Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice
et al., Virology Journal, doi:10.1186/s12985-021-01515-1, Feb 2021
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
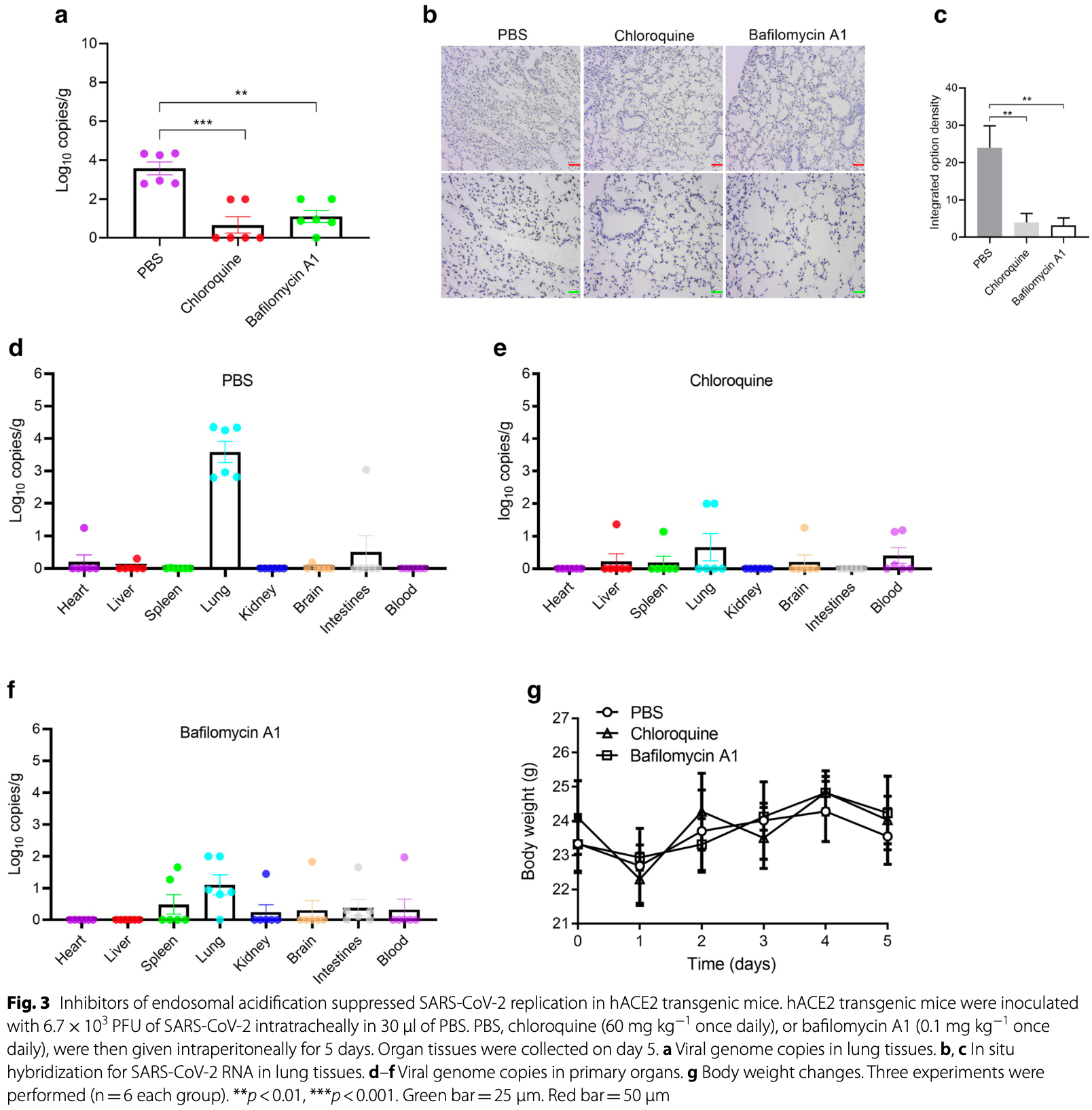
In vitro and mouse study showing that endosomal acidification inhibitors chloroquine, bafilomycin A1, and NH4CL suppress SARS-CoV-2 replication and relieve viral pneumonia. Authors found that these compounds significantly reduced SARS-CoV-2 viral yields in Vero E6, Huh-7, and 293T-ACE2 cells. In hACE2 transgenic mice, chloroquine and bafilomycin A1 reduced viral replication in lung tissues and alleviated pneumonia with reduced inflammatory infiltration and improved alveolar structure.
See also Yan et al., an in vitro study showing that endosomal acidification inhibitors like HCQ minimize SARS-CoV-2 infection by blocking viral internalization and RNA release in cells expressing ACE2. Authors utilized super-resolution structured illumination microscopy (SIM) to analyze the effects of CQ, HCQ, Bafilomycin A1 (BafA1), and Dynasore on the endocytic processes of SARS-CoV-2. All four compounds inhibited the internalization and degradation of the RBD-ACE2 complex in living cells, with BafA1 showing the highest inhibition rate.
39 preclinical studies support the efficacy of HCQ for COVID-19:
1.
Yan et al., Super-resolution imaging reveals the mechanism of endosomal acidification inhibitors against SARS-CoV-2 infection, ChemBioChem, doi:10.1002/cbic.202400404.
2.
Shang et al., Identification of Cathepsin L as the molecular target of hydroxychloroquine with chemical proteomics, Molecular & Cellular Proteomics, doi:10.1016/j.mcpro.2025.101314.
3.
González-Paz et al., Biophysical Analysis of Potential Inhibitors of SARS-CoV-2 Cell Recognition and Their Effect on Viral Dynamics in Different Cell Types: A Computational Prediction from In Vitro Experimental Data, ACS Omega, doi:10.1021/acsomega.3c06968.
4.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
5.
Guimarães Silva et al., Are Non-Structural Proteins From SARS-CoV-2 the Target of Hydroxychloroquine? An in Silico Study, ACTA MEDICA IRANICA, doi:10.18502/acta.v61i2.12533.
6.
Nguyen et al., The Potential of Ameliorating COVID-19 and Sequelae From Andrographis paniculata via Bioinformatics, Bioinformatics and Biology Insights, doi:10.1177/11779322221149622.
8.
Yadav et al., Repurposing the Combination Drug of Favipiravir, Hydroxychloroquine and Oseltamivir as a Potential Inhibitor Against SARS-CoV-2: A Computational Study, Research Square, doi:10.21203/rs.3.rs-628277/v1.
9.
Hussein et al., Molecular Docking Identification for the efficacy of Some Zinc Complexes with Chloroquine and Hydroxychloroquine against Main Protease of COVID-19, Journal of Molecular Structure, doi:10.1016/j.molstruc.2021.129979.
10.
Baildya et al., Inhibitory capacity of Chloroquine against SARS-COV-2 by effective binding with Angiotensin converting enzyme-2 receptor: An insight from molecular docking and MD-simulation studies, Journal of Molecular Structure, doi:10.1016/j.molstruc.2021.129891.
11.
Noureddine et al., Quantum chemical studies on molecular structure, AIM, ELF, RDG and antiviral activities of hybrid hydroxychloroquine in the treatment of COVID-19: molecular docking and DFT calculations, Journal of King Saud University - Science, doi:10.1016/j.jksus.2020.101334.
12.
Tarek et al., Pharmacokinetic Basis of the Hydroxychloroquine Response in COVID-19: Implications for Therapy and Prevention, European Journal of Drug Metabolism and Pharmacokinetics, doi:10.1007/s13318-020-00640-6.
13.
Rowland Yeo et al., Impact of Disease on Plasma and Lung Exposure of Chloroquine, Hydroxychloroquine and Azithromycin: Application of PBPK Modeling, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.1955.
14.
Hitti et al., Hydroxychloroquine attenuates double-stranded RNA-stimulated hyper-phosphorylation of tristetraprolin/ZFP36 and AU-rich mRNA stabilization, Immunology, doi:10.1111/imm.13835.
15.
Mohd Abd Razak et al., In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds, Natural Product Communications, doi:10.1177/1934578X231188861.
16.
Alsmadi et al., The In Vitro, In Vivo, and PBPK Evaluation of a Novel Lung-Targeted Cardiac-Safe Hydroxychloroquine Inhalation Aerogel, AAPS PharmSciTech, doi:10.1208/s12249-023-02627-3.
17.
Wen et al., Cholinergic α7 nAChR signaling suppresses SARS-CoV-2 infection and inflammation in lung epithelial cells, Journal of Molecular Cell Biology, doi:10.1093/jmcb/mjad048.
18.
Kamga Kapchoup et al., In vitro effect of hydroxychloroquine on pluripotent stem cells and their cardiomyocytes derivatives, Frontiers in Pharmacology, doi:10.3389/fphar.2023.1128382.
19.
Milan Bonotto et al., Cathepsin inhibitors nitroxoline and its derivatives inhibit SARS-CoV-2 infection, Antiviral Research, doi:10.1016/j.antiviral.2023.105655.
20.
Miao et al., SIM imaging resolves endocytosis of SARS-CoV-2 spike RBD in living cells, Cell Chemical Biology, doi:10.1016/j.chembiol.2023.02.001.
21.
Yuan et al., Hydroxychloroquine blocks SARS-CoV-2 entry into the endocytic pathway in mammalian cell culture, Communications Biology, doi:10.1038/s42003-022-03841-8.
22.
Faísca et al., Enhanced In Vitro Antiviral Activity of Hydroxychloroquine Ionic Liquids against SARS-CoV-2, Pharmaceutics, doi:10.3390/pharmaceutics14040877.
23.
Delandre et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445.
24.
Purwati et al., An in vitro study of dual drug combinations of anti-viral agents, antibiotics, and/or hydroxychloroquine against the SARS-CoV-2 virus isolated from hospitalized patients in Surabaya, Indonesia, PLOS One, doi:10.1371/journal.pone.0252302.
25.
Zhang et al., SARS-CoV-2 spike protein dictates syncytium-mediated lymphocyte elimination, Cell Death & Differentiation, doi:10.1038/s41418-021-00782-3.
26.
Dang et al., Structural basis of anti-SARS-CoV-2 activity of hydroxychloroquine: specific binding to NTD/CTD and disruption of LLPS of N protein, bioRxiv, doi:10.1101/2021.03.16.435741.
27.
Shang (B) et al., Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice, Virology Journal, doi:10.1186/s12985-021-01515-1.
28.
Wang et al., Chloroquine and hydroxychloroquine as ACE2 blockers to inhibit viropexis of 2019-nCoV Spike pseudotyped virus, Phytomedicine, doi:10.1016/j.phymed.2020.153333.
29.
Sheaff, R., A New Model of SARS-CoV-2 Infection Based on (Hydroxy)Chloroquine Activity, bioRxiv, doi:10.1101/2020.08.02.232892.
30.
Ou et al., Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2, PLOS Pathogens, doi:10.1371/journal.ppat.1009212.
31.
Andreani et al., In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect, Microbial Pathogenesis, doi:10.1016/j.micpath.2020.104228.
32.
Clementi et al., Combined Prophylactic and Therapeutic Use Maximizes Hydroxychloroquine Anti-SARS-CoV-2 Effects in vitro, Front. Microbiol., 10 July 2020, doi:10.3389/fmicb.2020.01704.
33.
Liu et al., Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discovery 6, 16 (2020), doi:10.1038/s41421-020-0156-0.
34.
Yao et al., In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin. Infect. Dis., 2020 Mar 9, doi:10.1093/cid/ciaa237.
Shang et al., 27 Feb 2021, peer-reviewed, 12 authors.
Contact: linjiaxiaoya@163.com (corresponding author), lixiao06@mails.jlu.edu.cn, skylee6226@163.com.
Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice
Virology Journal, doi:10.1186/s12985-021-01515-1
Background: Coronavirus disease 2019 (COVID-19) is caused by SARS-CoV-2 and broke out as a global pandemic in late 2019. The acidic pH environment of endosomes is believed to be essential for SARS-CoV-2 to be able to enter cells and begin replication. However, the clinical use of endosomal acidification inhibitors, typically chloroquine, has been controversial with this respect.
Methods: In this study, RT-qPCR method was used to detect the SARS-CoV-2N gene to evaluate viral replication. The CCK-8 assay was also used to evaluate the cytotoxic effect of SARS-CoV-2. In situ hybridization was used to examine the distribution of the SARS-CoV-2 gene in lung tissues. Hematoxylin and eosin staining was also used to evaluate virus-associated pathological changes in lung tissues. Results: In this study, analysis showed that endosomal acidification inhibitors, including chloroquine, bafilomycin A1 and NH 4 CL, significantly reduced the viral yields of SARS-CoV-2 in Vero E6, Huh-7 and 293T-ACE2 cells. Chloroquine and bafilomycin A1 also improved the viability and proliferation of Vero E6 cells after SARS-CoV-2 infection. Moreover, in the hACE2 transgenic mice model of SARS-CoV-2 infection, chloroquine and bafilomycin A1 reduced viral replication in lung tissues and alleviated viral pneumonia with reduced inflammatory exudation and infiltration in peribronchiolar and perivascular tissues, as well as improved structures of alveolar septum and pulmonary alveoli. Conclusions: Our research investigated the antiviral effects of endosomal acidification inhibitors against SARS-CoV-2 in several infection models and provides an experimental basis for further mechanistic studies and drug development.
Ethics approval and consent to participate The animal experimental protocols were approved by the Institutional Animal Care and Use Committee of the Academy of Military Medical Science (AMMS) and all efforts were made to minimize animal suffering and reduce the number of animals used for the experiments. The studies involving human participants were reviewed and approved by the Ethics Committee of the Chinese Academy of Military Medical Science (AMMS).
Consent for publication Not applicable.
Competing interests The authors declare no competing interests. • fast, convenient online submission • thorough peer review by experienced researchers in your field
• rapid publication on acceptance • support for research data, including large and complex data types • gold Open Access which fosters wider collaboration and increased citations maximum visibility for your research: over 100M website views per year
• At BMC, research is always in progress.
Learn more biomedcentral.com/submissions Ready to submit your research Ready to submit your research ? Choose BMC and benefit from: ? Choose BMC and benefit from:
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Bai, Gao, Zhang, Guan, Xu et al., BZML, a novel colchicine binding site inhibitor, overcomes multidrug resistance in A549/Taxol cells by inhibiting P-gp function and inducing mitotic catastrophe, Cancer Lett
Bao, Deng, Huang, Gao, Qin, The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice, Nature
Barrow, Nicola, Liu, Multiscale perspectives of virus entry via endocytosis, Virol J
Belouzard, Millet, Licitra, Whittaker, Mechanisms of coronavirus cell entry mediated by the viral spike protein, Viruses
Ferner, Aronson, Chloroquine and hydroxychloroquine in covid-19, BMJ (online)
Gorbalenya, Baker, Baric, Groot, Ziebuhr, The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2, Nat Microbiol
Heald-Sargent, Gallagher, Ready, set, fuse! The coronavirus spike protein and acquisition of fusion competence, Viruses
Hoffmann, Msbauer, Hofmann-Winkler, Kaul, Kleine-Weber et al., Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2, Nature
Kai, Kai, Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19, Hypertens Res
Kuba, Imai, Rao, Gao, Guo et al., A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury, Nat Med
Lan, Ge, Yu, Shan, Zhou et al., Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor, Nature
Liu, Cao, Xu, Wang, Wang, Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro
Maa, Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases, Pharmacol Res Perspect
Maisonnasse, Guedj, Contreras, Behillil, Grand, Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates, Nature
Ou, Liu, Lei, Li, Qian, Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV, Nat Commun
Runfeng, Yunlong, Jicheng, Weiqi, Zifeng, Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2), Pharmacol Res
Savarino, Trani, Donatelli, Cauda, Cassone, New insights into the antiviral effects of chloroquine, Lancet Infect Dis
Shang, Ye, Shi, Wan, Luo et al., Structural basis of receptor recognition by SARS-CoV-2, Nature
Sun, Tien, From endocytosis to membrane fusion: emerging roles of dynamin in virus entry, Crit Rev Microbiol
Wang, Cao, Zhang, Yang, Xiao, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wang, Yang, Liu, Guo, Zhang et al., SARS coronavirus entry into host cells through a novel clathrin-and caveolae-independent endocytic pathway, Cell Res
Xueting, Fei, Miao, Cheng, Baoying et al., In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Clin Infect Dis
Zhang, Li, Deng, Zhao, Huang et al., A thermostable mRNA vaccine against COVID-19, Cell
Zhou, Yang, Wang, Hu, Zhang et al., A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature
Zhu, Zhang, Li, Tan, A Novel Coronavirus from Patients with Pneumonia in China, N Engl J Med
DOI record:
{
"DOI": "10.1186/s12985-021-01515-1",
"ISSN": [
"1743-422X"
],
"URL": "http://dx.doi.org/10.1186/s12985-021-01515-1",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Coronavirus disease 2019 (COVID-19) is caused by SARS-CoV-2 and broke out as a global pandemic in late 2019. The acidic pH environment of endosomes is believed to be essential for SARS-CoV-2 to be able to enter cells and begin replication. However, the clinical use of endosomal acidification inhibitors, typically chloroquine, has been controversial with this respect.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>In this study, RT-qPCR method was used to detect the SARS-CoV-2N gene to evaluate viral replication. The CCK-8 assay was also used to evaluate the cytotoxic effect of SARS-CoV-2. In situ hybridization was used to examine the distribution of the SARS-CoV-2 gene in lung tissues. Hematoxylin and eosin staining was also used to evaluate virus-associated pathological changes in lung tissues.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>In this study, analysis showed that endosomal acidification inhibitors, including chloroquine, bafilomycin A1 and NH<jats:sub>4</jats:sub>CL, significantly reduced the viral yields of SARS-CoV-2 in Vero E6, Huh-7 and 293T-ACE2 cells. Chloroquine and bafilomycin A1 also improved the viability and proliferation of Vero E6 cells after SARS-CoV-2 infection. Moreover, in the hACE2 transgenic mice model of SARS-CoV-2 infection, chloroquine and bafilomycin A1 reduced viral replication in lung tissues and alleviated viral pneumonia with reduced inflammatory exudation and infiltration in peribronchiolar and perivascular tissues, as well as improved structures of alveolar septum and pulmonary alveoli.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Our research investigated the antiviral effects of endosomal acidification inhibitors against SARS-CoV-2 in several infection models and provides an experimental basis for further mechanistic studies and drug development.</jats:p>\n </jats:sec>",
"alternative-id": [
"1515"
],
"article-number": "46",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "12 December 2020"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "18 February 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "27 February 2021"
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The animal experimental protocols were approved by the Institutional Animal Care and Use Committee of the Academy of Military Medical Science (AMMS) and all efforts were made to minimize animal suffering and reduce the number of animals used for the experiments. The studies involving human participants were reviewed and approved by the Ethics Committee of the Chinese Academy of Military Medical Science (AMMS)."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Shang",
"given": "Chao",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zhuang",
"given": "Xinyu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "He",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Yiquan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Yilong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Jing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ge",
"given": "Chenchen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cong",
"given": "Jianan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Tingyu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tian",
"given": "Mingyao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jin",
"given": "Ningyi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Xiao",
"sequence": "additional"
}
],
"container-title": "Virology Journal",
"container-title-short": "Virol J",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
2,
27
]
],
"date-time": "2021-02-27T18:24:39Z",
"timestamp": 1614450279000
},
"deposited": {
"date-parts": [
[
2021,
2,
27
]
],
"date-time": "2021-02-27T18:25:03Z",
"timestamp": 1614450303000
},
"funder": [
{
"award": [
"Grant no. 20YF003"
],
"name": "the New coronavirus pneumonia (NCP) epidemic prevention and control emergency scientific research project in Changchun City"
},
{
"award": [
"Grant no. SQ2020YFF0417940"
],
"name": "the Key projects of science and technology boosting economy in 2020"
}
],
"indexed": {
"date-parts": [
[
2023,
6,
5
]
],
"date-time": "2023-06-05T18:29:44Z",
"timestamp": 1685989784052
},
"is-referenced-by-count": 33,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
2,
27
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
27
]
],
"date-time": "2021-02-27T00:00:00Z",
"timestamp": 1614384000000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
2,
27
]
],
"date-time": "2021-02-27T00:00:00Z",
"timestamp": 1614384000000
}
}
],
"link": [
{
"URL": "http://link.springer.com/content/pdf/10.1186/s12985-021-01515-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://link.springer.com/article/10.1186/s12985-021-01515-1/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://link.springer.com/content/pdf/10.1186/s12985-021-01515-1.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2021,
2,
27
]
]
},
"published-online": {
"date-parts": [
[
2021,
2,
27
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"doi-asserted-by": "crossref",
"key": "1515_CR1",
"unstructured": "Zhu N, Zhang D, Wang W, Li X, Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8)."
},
{
"DOI": "10.1038/s41564-020-0695-z",
"doi-asserted-by": "crossref",
"key": "1515_CR2",
"unstructured": "Gorbalenya AE, Baker SC, Baric RS, Groot RJD, Ziebuhr J.The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(5)."
},
{
"DOI": "10.1038/s41440-020-0455-8",
"author": "H Kai",
"doi-asserted-by": "publisher",
"first-page": "648",
"issue": "7",
"journal-title": "Hypertens Res",
"key": "1515_CR3",
"unstructured": "Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertens Res. 2020;43(7):648–54.",
"volume": "43",
"year": "2020"
},
{
"DOI": "10.3390/v4061011",
"author": "S Belouzard",
"doi-asserted-by": "publisher",
"first-page": "1011",
"issue": "6",
"journal-title": "Viruses",
"key": "1515_CR4",
"unstructured": "Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–33.",
"volume": "4",
"year": "2012"
},
{
"DOI": "10.3390/v4040557",
"doi-asserted-by": "crossref",
"key": "1515_CR5",
"unstructured": "Heald-Sargent T, Gallagher T. Ready, set, fuse! The coronavirus spike protein and acquisition of fusion competence. Viruses. 2012;4(4)."
},
{
"DOI": "10.1002/prp2.293",
"doi-asserted-by": "crossref",
"key": "1515_CR6",
"unstructured": "Al-Bari MAA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect. 2017;5(1)."
},
{
"author": "RE Ferner",
"first-page": "m1432",
"journal-title": "BMJ (online)",
"key": "1515_CR7",
"unstructured": "Ferner RE, Aronson JK. Chloroquine and hydroxychloroquine in covid-19. BMJ (online). 2020;369:m1432.",
"volume": "369",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(06)70361-9",
"author": "A Savarino",
"doi-asserted-by": "publisher",
"first-page": "67",
"issue": "2",
"journal-title": "Lancet Infect Dis",
"key": "1515_CR8",
"unstructured": "Savarino A, Trani LD, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6(2):67–9.",
"volume": "6",
"year": "2006"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"author": "M Wang",
"doi-asserted-by": "publisher",
"first-page": "269",
"issue": "3",
"journal-title": "Cell Res",
"key": "1515_CR9",
"unstructured": "Wang M, Cao R, Zhang L, Yang X, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–71.",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa237",
"doi-asserted-by": "crossref",
"key": "1515_CR10",
"unstructured": "Xueting Y, Fei Y, Miao Z, Cheng C, Baoying H, Peihua N, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020;71(15)."
},
{
"key": "1515_CR11",
"unstructured": "Hoffmann M, Msbauer K, Hofmann-Winkler H, Kaul A, Kleine-Weber H, Krüger N, et al. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature."
},
{
"DOI": "10.1038/s41586-020-2558-4",
"doi-asserted-by": "crossref",
"key": "1515_CR12",
"unstructured": "Maisonnasse P, Guedj J, Contreras V, Behillil S, Grand RL. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020:1–8."
},
{
"DOI": "10.1038/nm1267",
"author": "K Kuba",
"doi-asserted-by": "publisher",
"first-page": "875",
"issue": "8",
"journal-title": "Nat Med",
"key": "1515_CR13",
"unstructured": "Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875.",
"volume": "11",
"year": "2005"
},
{
"key": "1515_CR14",
"unstructured": "Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature."
},
{
"DOI": "10.1038/s41586-020-2179-y",
"doi-asserted-by": "crossref",
"key": "1515_CR15",
"unstructured": "Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807)."
},
{
"key": "1515_CR16",
"unstructured": "Bao L, Deng W, Huang B, Gao H, Qin C. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020:1–6."
},
{
"DOI": "10.1038/s41467-020-15562-9",
"doi-asserted-by": "crossref",
"key": "1515_CR17",
"unstructured": "Ou X, Liu Y, Lei X, Li P, Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11(1)."
},
{
"key": "1515_CR18",
"unstructured": "Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature."
},
{
"DOI": "10.1016/j.phrs.2020.104761",
"author": "L Runfeng",
"doi-asserted-by": "publisher",
"first-page": "104761",
"journal-title": "Pharmacol Res",
"key": "1515_CR19",
"unstructured": "Runfeng L, Yunlong H, Jicheng H, Weiqi P, Zifeng Y. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res. 2020;156:104761.",
"volume": "156",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.07.024",
"author": "NN Zhang",
"doi-asserted-by": "publisher",
"first-page": "1271",
"issue": "5",
"journal-title": "Cell",
"key": "1515_CR20",
"unstructured": "Zhang NN, Li XF, Deng YQ, Zhao H, Huang YJ, Yang G, et al. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182(5):1271-83e16.",
"volume": "182",
"year": "2020"
},
{
"DOI": "10.1016/j.canlet.2017.05.016",
"author": "Z Bai",
"doi-asserted-by": "publisher",
"first-page": "81",
"journal-title": "Cancer Lett",
"key": "1515_CR21",
"unstructured": "Bai Z, Gao M, Zhang H, Guan Q, Xu J, Li Y, et al. BZML, a novel colchicine binding site inhibitor, overcomes multidrug resistance in A549/Taxol cells by inhibiting P-gp function and inducing mitotic catastrophe. Cancer Lett. 2017;402:81–92.",
"volume": "402",
"year": "2017"
},
{
"DOI": "10.3109/1040841X.2012.694412",
"author": "Y Sun",
"doi-asserted-by": "publisher",
"first-page": "166",
"issue": "2",
"journal-title": "Crit Rev Microbiol",
"key": "1515_CR22",
"unstructured": "Sun Y, Tien P. From endocytosis to membrane fusion: emerging roles of dynamin in virus entry. Crit Rev Microbiol. 2013;39(2):166–79.",
"volume": "39",
"year": "2013"
},
{
"DOI": "10.1186/1743-422X-10-177",
"doi-asserted-by": "crossref",
"key": "1515_CR23",
"unstructured": "Barrow E, Nicola AV, Liu J. Multiscale perspectives of virus entry via endocytosis. Virol J. 2013;10."
},
{
"key": "1515_CR24",
"unstructured": "Liu J, Cao R, Xu M, Wang X, Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov."
},
{
"DOI": "10.1038/cr.2008.15",
"author": "H Wang",
"doi-asserted-by": "publisher",
"first-page": "290",
"issue": "2",
"journal-title": "Cell Res",
"key": "1515_CR25",
"unstructured": "Wang H, Yang P, Liu K, Guo F, Zhang Y, Zhang G, et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18(2):290.",
"volume": "18",
"year": "2008"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://virologyj.biomedcentral.com/articles/10.1186/s12985-021-01515-1"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "18"
}
