Cholinergic α7 nAChR signaling suppresses SARS-CoV-2 infection and inflammation in lung epithelial cells
et al., Journal of Molecular Cell Biology, doi:10.1093/jmcb/mjad048, Jul 2023
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
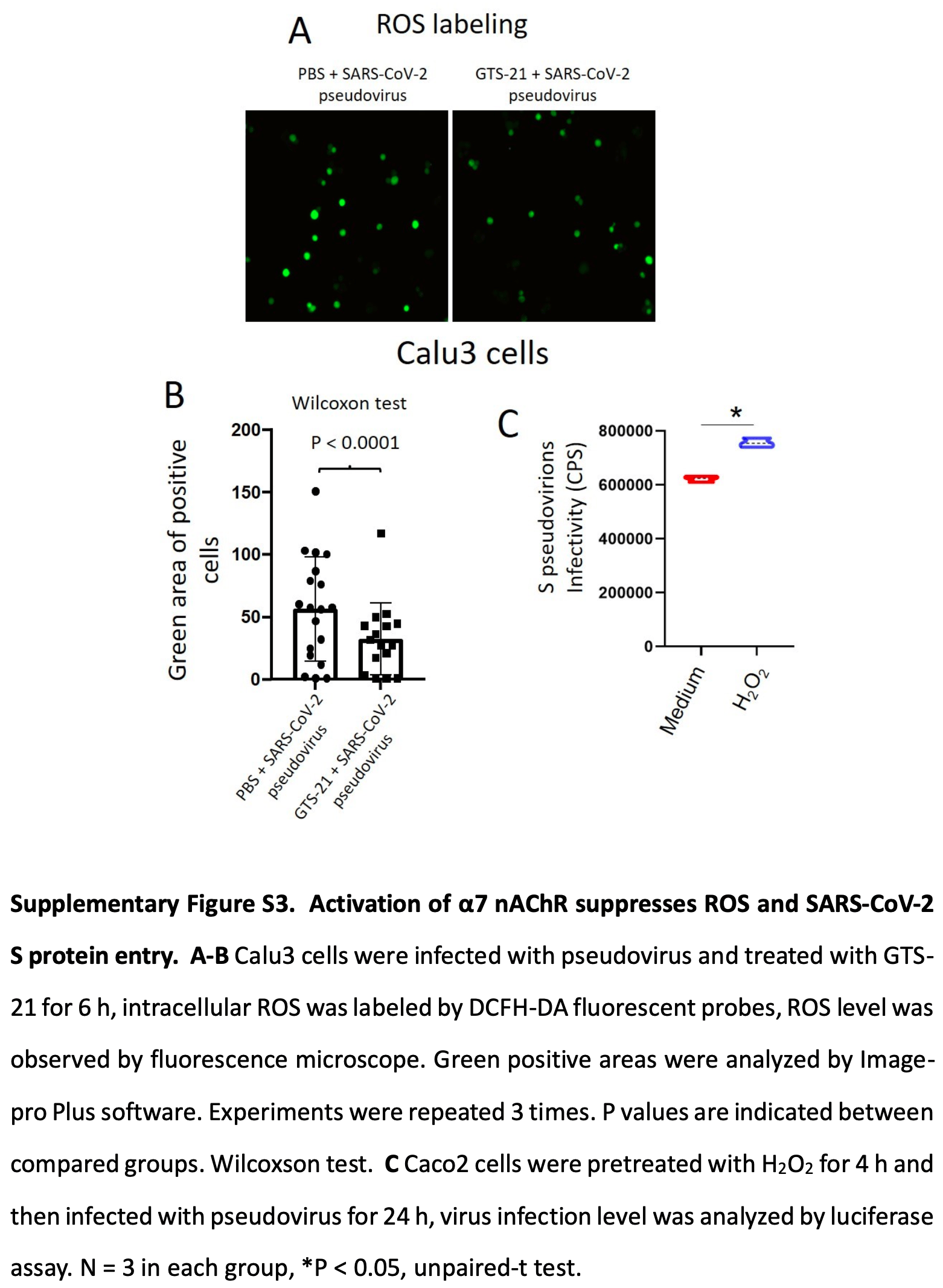
In vitro and mouse study showing that activating α7 nAChR with the agonist GTS-21 reduced oxidative stress and inflammation, and reduced live virus infection in lung epithelial cells.
The results provide some mechanistic insight into how HCQ may inhibit SARS-CoV-2 infection, by acting through the α7 nAChR pathway. Previous in silico studies predict HCQ blocks SARS-CoV-2 binding to both ACE2 and α7 nAChR. This study provides experimental evidence that activating α7 nAChR decreases ACE2 expression and reduces SARS-CoV-2 infection.
Activating the α7 nAChR pathway (with agonists) or protecting it from viral interference (with HCQ for example) could be viable therapeutic approaches against COVID-19.
39 preclinical studies support the efficacy of HCQ for COVID-19:
1.
Shang et al., Identification of Cathepsin L as the molecular target of hydroxychloroquine with chemical proteomics, Molecular & Cellular Proteomics, doi:10.1016/j.mcpro.2025.101314.
2.
González-Paz et al., Biophysical Analysis of Potential Inhibitors of SARS-CoV-2 Cell Recognition and Their Effect on Viral Dynamics in Different Cell Types: A Computational Prediction from In Vitro Experimental Data, ACS Omega, doi:10.1021/acsomega.3c06968.
3.
Alkafaas et al., A study on the effect of natural products against the transmission of B.1.1.529 Omicron, Virology Journal, doi:10.1186/s12985-023-02160-6.
4.
Guimarães Silva et al., Are Non-Structural Proteins From SARS-CoV-2 the Target of Hydroxychloroquine? An in Silico Study, ACTA MEDICA IRANICA, doi:10.18502/acta.v61i2.12533.
5.
Nguyen et al., The Potential of Ameliorating COVID-19 and Sequelae From Andrographis paniculata via Bioinformatics, Bioinformatics and Biology Insights, doi:10.1177/11779322221149622.
7.
Yadav et al., Repurposing the Combination Drug of Favipiravir, Hydroxychloroquine and Oseltamivir as a Potential Inhibitor Against SARS-CoV-2: A Computational Study, Research Square, doi:10.21203/rs.3.rs-628277/v1.
8.
Hussein et al., Molecular Docking Identification for the efficacy of Some Zinc Complexes with Chloroquine and Hydroxychloroquine against Main Protease of COVID-19, Journal of Molecular Structure, doi:10.1016/j.molstruc.2021.129979.
9.
Baildya et al., Inhibitory capacity of Chloroquine against SARS-COV-2 by effective binding with Angiotensin converting enzyme-2 receptor: An insight from molecular docking and MD-simulation studies, Journal of Molecular Structure, doi:10.1016/j.molstruc.2021.129891.
10.
Noureddine et al., Quantum chemical studies on molecular structure, AIM, ELF, RDG and antiviral activities of hybrid hydroxychloroquine in the treatment of COVID-19: molecular docking and DFT calculations, Journal of King Saud University - Science, doi:10.1016/j.jksus.2020.101334.
11.
Tarek et al., Pharmacokinetic Basis of the Hydroxychloroquine Response in COVID-19: Implications for Therapy and Prevention, European Journal of Drug Metabolism and Pharmacokinetics, doi:10.1007/s13318-020-00640-6.
12.
Rowland Yeo et al., Impact of Disease on Plasma and Lung Exposure of Chloroquine, Hydroxychloroquine and Azithromycin: Application of PBPK Modeling, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.1955.
13.
Hitti et al., Hydroxychloroquine attenuates double-stranded RNA-stimulated hyper-phosphorylation of tristetraprolin/ZFP36 and AU-rich mRNA stabilization, Immunology, doi:10.1111/imm.13835.
14.
Yan et al., Super-resolution imaging reveals the mechanism of endosomal acidification inhibitors against SARS-CoV-2 infection, ChemBioChem, doi:10.1002/cbic.202400404.
15.
Mohd Abd Razak et al., In Vitro Anti-SARS-CoV-2 Activities of Curcumin and Selected Phenolic Compounds, Natural Product Communications, doi:10.1177/1934578X231188861.
16.
Alsmadi et al., The In Vitro, In Vivo, and PBPK Evaluation of a Novel Lung-Targeted Cardiac-Safe Hydroxychloroquine Inhalation Aerogel, AAPS PharmSciTech, doi:10.1208/s12249-023-02627-3.
17.
Wen et al., Cholinergic α7 nAChR signaling suppresses SARS-CoV-2 infection and inflammation in lung epithelial cells, Journal of Molecular Cell Biology, doi:10.1093/jmcb/mjad048.
18.
Kamga Kapchoup et al., In vitro effect of hydroxychloroquine on pluripotent stem cells and their cardiomyocytes derivatives, Frontiers in Pharmacology, doi:10.3389/fphar.2023.1128382.
19.
Milan Bonotto et al., Cathepsin inhibitors nitroxoline and its derivatives inhibit SARS-CoV-2 infection, Antiviral Research, doi:10.1016/j.antiviral.2023.105655.
20.
Miao et al., SIM imaging resolves endocytosis of SARS-CoV-2 spike RBD in living cells, Cell Chemical Biology, doi:10.1016/j.chembiol.2023.02.001.
21.
Yuan et al., Hydroxychloroquine blocks SARS-CoV-2 entry into the endocytic pathway in mammalian cell culture, Communications Biology, doi:10.1038/s42003-022-03841-8.
22.
Faísca et al., Enhanced In Vitro Antiviral Activity of Hydroxychloroquine Ionic Liquids against SARS-CoV-2, Pharmaceutics, doi:10.3390/pharmaceutics14040877.
23.
Delandre et al., Antiviral Activity of Repurposing Ivermectin against a Panel of 30 Clinical SARS-CoV-2 Strains Belonging to 14 Variants, Pharmaceuticals, doi:10.3390/ph15040445.
24.
Purwati et al., An in vitro study of dual drug combinations of anti-viral agents, antibiotics, and/or hydroxychloroquine against the SARS-CoV-2 virus isolated from hospitalized patients in Surabaya, Indonesia, PLOS One, doi:10.1371/journal.pone.0252302.
25.
Zhang et al., SARS-CoV-2 spike protein dictates syncytium-mediated lymphocyte elimination, Cell Death & Differentiation, doi:10.1038/s41418-021-00782-3.
26.
Dang et al., Structural basis of anti-SARS-CoV-2 activity of hydroxychloroquine: specific binding to NTD/CTD and disruption of LLPS of N protein, bioRxiv, doi:10.1101/2021.03.16.435741.
27.
Shang (B) et al., Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice, Virology Journal, doi:10.1186/s12985-021-01515-1.
28.
Wang et al., Chloroquine and hydroxychloroquine as ACE2 blockers to inhibit viropexis of 2019-nCoV Spike pseudotyped virus, Phytomedicine, doi:10.1016/j.phymed.2020.153333.
29.
Sheaff, R., A New Model of SARS-CoV-2 Infection Based on (Hydroxy)Chloroquine Activity, bioRxiv, doi:10.1101/2020.08.02.232892.
30.
Ou et al., Hydroxychloroquine-mediated inhibition of SARS-CoV-2 entry is attenuated by TMPRSS2, PLOS Pathogens, doi:10.1371/journal.ppat.1009212.
31.
Andreani et al., In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect, Microbial Pathogenesis, doi:10.1016/j.micpath.2020.104228.
32.
Clementi et al., Combined Prophylactic and Therapeutic Use Maximizes Hydroxychloroquine Anti-SARS-CoV-2 Effects in vitro, Front. Microbiol., 10 July 2020, doi:10.3389/fmicb.2020.01704.
33.
Liu et al., Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro, Cell Discovery 6, 16 (2020), doi:10.1038/s41421-020-0156-0.
34.
Yao et al., In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Clin. Infect. Dis., 2020 Mar 9, doi:10.1093/cid/ciaa237.
Wen et al., 25 Jul 2023, peer-reviewed, 5 authors.
Contact: zhaojincun@gird.cn, xsu@ips.ac.cn.
Cholinergic α7 nAChR signaling suppresses SARS-CoV-2 infection and inflammation in lung epithelial cells
doi:10.1093/jmcb/mjad048/7231085
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced coronavirus disease 2019 (COVID-19) has caused more than 6 million deaths and poses a huge threat to the global economy and public health. SARS-CoV-2 enters lung epithelial cells depending on S protein binding with angiotensin converting enzyme 2 (ACE2). In-silico studies have shown that both SARS-CoV and SARS-CoV-2 S glycoproteins can interact with the extracellular domain of α7 nicotinic acetylcholine receptor (nAChR). Hydroxychloroquine, a known SARS-CoV-2 inhibitor, acts at the entry stage of SARS-CoV-2 by blocking the virus-binding sites on the two receptors, ACE2 and α7 nAChR (Navya and Hosur, 2021). Given that α7 nAChR possesses anti-inflammatory effects and could interact with the SARS-CoV-2 S protein,
References
Azabou, Bao, Bounab, Vagus Nerve Stimulation: A Potential Adjunct Therapy for COVID-19, Frontiers in medicine
Bonaz, Sinniger, Pellissier, Targeting the cholinergic anti-inflammatory pathway with vagus nerve stimulation in patients with Covid-19?, Bioelectronic medicine
Hsieh, Wang, Wu, Reactive Oxygen Species-Dependent c-Fos/Activator Protein 1 Induction Upregulates Heme Oxygenase-1 Expression by Bradykinin in Brain Astrocytes, Antioxidants & redox signaling
Kipshidze, Chekanov, Kipshidze, Transpulmonary electrotherapy for reduction of lung viral load of SARS-CoV-2 in patients with COVID-19, Medical hypotheses
Kox, Pompe, Peters, alpha7 nicotinic acetylcholine receptor agonist GTS-21 attenuates ventilator-induced tumour necrosis factor-alpha production and lung injury, Br J Anaesth
Li, Li, Wang, SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling, Biochimica et biophysica acta. Molecular basis of disease
DOI record:
{
"DOI": "10.1093/jmcb/mjad048",
"ISSN": [
"1674-2788",
"1759-4685"
],
"URL": "http://dx.doi.org/10.1093/jmcb/mjad048",
"author": [
{
"affiliation": [
{
"name": "Unit of Respiratory Infection and Immunity, Institut Pasteur of Shanghai, Chinese Academy of Sciences , Shanghai 200031 , China"
},
{
"name": "CAS Key Laboratory of Molecular Virology and Immunology, Institut Pasteur of Shanghai, Chinese Academy of Sciences , Shanghai 200031 , China"
},
{
"name": "University of Chinese Academy of Sciences , Beijing 100049 , China"
}
],
"family": "Wen",
"given": "Jing",
"sequence": "first"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Respiratory Disease, National Clinical Research Centre for Respiratory Disease, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University , Guangzhou 510120 , China"
}
],
"family": "Sun",
"given": "Jing",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Respiratory Disease, National Clinical Research Centre for Respiratory Disease, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University , Guangzhou 510120 , China"
}
],
"family": "Tang",
"given": "Yanhong",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "State Key Laboratory of Respiratory Disease, National Clinical Research Centre for Respiratory Disease, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University , Guangzhou 510120 , China"
}
],
"family": "Zhao",
"given": "Jincun",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-8692-8365",
"affiliation": [
{
"name": "Unit of Respiratory Infection and Immunity, Institut Pasteur of Shanghai, Chinese Academy of Sciences , Shanghai 200031 , China"
},
{
"name": "CAS Key Laboratory of Molecular Virology and Immunology, Institut Pasteur of Shanghai, Chinese Academy of Sciences , Shanghai 200031 , China"
},
{
"name": "University of Chinese Academy of Sciences , Beijing 100049 , China"
}
],
"authenticated-orcid": false,
"family": "Su",
"given": "Xiao",
"sequence": "additional"
}
],
"container-title": "Journal of Molecular Cell Biology",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
7,
26
]
],
"date-time": "2023-07-26T03:39:02Z",
"timestamp": 1690342742000
},
"deposited": {
"date-parts": [
[
2023,
7,
26
]
],
"date-time": "2023-07-26T03:39:03Z",
"timestamp": 1690342743000
},
"indexed": {
"date-parts": [
[
2023,
7,
27
]
],
"date-time": "2023-07-27T04:29:17Z",
"timestamp": 1690432157617
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
7,
25
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "am",
"delay-in-days": 1,
"start": {
"date-parts": [
[
2023,
7,
26
]
],
"date-time": "2023-07-26T00:00:00Z",
"timestamp": 1690329600000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/jmcb/advance-article-pdf/doi/10.1093/jmcb/mjad048/50961624/mjad048.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/jmcb/advance-article-pdf/doi/10.1093/jmcb/mjad048/50961624/mjad048.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2023,
7,
25
]
]
},
"published-online": {
"date-parts": [
[
2023,
7,
25
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/jmcb/advance-article/doi/10.1093/jmcb/mjad048/7231085"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Cell Biology",
"Genetics",
"Molecular Biology",
"General Medicine"
],
"subtitle": [],
"title": "Cholinergic α7 nAChR signaling suppresses SARS-CoV-2 infection and inflammation in lung epithelial cells",
"type": "journal-article"
}
