The enhanced bioavailability of free curcumin and bioactive-metabolite tetrahydrocurcumin from a dispersible, oleoresin-based turmeric formulation
et al., Medicine, doi:10.1097/MD.0000000000026601, Jul 2021
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
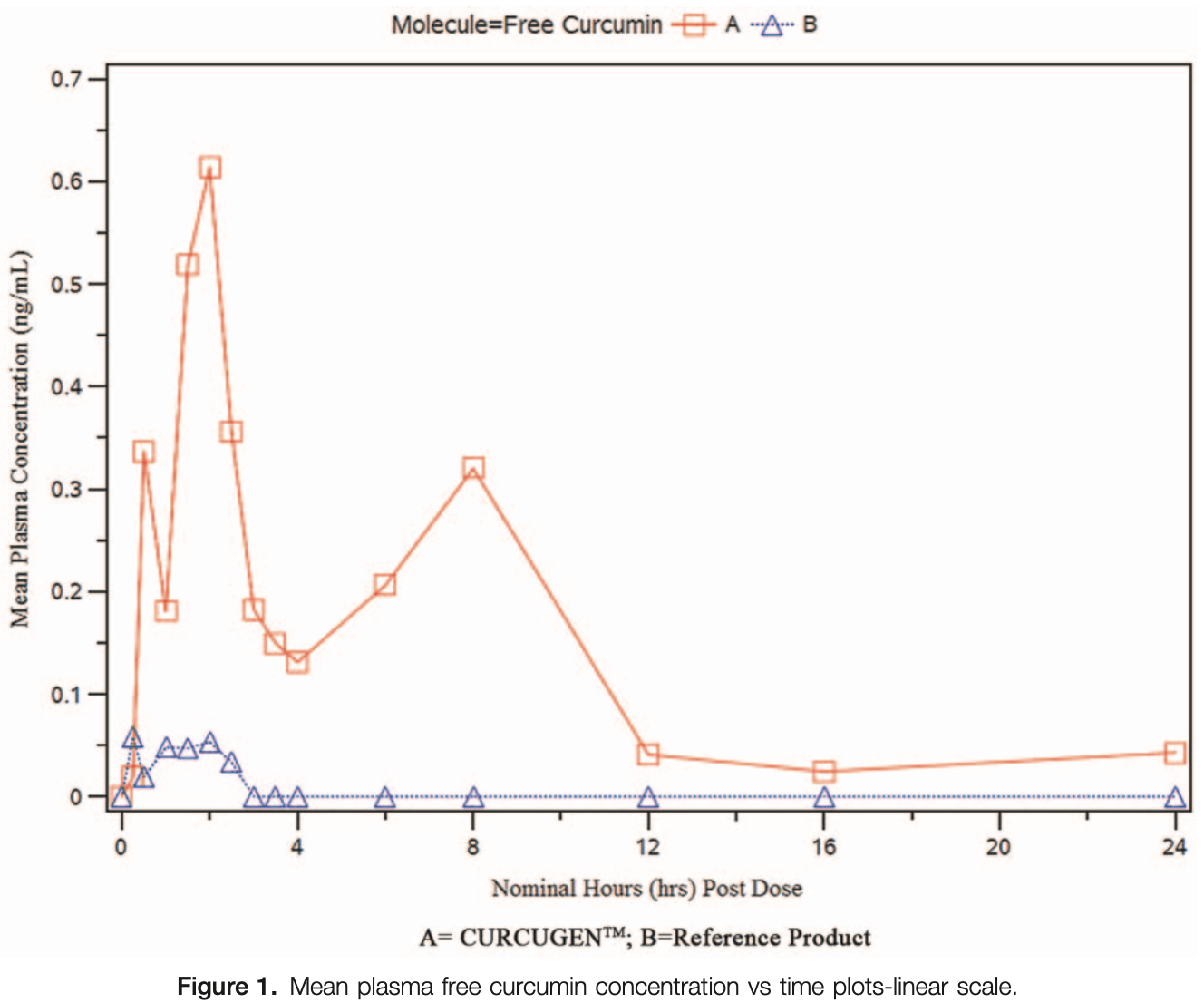
Bioavailability RCT comparing CURCUGEN, a 50% curcuminoids-concentrated turmeric extract, with curcuminoids 95% standardized extract (C-95), showing significant improvements in bioavailability.
1.
Kamble et al., Nanoparticulate curcumin spray imparts prophylactic and therapeutic properties against SARS-CoV-2, Emergent Materials, doi:10.1007/s42247-024-00754-6.
2.
Vaiss et al., Curcumin and quercetin co-encapsulated in nanoemulsions for nasal administration: a promising therapeutic and prophylactic treatment for viral respiratory infections, European Journal of Pharmaceutical Sciences, doi:10.1016/j.ejps.2024.106766.
Panda et al., 9 Jul 2021, peer-reviewed, 4 authors.
The enhanced bioavailability of free curcumin and bioactive-metabolite tetrahydrocurcumin from a dispersible, oleoresin-based turmeric formulation
Medicine, doi:10.1097/md.0000000000026601
Background: Curcuminoids have been widely studied for human health and disease applications, yet bioavailability remains a hurdle to actualizing all the benefits ascribed to them. The lack of standardization in analysis method, confusion about what constitutes an ideal analyte, and conflicting thoughts around dosing strategies have made it difficult to draw parity between bioavailability and bioactivity and establish a baseline for formulation comparisons. Methods: This randomized double-blinded, 2-way cross over, single oral dose, comparative bioavailability study differentially evaluates curcumin at the time of its absorption and along various biotransformation pathways, to include free curcumin, the readily usable form of curcumin; individual and composite totals of curcumin and its analogues as exogenously cleaved conjugates, for example, total curcumin, total demethoxycurcumin (DMC), total bisdemethoxycurcumin (BDMC), and total curcuminoids respectively; and the bioactive metabolite of curcumin, total tetrahydrocurcumin (THC). As a primary study objective, the relative bioavailability of CURCUGEN, a novel dispersible, 50% curcuminoids-concentrated turmeric extract was compared to the standard curcumin reference product, curcuminoids 95% standardized extract (C-95), using the maximum concentration (C max ), and area under the curve (AUC 0-t ) of free curcumin, total curcumin, total DMC, total BDMC and the curcumin active metabolite, as total THC.
Results: The evaluation of free curcumin demonstrated that the C max and AUC 0-t of the CURCUGEN was 16.1 times and 39 times higher than the C max and AUC 0-t of C-95. Furthermore, total curcumin, total DMC, total BDMC, and total curcuminoids resulted in AUC 0-t of the CURCUGEN at 49.5-, 43.5-, 46.8-, and 52.5-fold higher than C-95, respectively. The relative bioavailability of CURCUGEN for total THC was found to be 31 times higher when compared to C-95.
Conclusion: As the first human pharmacokinetics study to apply best-practice recommendations and pharmaceutically-aligned guidance in the comprehensive evaluation of a novel curcuminoids formulation, we have established the novelty of said formulation while better standardizing for the common variances and discrepancies between curcuminoids and their derivatives in the literature and commercial marketing, alike. Abbreviations: AUC = area under the curve, BDMC = bisdemethoxycurcumin, C-95 = curcuminoids 95% standardized extract, C max = maximum concentration, DMC = demethoxycurcumin, THC = tetrahydrocurcumin, T max = time to maximum serum concentration.
Author contributions Conceptualization: Sanjib Kumar Panda
References
Aggarwal, Deb, Prasad, Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses, Molecules
Aggarwal, Harikumar, Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases, Int J Biochem Cell Biol
Anand, Kunnumakkara, Newman, Bioavailability of curcumin: problems and promises, Mol Pharm
Bartholome, Haenen, Hollman, Deconjugation kinetics of glucuronidated phase II flavonoid metabolites by b-glucuronidase from neutrophils, Drug Metab Pharmacokinet
Cas, Ghidoni, Dietary curcumin: correlation between bioavailability and health potential, Nutrients
Cder, Guidance for the Industry: Bioavailability and Bioequivalence Studies Submitted in NDAs or INDs -General Considerations
Cheng, Hsu, Lin, Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions, Anticancer Res
Cuomo, Appendino, Dern, Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation, J Nat Prod
Douglass, Clouatre, Beyond yellow curry: assessing commercial curcumin absorption technologies, J Am Coll Nutr
Gordon, Luis, Ashley, Oxidative transformation of demethoxy-and bisdemethoxycurcumin: products, mechanism of formation, and poisoning of human topoisomerase IIa, Chem Res Toxicol
Han, Chung, Robertson, Ranjan, Bondada, Curcumin causes the growth arrest and apoptosis of B cell lymphoma by downregulation of egr-1, c-myc, bcl-XL, NF-kappa B, and p53, Clin Immunol
Hatamipour, Ramezani, Tabassi, Johnston, Ramezani et al., Demethoxycurcumin: a naturally occurring curcumin analogue with antitumor properties, J Cell Physiol
Jin, Du, Bisdemethoxycurcumin protects against renal fibrosis via activation of fibroblast apoptosis, Eur J Pharmacol Mar
Jäger, Lowery, Calvanese, Comparative absorption of curcumin formulations, Nutr J
Kim, Kang, Lee, Hepatoprotective effect and synergism of bisdemethoycurcumin against MCD diet-induced nonalcoholic fatty liver disease in mice, PLoS One
Kotra, Satyabanta, Goswami, A critical review of analytical methods for determination of curcuminoids in turmeric, J Food Sci Technol
Kumar, Jacob, Subash, Enhanced bioavailability and relative distribution of free (unconjugated) curcuminoids following the oral administration of a food-grade formulation with fenugreek dietary fibre: a randomised double-blind crossover study, J Funct Foods
Kunihiro, Luis, Brickey, Beta-glucuronidase catalyzes deconjugation and activation of curcumin-glucuronide in bone, J Nat Prod
Kunnumakkara, Bordoloi, Padmavathi, Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases, Br J Pharmacol
Lai, Chen, Lee, Bisdemethoxycurcumin inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat diet-fed C57BL/6 mice, J Agric Food Chem
Lao, Ruffin, Normolle, Dose escalation of a curcuminoid formulation, BMC Complement Altern Med March
Liu, Lou, Zhao, Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin, J Pharm Biomed Anal
Matsunaga, Endo, Soda, Potent and selective inhibition of the tumor marker AKR1B10 by bisdemethoxycurcumin: probing the active site of the enzyme with molecular modeling and site-directed mutagenesis, Biochem Biophys Res Commun
Meo, Margarucci, Galderisi, Crispi, Peluso, Curcumin, gut microbiota, and neuroprotection, Nutrients
Mishra, Palanivelu, The effect of curcumin (turmeric) on Alzheimer's disease: an overview, Ann Indian Acad Neurol
Nasef, Loveday, Golding, Food matrix and co-presence of turmeric compounds influence bioavailability of curcumin in healthy humans, Food & Function
Panda, None, Medicine
Panda, None, Medicine
Rao, Regulation of COX and LOX by curcumin, Adv Exp Med Biol
Sasaki, Sunagawa, Takahasi, Innovative preparation of curcumin for improved oral bioavailability, Biol Pharm Bull
Schiborr, Kocher, Behnam, Jandasek, Toelstede et al., The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes, Mol Nutr Food Res
Shah, Nawaz, Pertani, Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor-and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca 2+ signaling, Biochem Pharmacol
Shimoi, Nakayama, Glucuronidase deconjugation in inflammation, Methods Enzymol
Shoba, Joy, Joseph, Majeed, Rajendran et al., Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers, Planta Med
Singh, Tripathi, Rai, An appraisal of the bioavailability enhancers in Ayurveda in the light of recent pharmacological advances, Ayu
Stohs, Chen, Preuss, The fallacy of enzymatic hydrolysis for the determination of bioactive curcumin in plasma samples as an indication of bioavailability: a comparative study, BMC Complement Altern Med
Stohs, Chen, Ray, Ji, Bucci et al., Highly bioavailable forms of curcumin and promising avenues for curcumin-based research and application: a review, Molecules
Vareed, Kakarala, Ruffin, Pharmacokinetics of curcumin conjugate metabolism in healthy human subjects, Cancer Epidemiol Biomarkers Prev
Yoshiteru, Hashimoto, Tomita-Yokotani, Kobayashi, Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism, Proc Natl Acad Sci U S A
Yue, Cheng, Yu, The role of turmerones on curcumin transportation and P-glycoprotein activities in intestinal Caco-2 cells, J Med Food
Zhang, Xing, Zhao, Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs, Pharmaceutics
DOI record:
{
"DOI": "10.1097/md.0000000000026601",
"ISSN": [
"0025-7974",
"1536-5964"
],
"URL": "http://dx.doi.org/10.1097/MD.0000000000026601",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background:</jats:title>\n <jats:p>Curcuminoids have been widely studied for human health and disease applications, yet bioavailability remains a hurdle to actualizing all the benefits ascribed to them. The lack of standardization in analysis method, confusion about what constitutes an ideal analyte, and conflicting thoughts around dosing strategies have made it difficult to draw parity between bioavailability and bioactivity and establish a baseline for formulation comparisons.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods:</jats:title>\n <jats:p>This randomized double-blinded, 2-way cross over, single oral dose, comparative bioavailability study differentially evaluates curcumin at the time of its absorption and along various biotransformation pathways, to include free curcumin, the readily usable form of curcumin; individual and composite totals of curcumin and its analogues as exogenously cleaved conjugates, for example, total curcumin, total demethoxycurcumin (DMC), total bisdemethoxycurcumin (BDMC), and total curcuminoids respectively; and the bioactive metabolite of curcumin, total tetrahydrocurcumin (THC). As a primary study objective, the relative bioavailability of CURCUGEN, a novel dispersible, 50% curcuminoids-concentrated turmeric extract was compared to the standard curcumin reference product, curcuminoids 95% standardized extract (C-95), using the maximum concentration (<jats:italic toggle=\"yes\">C</jats:italic>\n <jats:sub>max</jats:sub>), and area under the curve (AUC<jats:sub>0-t</jats:sub>) of free curcumin, total curcumin, total DMC, total BDMC and the curcumin active metabolite, as total THC.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results:</jats:title>\n <jats:p>The evaluation of free curcumin demonstrated that the <jats:italic toggle=\"yes\">C</jats:italic>\n <jats:sub>max</jats:sub> and AUC<jats:sub>0-t</jats:sub> of the CURCUGEN was 16.1 times and 39 times higher than the <jats:italic toggle=\"yes\">C</jats:italic>\n <jats:sub>max</jats:sub> and AUC<jats:sub>0-t</jats:sub> of C-95. Furthermore, total curcumin, total DMC, total BDMC, and total curcuminoids resulted in AUC<jats:sub>0-t</jats:sub> of the CURCUGEN at 49.5-, 43.5-, 46.8-, and 52.5-fold higher than C-95, respectively. The relative bioavailability of CURCUGEN for total THC was found to be 31 times higher when compared to C-95.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion:</jats:title>\n <jats:p>As the first human pharmacokinetics study to apply best-practice recommendations and pharmaceutically-aligned guidance in the comprehensive evaluation of a novel curcuminoids formulation, we have established the novelty of said formulation while better standardizing for the common variances and discrepancies between curcuminoids and their derivatives in the literature and commercial marketing, alike.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Olene Life Sciences Private Limited, Chennai, Tamil Nadu, India"
}
],
"family": "Panda",
"given": "Sanjib Kumar",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Olene Life Sciences Private Limited, Chennai, Tamil Nadu, India"
}
],
"family": "Nirvanashetty",
"given": "Somashekara",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Research, Vimta Labs Ltd, Hyderabad, Telangana, India"
}
],
"family": "Missamma",
"given": "M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6269-9035",
"affiliation": [
{
"name": "Dolcas Biotech LLC, Landing, NJ."
}
],
"authenticated-orcid": false,
"family": "Jackson-Michel",
"given": "Shavon",
"sequence": "additional"
}
],
"container-title": "Medicine",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
7,
7
]
],
"date-time": "2021-07-07T17:58:38Z",
"timestamp": 1625680718000
},
"deposited": {
"date-parts": [
[
2023,
9,
16
]
],
"date-time": "2023-09-16T05:55:44Z",
"timestamp": 1694843744000
},
"funder": [
{
"award": [
"no"
],
"name": "DolCas Biotech, LLC"
}
],
"indexed": {
"date-parts": [
[
2024,
2,
5
]
],
"date-time": "2024-02-05T12:30:46Z",
"timestamp": 1707136246642
},
"is-referenced-by-count": 10,
"issue": "27",
"issued": {
"date-parts": [
[
2021,
7,
9
]
]
},
"journal-issue": {
"issue": "27",
"published-online": {
"date-parts": [
[
2021
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
9
]
],
"date-time": "2021-07-09T00:00:00Z",
"timestamp": 1625788800000
}
}
],
"link": [
{
"URL": "https://journals.lww.com/10.1097/MD.0000000000026601",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "276",
"original-title": [],
"page": "e26601",
"prefix": "10.1097",
"published": {
"date-parts": [
[
2021,
7,
9
]
]
},
"published-online": {
"date-parts": [
[
2021,
7,
9
]
]
},
"publisher": "Ovid Technologies (Wolters Kluwer Health)",
"reference": [
{
"DOI": "10.4103/0972-2327.40220",
"article-title": "The effect of curcumin (turmeric) on Alzheimer's disease: an overview",
"author": "Mishra",
"doi-asserted-by": "crossref",
"first-page": "13",
"journal-title": "Ann Indian Acad Neurol",
"key": "R1-20230916",
"volume": "11",
"year": "2008"
},
{
"DOI": "10.4103/ayu.AYU_11_15",
"article-title": "An appraisal of the bioavailability enhancers in Ayurveda in the light of recent pharmacological advances",
"author": "Singh",
"doi-asserted-by": "crossref",
"first-page": "03",
"journal-title": "Ayu",
"key": "R2-20230916",
"volume": "37",
"year": "2016"
},
{
"DOI": "10.1073/pnas.1016217108",
"article-title": "Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism",
"author": "Yoshiteru",
"doi-asserted-by": "crossref",
"first-page": "6615",
"journal-title": "Proc Natl Acad Sci U S A",
"key": "R3-20230916",
"volume": "108",
"year": "2011"
},
{
"DOI": "10.3390/molecules20010185",
"article-title": "Curcumin differs from tetrahydrocurcumin for molecular targets, signaling pathways and cellular responses",
"author": "Aggarwal",
"doi-asserted-by": "crossref",
"first-page": "185",
"journal-title": "Molecules",
"key": "R4-20230916",
"volume": "20",
"year": "2014"
},
{
"DOI": "10.1111/bph.13621",
"article-title": "Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases",
"author": "Kunnumakkara",
"doi-asserted-by": "crossref",
"first-page": "1325",
"journal-title": "Br J Pharmacol",
"key": "R5-20230916",
"volume": "174",
"year": "2017"
},
{
"DOI": "10.1006/clim.1999.4769",
"article-title": "Curcumin causes the growth arrest and apoptosis of B cell lymphoma by downregulation of egr-1, c-myc, bcl-XL, NF-kappa B, and p53",
"author": "Han",
"doi-asserted-by": "crossref",
"first-page": "152",
"journal-title": "Clin Immunol",
"key": "R6-20230916",
"volume": "93",
"year": "1999"
},
{
"DOI": "10.1007/978-0-387-46401-5_9",
"article-title": "Regulation of COX and LOX by curcumin",
"author": "Rao",
"doi-asserted-by": "crossref",
"first-page": "213",
"journal-title": "Adv Exp Med Biol",
"key": "R7-20230916",
"volume": "595",
"year": "2007"
},
{
"DOI": "10.1016/S0006-2952(99)00206-3",
"article-title": "Inhibitory effect of curcumin, a food spice from turmeric, on platelet-activating factor- and arachidonic acid-mediated platelet aggregation through inhibition of thromboxane formation and Ca2+ signaling",
"author": "Shah",
"doi-asserted-by": "crossref",
"first-page": "1167",
"journal-title": "Biochem Pharmacol",
"key": "R8-20230916",
"volume": "58",
"year": "1999"
},
{
"DOI": "10.1016/j.biocel.2008.06.010",
"article-title": "Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases",
"author": "Aggarwal",
"doi-asserted-by": "crossref",
"first-page": "40",
"journal-title": "Int J Biochem Cell Biol",
"key": "R9-20230916",
"volume": "41",
"year": "2009"
},
{
"article-title": "Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions",
"author": "Cheng",
"first-page": "2895",
"journal-title": "Anticancer Res",
"key": "R10-20230916",
"volume": "21",
"year": "2001"
},
{
"DOI": "10.1186/1472-6882-6-10",
"article-title": "Dose escalation of a curcuminoid formulation",
"author": "Lao",
"doi-asserted-by": "crossref",
"first-page": "10",
"journal-title": "BMC Complement Altern Med March",
"key": "R11-20230916",
"volume": "6",
"year": "2006"
},
{
"DOI": "10.1055/s-2006-957450",
"article-title": "Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers",
"author": "Shoba",
"doi-asserted-by": "crossref",
"first-page": "353",
"journal-title": "Planta Med",
"key": "R12-20230916",
"volume": "64",
"year": "1998"
},
{
"DOI": "10.3390/molecules25061397",
"article-title": "Highly bioavailable forms of curcumin and promising avenues for curcumin-based research and application: a review",
"author": "Stohs",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "Molecules",
"key": "R13-20230916",
"volume": "25",
"year": "2020"
},
{
"DOI": "10.1080/07315724.2014.950392",
"article-title": "Beyond yellow curry: assessing commercial curcumin absorption technologies",
"author": "Douglass",
"doi-asserted-by": "crossref",
"first-page": "347",
"journal-title": "J Am Coll Nutr",
"key": "R14-20230916",
"volume": "34",
"year": "2015"
},
{
"DOI": "10.3390/pharmaceutics10030074",
"article-title": "Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "74",
"journal-title": "Pharmaceutics",
"key": "R15-20230916",
"volume": "10",
"year": "2018"
},
{
"DOI": "10.1089/jmf.2011.1845",
"article-title": "The role of turmerones on curcumin transportation and P-glycoprotein activities in intestinal Caco-2 cells",
"author": "Yue",
"doi-asserted-by": "crossref",
"first-page": "242",
"journal-title": "J Med Food",
"key": "R16-20230916",
"volume": "15",
"year": "2012"
},
{
"DOI": "10.1039/C9FO01063G",
"article-title": "Food matrix and co-presence of turmeric compounds influence bioavailability of curcumin in healthy humans",
"author": "Nasef",
"doi-asserted-by": "crossref",
"first-page": "4584",
"journal-title": "Food & Function",
"key": "R17-20230916",
"volume": "10",
"year": "2019"
},
{
"DOI": "10.1002/jcp.27029",
"article-title": "Demethoxycurcumin: a naturally occurring curcumin analogue with antitumor properties",
"author": "Hatamipour",
"doi-asserted-by": "crossref",
"first-page": "9247",
"journal-title": "J Cell Physiol",
"key": "R18-20230916",
"volume": "233",
"year": "2018"
},
{
"DOI": "10.1016/j.bbrc.2009.08.107",
"article-title": "Potent and selective inhibition of the tumor marker AKR1B10 by bisdemethoxycurcumin: probing the active site of the enzyme with molecular modeling and site-directed mutagenesis",
"author": "Matsunaga",
"doi-asserted-by": "crossref",
"first-page": "128",
"journal-title": "Biochem Biophys Res Commun",
"key": "R19-20230916",
"volume": "389",
"year": "2009"
},
{
"DOI": "10.1021/acs.jafc.5b05577",
"article-title": "Bisdemethoxycurcumin inhibits adipogenesis in 3T3-L1 preadipocytes and suppresses obesity in high-fat diet-fed C57BL/6 mice",
"author": "Lai",
"doi-asserted-by": "crossref",
"first-page": "821",
"journal-title": "J Agric Food Chem",
"key": "R20-20230916",
"volume": "64",
"year": "2016"
},
{
"article-title": "Hepatoprotective effect and synergism of bisdemethoycurcumin against MCD diet-induced nonalcoholic fatty liver disease in mice",
"author": "Kim",
"first-page": "01",
"journal-title": "PLoS One",
"key": "R21-20230916",
"volume": "11",
"year": "2016"
},
{
"DOI": "10.1016/j.ejphar.2019.01.012",
"article-title": "Bisdemethoxycurcumin protects against renal fibrosis via activation of fibroblast apoptosis",
"author": "Jin",
"doi-asserted-by": "crossref",
"first-page": "26",
"journal-title": "Eur J Pharmacol Mar",
"key": "R22-20230916",
"volume": "847",
"year": "2019"
},
{
"DOI": "10.1021/acs.chemrestox.5b00009",
"article-title": "Oxidative transformation of demethoxy- and bisdemethoxycurcumin: products, mechanism of formation, and poisoning of human topoisomerase IIα",
"author": "Gordon",
"doi-asserted-by": "crossref",
"first-page": "989",
"journal-title": "Chem Res Toxicol",
"key": "R23-20230916",
"volume": "28",
"year": "2015"
},
{
"DOI": "10.1007/s13197-019-03986-1",
"article-title": "A critical review of analytical methods for determination of curcuminoids in turmeric",
"author": "Kotra",
"doi-asserted-by": "crossref",
"first-page": "5153",
"journal-title": "J Food Sci Technol",
"key": "R24-20230916",
"volume": "56",
"year": "2019"
},
{
"DOI": "10.1021/np1007262",
"article-title": "Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation",
"author": "Cuomo",
"doi-asserted-by": "crossref",
"first-page": "664",
"journal-title": "J Nat Prod",
"key": "R25-20230916",
"volume": "74",
"year": "2011"
},
{
"DOI": "10.1186/1475-2891-13-11",
"article-title": "Comparative absorption of curcumin formulations",
"author": "Jäger",
"doi-asserted-by": "crossref",
"first-page": "01",
"journal-title": "Nutr J",
"key": "R26-20230916",
"volume": "13",
"year": "2014"
},
{
"DOI": "10.1021/mp700113r",
"article-title": "Bioavailability of curcumin: problems and promises",
"author": "Anand",
"doi-asserted-by": "crossref",
"first-page": "807",
"journal-title": "Mol Pharm",
"key": "R27-20230916",
"volume": "4",
"year": "2007"
},
{
"DOI": "10.3390/nu11092147",
"article-title": "Dietary curcumin: correlation between bioavailability and health potential",
"author": "Dei Cas",
"doi-asserted-by": "crossref",
"first-page": "2147",
"journal-title": "Nutrients",
"key": "R29-20230916",
"volume": "11",
"year": "2019"
},
{
"DOI": "10.1016/j.jff.2016.01.039",
"article-title": "Enhanced bioavailability and relative distribution of free (unconjugated) curcuminoids following the oral administration of a food-grade formulation with fenugreek dietary fibre: a randomised double-blind crossover study",
"author": "Kumar",
"doi-asserted-by": "crossref",
"first-page": "578",
"journal-title": "J Funct Foods",
"key": "R30-20230916",
"volume": "22",
"year": "2016"
},
{
"DOI": "10.1186/s12906-019-2699-x",
"article-title": "The fallacy of enzymatic hydrolysis for the determination of bioactive curcumin in plasma samples as an indication of bioavailability: a comparative study",
"author": "Stohs",
"doi-asserted-by": "crossref",
"first-page": "293",
"journal-title": "BMC Complement Altern Med",
"key": "R31-20230916",
"volume": "19",
"year": "2019"
},
{
"DOI": "10.1158/1055-9965.EPI-07-2693",
"article-title": "Pharmacokinetics of curcumin conjugate metabolism in healthy human subjects",
"author": "Vareed",
"doi-asserted-by": "crossref",
"first-page": "1411",
"journal-title": "Cancer Epidemiol Biomarkers Prev",
"key": "R32-20230916",
"volume": "17",
"year": "2008"
},
{
"DOI": "10.1002/mnfr.201300724",
"article-title": "The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes",
"author": "Schiborr",
"doi-asserted-by": "crossref",
"first-page": "516",
"journal-title": "Mol Nutr Food Res",
"key": "R33-20230916",
"volume": "58",
"year": "2014"
},
{
"DOI": "10.1248/bpb.34.660",
"article-title": "Innovative preparation of curcumin for improved oral bioavailability",
"author": "Sasaki",
"doi-asserted-by": "crossref",
"first-page": "660",
"journal-title": "Biol Pharm Bull",
"key": "R34-20230916",
"volume": "34",
"year": "2011"
},
{
"DOI": "10.1021/acs.jnatprod.8b00873",
"article-title": "Beta-glucuronidase catalyzes deconjugation and activation of curcumin-glucuronide in bone",
"author": "Kunihiro",
"doi-asserted-by": "crossref",
"first-page": "500",
"journal-title": "J Nat Prod",
"key": "R35-20230916",
"volume": "82",
"year": "2019"
},
{
"DOI": "10.2133/dmpk.DMPK-10-RG-002",
"article-title": "Deconjugation kinetics of glucuronidated phase II flavonoid metabolites by β-glucuronidase from neutrophils",
"author": "Bartholome",
"doi-asserted-by": "crossref",
"first-page": "379",
"journal-title": "Drug Metab Pharmacokinet",
"key": "R36-20230916",
"volume": "25",
"year": "2010"
},
{
"DOI": "10.1016/S0076-6879(05)00015-7",
"article-title": "Glucuronidase deconjugation in inflammation",
"author": "Shimoi",
"doi-asserted-by": "crossref",
"first-page": "263",
"journal-title": "Methods Enzymol",
"key": "R37-20230916",
"volume": "400",
"year": "2005"
},
{
"DOI": "10.1016/j.jpba.2005.09.032",
"article-title": "Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin",
"author": "Liu",
"doi-asserted-by": "crossref",
"first-page": "720",
"journal-title": "J Pharm Biomed Anal",
"key": "R38-20230916",
"volume": "40",
"year": "2006"
},
{
"DOI": "10.3390/nu11102426",
"article-title": "Curcumin, gut microbiota, and neuroprotection",
"author": "Di Meo",
"doi-asserted-by": "crossref",
"first-page": "2426",
"journal-title": "Nutrients",
"key": "R39-20230916",
"volume": "11",
"year": "2019"
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.lww.com/10.1097/MD.0000000000026601"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The enhanced bioavailability of free curcumin and bioactive-metabolite tetrahydrocurcumin from a dispersible, oleoresin-based turmeric formulation",
"type": "journal-article",
"volume": "100"
}
