, N. Engl. J. Med, doi:10.1056/NEJMoa2007764
Ben-Zvi, Kivity, Langevitz, Shoenfeld, Hydroxychloroquine: From Malaria to Autoimmunity, Clin. Rev. Allergy Immunol, doi:10.1007/s12016-010-8243-x
Billington, Reid, Benefits of Bisphosphonate Therapy: Beyond the Skeleton, Curr. Osteoporos. Rep
Burki, The Role of Antiviral Treatment in the COVID-19 Pandemic, Lancet Respir. Med, doi:10.1016/S2213-2600(22)00011-X
Carrera, Santos, Costa, Rebelo, Marrucho et al., Highly Water Soluble Room Temperature Superionic Liquids of APIs, New J. Chem, doi:10.1039/C7NJ01398A
Childs, Chyall, Dunlap, Smolenskaya, Stahly et al., Crystal Engineering Approach to Forming Cocrystals of Amine Hydrochlorides with Organic Acids. Molecular Complexes of Fluoxetine Hydrochloride with Benzoic, Succinic, and Fumaric Acids, J. Am. Chem. Soc, doi:10.1021/ja048114o
Cole, Li, El-Zahab, Janes, Hayes et al., Synthesis, and Biological Evaluation of β-Lactam Antibiotic-Based Imidazolium-and Pyridinium-Type Ionic Liquids, Chem. Biol. Drug Des, doi:10.1111/j.1747-0285.2011.01114.x
Dean, Turanjanin, Yoshizawa-Fujita, Macfarlane, Scott, Exploring an Anti-Crystal Engineering Approach to the Preparation of Pharmaceutically Active Ionic Liquids, Cryst. Growth Des, doi:10.1021/cg8009496
Egorova, Gordeev, Ananikov, Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine, Chem. Rev, doi:10.1021/acs.chemrev.6b00562
El Keshky, Basyouni, Al Sabban, Corrigendum: Getting through COVID-19: The Pandemic's Impact on the Psychology of Sustainability, Quality of Life, and the Global Economy-A Systematic Review, Front. Psychol, doi:10.3389/fpsyg.2021.700815
Elder, Holm, De Diego, Use of Pharmaceutical Salts and Cocrystals to Address the Issue of Poor Solubility, Int. J. Pharm, doi:10.1016/j.ijpharm.2012.11.028
Eng, Chew, Jin, Chua, In Vitro Inhibition of Human Influenza A Virus Replication by Chloroquine, Virol. J, doi:10.1186/1743-422X-3-39
Fan, Zhang, Liu, Yang, Zheng et al., Connecting Hydroxychloroquine in Vitro Antiviral Activity to in Vivo Concentration for Prediction of Antiviral Effect: A Critical Step in Treating Patients with Coronavirus Disease, Clin. Infect. Dis, doi:10.1093/cid/ciaa623
Fanouriakis, Kostopoulou, Alunno, Aringer, Bajema et al., Update of the EULAR Recommendations for the Management of Systemic Lupus Erythematosus, Ann. Rheum. Dis, doi:10.1136/annrheumdis-2019-215089
Ferraz, Silva, Dias, Dias, Santos et al., Synthesis and Antibacterial Activity of Ionic Liquids and Organic Salts Based on Penicillin g and Amoxicillin Hydrolysate Derivatives against Resistant Bacteria, Pharmaceutics, doi:10.3390/pharmaceutics12030221
Ferraz, Teixeira, Rodrigues, Fernandes, Prudêncio et al., Antibacterial Activity of Ionic Liquids Based on Ampicillin against Resistant Bacteria, RSC Adv, doi:10.1039/C3RA44286A
Flannery, Chatterjee, Winzeler, Antimalarial Drug Discovery-Approaches and Progress towards New Medicines, Nat. Rev. Microbiol, doi:10.1038/nrmicro3138
Florindo, Costa, Matos, Nunes, Matias et al., Novel Organic Salts Based on Fluoroquinolone Drugs: Synthesis, Bioavailability and Toxicological Profiles, Int. J. Pharm, doi:10.1016/j.ijpharm.2014.04.034
Gard, Ennis, Summerfield, 1-Octanol/Water Partition Coefficients of Dialkylated Methylimidazolium Halide Salts, Glob. J. Sci. Front. Res. B Chem
Gbinigie, Fri, Should Chloroquine and Hydroxychloroquine Be Used to Treat COVID-19? A Rapid Review, BJGP Open, doi:10.3399/bjgpopen20X101069
Gindri, Siddiqui, Bhardwaj, Rodriguez, Palmer et al., Dicationic Imidazolium-Based Ionic Liquids: A New Strategy for Non-Toxic and Antimicrobial Materials, RSC Adv
Gordon, Amissah-Arthur, Gayed, Brown, Bruce et al., The British Society for Rheumatology Guideline for the Management of Systemic Lupus Erythematosus in Adults, Rheumatology, doi:10.1093/rheumatology/kex286
Guglielmero, Mezzetta, Guazzelli, Pomelli, ; D'andrea et al., Systematic Synthesis and Properties Evaluation of Dicationic Ionic Liquids, and a Glance into a Potential New Field, Front. Chem, doi:10.3389/fchem.2018.00612
Hoffmann, Kleine-Weber, Schroeder, Krüger, Herrler et al., SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Hough, Smiglak, Rodríguez, Swatloski, Spear et al., The Third Evolution of Ionic Liquids: Active Pharmaceutical Ingredients, New J. Chem, doi:10.1039/b706677p
Huttunen, Raunio, Rautio, Prodrugs-from Serendipity to Rational Design, Pharmacol. Rev
Hz, -G, -f, H-j) ppm. 13 C NMR (126 MHz
Hz, -G, -f, H-j) ppm. 13 C NMR (126 MHz
Hz, 6H, H-f) ppm. 13 C NMR (126 MHz
Hz, 6H, H-f) ppm. 13 C NMR (126 MHz
Hz, 6H, H-f) ppm. 13 C NMR (126 MHz
Hz, H-e, H-g
Hz, None
Hz, None
Hz, None
Hz, None, 2H, H-b
Hz, None, 2H, H-b″
Hz, None, 2H, H-b″
Hz, None, H
Hz, None, H
Hz, None, H
Hz, None, H
Hz, None, H
Hz, None, H
Hz, None, H
Hz, None, H
Hz, None, H
Hz, None, H-21
Hz, None, H-21
Hz, None, H-21
Hz, None, J =
Hz, None, J =
Hz, None, J =
Hz, None, J = 6
Hz, None, J = 6
Hz, None, J = 6
Keyaerts, Li, Vijgen, Rysman, Verbeeck et al., Antiviral Activity of Chloroquine against Human Coronavirus OC43 Infection in Newborn Mice, Antimicrob. Agents Chemother, doi:10.1128/AAC.01509-08
Keyaerts, Vijgen, Maes, Neyts, Van Ranst, In Vitro Inhibition of Severe Acute Respiratory Syndrome Coronavirus by Chloroquine, Biochem. Biophys. Res. Commun, doi:10.1016/j.bbrc.2004.08.085
Leuner, Dressman, Improving Drug Solubility for Oral Delivery Using Solid Dispersions, Eur. J. Pharm. Biopharm, doi:10.1016/S0939-6411(00)00076-X
Liu, Cao, Xu, Wang, Zhang et al., Hydroxychloroquine, a Less Toxic Derivative of Chloroquine, Is Effective in Inhibiting SARS-CoV-2 Infection In Vitro, Cell Discov, doi:10.1038/s41421-020-0156-0
Lu, Zhao, Li, Niu, Yang et al., Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding, Lancet
Madeira, Alves, Silva, Florindo, Costa et al., Fluoroquinolone-Based Organic Salts and Ionic Liquids as Highly Bioavailable Broad-Spectrum Antimicrobials, Proceedings, doi:10.3390/iecp2020-08649
Mero, Mezzetta, Nowicki, Łuczak, Guazzelli, Betaine and L-Carnitine Ester Bromides: Synthesis and Comparative Study of Their Thermal Behaviour and Surface Activity, J. Mol. Liq, doi:10.1016/j.molliq.2021.115988
Mezzetta, Perillo, Guazzelli, Chiappe, Thermal Behavior Analysis as a Valuable Tool for Comparing Ionic Liquids of Different Classes, J. Therm. Anal. Calorim, doi:10.1007/s10973-019-08951-w
Murgolo, Therien, Howell, Klein, Koeplinger et al., SARS-CoV-2 Tropism, Entry, Replication, and Propagation: Considerations for Drug Discovery and Development, PLoS Pathog, doi:10.1371/journal.ppat.1009225
Nmr, 6 ) δ 171, DMSO-d
Nmr, 6 ) δ 8.71, DMSO-d
Nmr, d 6 ) δ 8.60, J = 9.1 Hz, 1H, H-15
Nmr, d6) δ 8.60, DMSO
Nmr, d6) δ 8.60, DMSO
Nmr, δ 8.71, DMSO-d6)
Nmr, δ 8.71, DMSO-d6)
Ohkuma, Poole, Fluorescence Probe Measurement of the Intralysosomal PH in Living Cells and the Perturbation of PH by Various Agents, Proc. Natl. Acad. Sci, doi:10.1073/pnas.75.7.3327
Pan, Peto, Henao-Restrepo, Preziosi, Sathiyamoorthy et al., Repurposed Antiviral Drugs for COVID-19-Interim WHO Solidarity Trial Results, N. Engl. J. Med
Patil, Talebi, Xu, Bhawal, Armstrong, Synthesis of Thermally Stable Geminal Dicationic Ionic Liquids and Related Ionic Compounds: An Examination of Physicochemical Properties by Structural Modification, Chem. Mater, doi:10.1021/acs.chemmater.6b01247
Paton, Aboulhab, Hydroxychloroquine, Hydroxyurea and Didanosine as Initial Therapy for HIV-Infected Patients with Low Viral Load: Safety, Efficacy and Resistance Profile after 144 Weeks, HIV Med, doi:10.1111/j.1468-1293.2005.00259.x
Pedro, Freire, Silvestre, Freire, The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications, Int. J. Mol. Sci, doi:10.3390/ijms21218298
Pons-Estel, Bonfa, Soriano, Cardiel, Izcovich et al., First Latin American Clinical Practice Guidelines for the Treatment of Systemic Lupus Erythematosus: Latin American Group for the Study of Lupus (GLADEL, Grupo Latino Americano de Estudio Del Lupus)-Pan-American League of Associations of Rheumatology (PANLAR), Ann. Rheum. Dis, doi:10.1136/annrheumdis-2018-213512
Ratajczak, Orville-Thomas, Molecular Interactions
Reed, Muench, A Simple Method of Estimating Fifty per Cent Endpoints, Am. J. Epidemiol, doi:10.1093/oxfordjournals.aje.a118408
Ridley, Medical Need, Scientific Opportunity and the Drive for Antimalarial Drugs, Nature, doi:10.1038/415686a
Santos, Alves, Silva, Florindo, Costa et al., Antimicrobial Activities of Highly Bioavailable Organic Salts and Ionic Liquids from Fluoroquinolones, Pharmaceutics, doi:10.3390/pharmaceutics12080694
Santos, Branco, Duarte, Organic Salts Based on Isoniazid Drug: Synthesis, Bioavailability and Cytotoxicity Studies, Pharmaceutics, doi:10.3390/pharmaceutics12100952
Santos, Raposo, Carrera, Costa, Dionísio et al., Ionic Liquids and Salts from Ibuprofen as Promising Innovative Formulations of an Old Drug, ChemMedChem, doi:10.1002/cmdc.201900040
Schilling, White, Does Hydroxychloroquine Still Have Any Role in the COVID-19 Pandemic? Expert, Opin. Pharmacother, doi:10.1080/14656566.2021.1898589
Serajuddin, Salt Formation to Improve Drug Solubility, Adv. Drug Deliv. Rev, doi:10.1016/j.addr.2007.05.010
Shibata, Aoki, Tsurumi, Sugiura, Nishiyama et al., Mechanism of Uncoating of Influenza B Virus in MDCK Cells: Action of Chloroquine, J. Gen. Virol, doi:10.1099/0022-1317-64-5-1149
Shukla, Archibald, Shukla, Mehta, Cherabuddi, Chloroquine and Hydroxychloroquine in the Context of COVID-19, Drugs Context, doi:10.7573/dic.2020-4-5
Skittrall, Wilson, Smielewska, Parmar, Fortune et al., Specificity and Positive Predictive Value of SARS-CoV-2 Nucleic Acid Amplification Testing in a Low-Prevalence Setting, Clin. Microbiol. Infect, doi:10.1016/j.cmi.2020.10.003
Smolen, Landewé, Bijlsma, Burmester, Dougados et al., EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2019 Update, Ann. Rheum. Dis, doi:10.1136/annrheumdis-2019-216655
Stella, Prodrugs: Some Thoughts and Current Issues, J. Pharm. Sci, doi:10.1002/jps.22205
Tampucci, Guazzelli, Burgalassi, Carpi, Chetoni et al., PH-Responsive Nanostructures Based on Surface Active Fatty Acid-Protic Ionic Liquids for Imiquimod Delivery in Skin Cancer Topical Therapy, Pharmaceutics, doi:10.3390/pharmaceutics12111078
Tao, Tzou, Nouhin, Bonilla, Jagannathan et al., SARS-CoV-2 Antiviral Therapy, Clin. Microbiol. Rev, doi:10.1128/CMR.00109-21
Teixeira, Santos, Branco, Costa-Rodrigues, Etidronate-Based Organic Salts and Ionic Liquids: In Vitro Effects on Bone Metabolism, Int. J. Pharm, doi:10.1016/j.ijpharm.2021.121262
Teixeira, Santos, Fernandes, Costa-Rodrigues, Branco, Alendronic Acid as Ionic Liquid: New Perspective on Osteosarcoma, Pharmaceutics, doi:10.3390/pharmaceutics12030293
Teixeira, Santos, Ferraz, Prudêncio, Fernandes et al., A Novel Approach for Bisphosphonates: Ionic Liquids and Organic Salts from Zoledronic Acid, ChemMedChem, doi:10.1002/cmdc.201900397
Torchilin, Recent Advances with Liposomes as Pharmaceutical Carriers, Nat. Rev. Drug Discov, doi:10.1038/nrd1632
Uzunova, Filipova, Pavlova, Vekov, Insights into Antiviral Mechanisms of Remdesivir, Lopinavir/Ritonavir and Chloroquine/Hydroxychloroquine Affecting the New SARS-CoV-2, Biomed. Pharmacother, doi:10.1016/j.biopha.2020.110668
Vasconcelos, Sarmento, Costa, Solid Dispersions as Strategy to Improve Oral Bioavailability of Poor Water Soluble Drugs, Drug Discov. Today, doi:10.1016/j.drudis.2007.09.005
Vincent, Bergeron, Benjannet, Erickson, Rollin et al., Chloroquine Is a Potent Inhibitor of SARS Coronavirus Infection and Spread, Virol. J, doi:10.1186/1743-422X-2-69
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus (2019-NCoV) in Vitro, Cell Res, doi:10.1038/s41422-020-0282-0
Wu, Zhao, Yu, Chen, Wang et al., A New Coronavirus Associated with Human Respiratory Disease in China, Nature, doi:10.1038/s41586-020-2008-3
Yao, Ye, Zhang, Cui, Huang et al., In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin. Infect. Dis, doi:10.1093/cid/ciaa237
Ziegler, Unanue, Decrease in Macrophage Antigen Catabolism Caused by Ammonia and Chloroquine Is Associated with Inhibition of Antigen Presentation to T Cells, Proc. Natl. Acad. Sci, doi:10.1073/pnas.79.1.175
DOI record:
{
"DOI": "10.3390/pharmaceutics14040877",
"ISSN": [
"1999-4923"
],
"URL": "http://dx.doi.org/10.3390/pharmaceutics14040877",
"abstract": "<jats:p>The development of effective antiviral drugs against SARS-CoV-2 is urgently needed and a global health priority. In light of the initial data regarding the repurposing of hydroxychloroquine (HCQ) to tackle this coronavirus, herein we present a quantitative synthesis and spectroscopic and thermal characterization of seven HCQ room temperature ionic liquids (HCQ-ILs) obtained by direct protonation of the base with two equivalents of organic sulfonic, sulfuric and carboxylic acids of different polarities. Two non-toxic and hydrophilic HCQ-ILs, in particular, [HCQH2][C1SO3]2 and [HCQH2][GlcCOO]2, decreased the virus-induced cytopathic effect by two-fold in comparison with the original drug, [HCQH2][SO4]. Despite there being no significant differences in viral RNA production between the three compounds, progeny virus production was significantly affected (p < 0.05) by [HCQH2][GlcCOO]2. Overall, the data suggest that the in vitro antiviral activities of the HCQ-ILs are most likely the result of specific intra- and intermolecular interactions and not so much related with their hydrophilic or lipophilic character. This work paves the way for the development of future novel ionic formulations of hydroxychloroquine with enhanced physicochemical properties.</jats:p>",
"alternative-id": [
"pharmaceutics14040877"
],
"author": [
{
"affiliation": [],
"family": "Faísca",
"given": "Francisco",
"sequence": "first"
},
{
"affiliation": [],
"family": "Correia",
"given": "Vanessa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8104-7008",
"affiliation": [],
"authenticated-orcid": false,
"family": "Petrovski",
"given": "Željko",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2520-1151",
"affiliation": [],
"authenticated-orcid": false,
"family": "Branco",
"given": "Luís C.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0138-0944",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rebelo-de-Andrade",
"given": "Helena",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8973-1595",
"affiliation": [],
"authenticated-orcid": false,
"family": "Santos",
"given": "Miguel M.",
"sequence": "additional"
}
],
"container-title": "Pharmaceutics",
"container-title-short": "Pharmaceutics",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
4,
19
]
],
"date-time": "2022-04-19T06:39:31Z",
"timestamp": 1650350371000
},
"deposited": {
"date-parts": [
[
2022,
4,
19
]
],
"date-time": "2022-04-19T06:53:02Z",
"timestamp": 1650351182000
},
"funder": [
{
"DOI": "10.13039/501100001871",
"award": [
"UIDB/50006/2020",
"UIDP/50006/2020",
"Research 4 COVID-19 nº 582",
"PTDC/QUI-QOR/32406/2017",
"RECI/BBBBQB/0230/2012"
],
"doi-asserted-by": "publisher",
"name": "Fundação para a Ciência e Tecnologia"
}
],
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T21:58:22Z",
"timestamp": 1712613502099
},
"is-referenced-by-count": 5,
"issue": "4",
"issued": {
"date-parts": [
[
2022,
4,
17
]
]
},
"journal-issue": {
"issue": "4",
"published-online": {
"date-parts": [
[
2022,
4
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
4,
17
]
],
"date-time": "2022-04-17T00:00:00Z",
"timestamp": 1650153600000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/1999-4923/14/4/877/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "877",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2022,
4,
17
]
]
},
"published-online": {
"date-parts": [
[
2022,
4,
17
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref1",
"unstructured": "WHO Coronavirus (COVID-19) Dashboardhttps://covid19.who.int/"
},
{
"DOI": "10.3389/fpsyg.2021.700815",
"doi-asserted-by": "publisher",
"key": "ref2"
},
{
"DOI": "10.1128/CMR.00109-21",
"doi-asserted-by": "publisher",
"key": "ref3"
},
{
"key": "ref4"
},
{
"key": "ref5",
"unstructured": "COVID-19 Treatmentshttps://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/covid-19-treatments"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "ref6"
},
{
"DOI": "10.1056/NEJMoa2023184",
"article-title": "Repurposed Antiviral Drugs for COVID-19—Interim WHO Solidarity Trial Results",
"author": "Pan",
"doi-asserted-by": "crossref",
"first-page": "497",
"journal-title": "N. Engl. J. Med.",
"key": "ref7",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(22)00011-X",
"doi-asserted-by": "publisher",
"key": "ref8"
},
{
"DOI": "10.1371/journal.ppat.1009225",
"doi-asserted-by": "publisher",
"key": "ref9"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "ref10"
},
{
"DOI": "10.1038/s41421-020-0156-0",
"doi-asserted-by": "publisher",
"key": "ref11"
},
{
"DOI": "10.1093/cid/ciaa237",
"doi-asserted-by": "publisher",
"key": "ref12"
},
{
"DOI": "10.1136/annrheumdis-2019-216655",
"doi-asserted-by": "publisher",
"key": "ref13"
},
{
"DOI": "10.1136/annrheumdis-2019-215089",
"doi-asserted-by": "publisher",
"key": "ref14"
},
{
"DOI": "10.1136/annrheumdis-2018-213512",
"doi-asserted-by": "publisher",
"key": "ref15"
},
{
"DOI": "10.1093/rheumatology/kex286",
"doi-asserted-by": "publisher",
"key": "ref16"
},
{
"DOI": "10.1007/s12016-010-8243-x",
"doi-asserted-by": "publisher",
"key": "ref17"
},
{
"DOI": "10.1186/1743-422X-3-39",
"doi-asserted-by": "publisher",
"key": "ref18"
},
{
"DOI": "10.1099/0022-1317-64-5-1149",
"doi-asserted-by": "publisher",
"key": "ref19"
},
{
"DOI": "10.1111/j.1468-1293.2005.00259.x",
"doi-asserted-by": "publisher",
"key": "ref20"
},
{
"DOI": "10.1016/j.bbrc.2004.08.085",
"doi-asserted-by": "publisher",
"key": "ref21"
},
{
"DOI": "10.1186/1743-422X-2-69",
"doi-asserted-by": "publisher",
"key": "ref22"
},
{
"DOI": "10.1128/AAC.01509-08",
"doi-asserted-by": "publisher",
"key": "ref23"
},
{
"DOI": "10.1038/nrmicro3138",
"doi-asserted-by": "publisher",
"key": "ref24"
},
{
"DOI": "10.1038/415686a",
"doi-asserted-by": "publisher",
"key": "ref25"
},
{
"DOI": "10.1073/pnas.75.7.3327",
"doi-asserted-by": "publisher",
"key": "ref26"
},
{
"DOI": "10.1073/pnas.79.1.175",
"doi-asserted-by": "publisher",
"key": "ref27"
},
{
"DOI": "10.1038/s41586-020-2008-3",
"doi-asserted-by": "publisher",
"key": "ref28"
},
{
"DOI": "10.1016/S0140-6736(20)30251-8",
"doi-asserted-by": "publisher",
"key": "ref29"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"doi-asserted-by": "publisher",
"key": "ref30"
},
{
"DOI": "10.1016/j.biopha.2020.110668",
"doi-asserted-by": "publisher",
"key": "ref31"
},
{
"DOI": "10.1080/14656566.2021.1898589",
"doi-asserted-by": "publisher",
"key": "ref32"
},
{
"DOI": "10.3399/bjgpopen20X101069",
"doi-asserted-by": "publisher",
"key": "ref33"
},
{
"DOI": "10.7573/dic.2020-4-5",
"doi-asserted-by": "publisher",
"key": "ref34"
},
{
"DOI": "10.1093/cid/ciaa623",
"doi-asserted-by": "publisher",
"key": "ref35"
},
{
"DOI": "10.1002/jps.22205",
"doi-asserted-by": "publisher",
"key": "ref36"
},
{
"DOI": "10.1124/pr.110.003459",
"doi-asserted-by": "publisher",
"key": "ref37"
},
{
"DOI": "10.1021/ja048114o",
"doi-asserted-by": "publisher",
"key": "ref38"
},
{
"DOI": "10.1021/cg8009496",
"doi-asserted-by": "publisher",
"key": "ref39"
},
{
"DOI": "10.1016/j.ijpharm.2012.11.028",
"doi-asserted-by": "publisher",
"key": "ref40"
},
{
"DOI": "10.1016/S0939-6411(00)00076-X",
"doi-asserted-by": "publisher",
"key": "ref41"
},
{
"DOI": "10.1016/j.drudis.2007.09.005",
"doi-asserted-by": "publisher",
"key": "ref42"
},
{
"DOI": "10.1038/nrd1632",
"doi-asserted-by": "publisher",
"key": "ref43"
},
{
"DOI": "10.1016/j.addr.2007.05.010",
"doi-asserted-by": "publisher",
"key": "ref44"
},
{
"DOI": "10.3390/ijms21218298",
"doi-asserted-by": "publisher",
"key": "ref45"
},
{
"DOI": "10.1016/j.molliq.2021.115988",
"doi-asserted-by": "publisher",
"key": "ref46"
},
{
"DOI": "10.3390/pharmaceutics12111078",
"doi-asserted-by": "publisher",
"key": "ref47"
},
{
"DOI": "10.1039/b706677p",
"doi-asserted-by": "publisher",
"key": "ref48"
},
{
"DOI": "10.1021/acs.chemrev.6b00562",
"doi-asserted-by": "publisher",
"key": "ref49"
},
{
"DOI": "10.1111/j.1747-0285.2011.01114.x",
"doi-asserted-by": "publisher",
"key": "ref50"
},
{
"DOI": "10.1016/j.ijpharm.2014.04.034",
"doi-asserted-by": "publisher",
"key": "ref51"
},
{
"DOI": "10.3390/pharmaceutics12080694",
"doi-asserted-by": "publisher",
"key": "ref52"
},
{
"DOI": "10.3390/iecp2020-08649",
"doi-asserted-by": "publisher",
"key": "ref53"
},
{
"DOI": "10.3390/pharmaceutics12030221",
"doi-asserted-by": "publisher",
"key": "ref54"
},
{
"DOI": "10.3390/pharmaceutics12100952",
"doi-asserted-by": "publisher",
"key": "ref55"
},
{
"DOI": "10.1002/cmdc.201900397",
"doi-asserted-by": "publisher",
"key": "ref56"
},
{
"DOI": "10.3390/pharmaceutics12030293",
"doi-asserted-by": "publisher",
"key": "ref57"
},
{
"DOI": "10.1016/j.ijpharm.2021.121262",
"doi-asserted-by": "publisher",
"key": "ref58"
},
{
"DOI": "10.1002/cmdc.201900040",
"doi-asserted-by": "publisher",
"key": "ref59"
},
{
"DOI": "10.1039/C7NJ01398A",
"doi-asserted-by": "publisher",
"key": "ref60"
},
{
"DOI": "10.1039/C3RA44286A",
"doi-asserted-by": "publisher",
"key": "ref61"
},
{
"DOI": "10.1007/s11914-020-00612-4",
"doi-asserted-by": "publisher",
"key": "ref62"
},
{
"DOI": "10.3389/fchem.2018.00612",
"doi-asserted-by": "publisher",
"key": "ref63"
},
{
"DOI": "10.1021/acs.chemmater.6b01247",
"doi-asserted-by": "publisher",
"key": "ref64"
},
{
"DOI": "10.1007/s10973-019-08951-w",
"doi-asserted-by": "publisher",
"key": "ref65"
},
{
"DOI": "10.1039/C4RA09906K",
"doi-asserted-by": "publisher",
"key": "ref66"
},
{
"article-title": "1-Octanol/Water Partition Coefficients of Dialkylated Methylimidazolium Halide Salts",
"author": "Gard",
"first-page": "6-1-6",
"journal-title": "Glob. J. Sci. Front. Res. B Chem.",
"key": "ref67",
"volume": "15",
"year": "2015"
},
{
"key": "ref68",
"series-title": "Laboratory Biosafety Guidance Related to Coronavirus Disease (COVID-19): Interim Guidance, 28 January 2021",
"year": "2021"
},
{
"DOI": "10.1093/oxfordjournals.aje.a118408",
"doi-asserted-by": "publisher",
"key": "ref69"
},
{
"DOI": "10.1016/j.cmi.2020.10.003",
"doi-asserted-by": "publisher",
"key": "ref70"
},
{
"DOI": "10.1007/s11743-006-1000-8",
"doi-asserted-by": "publisher",
"key": "ref71"
},
{
"author": "Ratajczak",
"key": "ref72",
"series-title": "Molecular Interactions",
"year": "1980"
}
],
"reference-count": 72,
"references-count": 72,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/1999-4923/14/4/877"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Enhanced In Vitro Antiviral Activity of Hydroxychloroquine Ionic Liquids against SARS-CoV-2",
"type": "journal-article",
"volume": "14"
}
