Oral nano-curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease-19 patients: An open label nonrandomized clinical trial
et al., Phytotherapy Research, doi:10.1002/ptr.7004
, Jan 2021
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
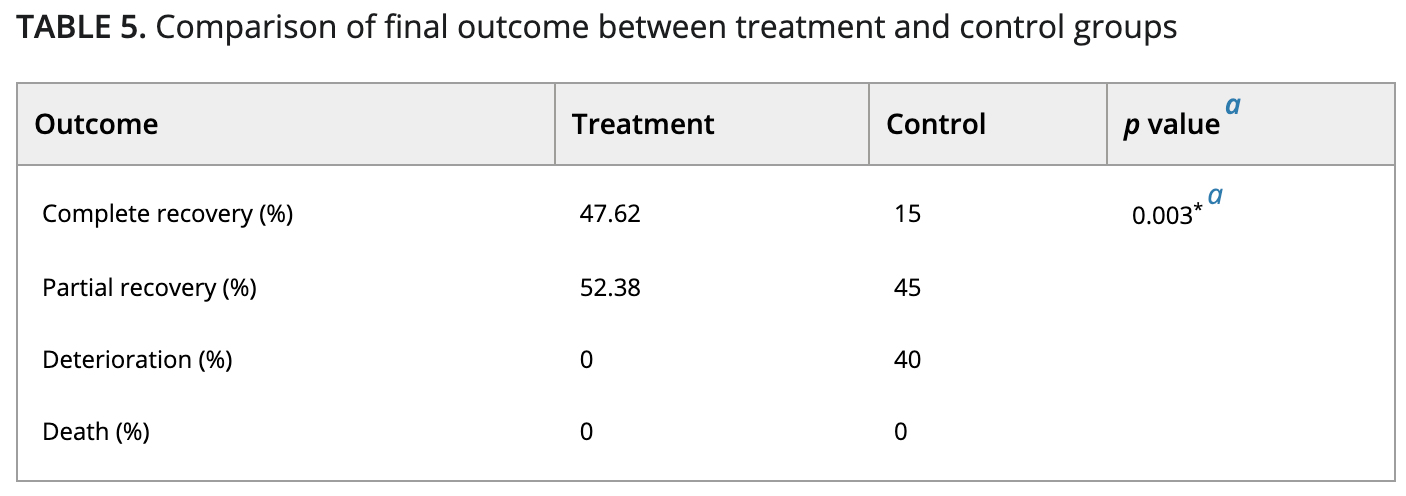
Small prospective nonrandomized trial with 41 patients, 21 treated with curcumin, showing lower disease progression and faster recovery with treatment. IRCT20200408046990N1.
This is the 2nd of 28 COVID-19 controlled studies for curcumin, which collectively show efficacy with p=0.0000000061.
21 studies are RCTs, which show efficacy with p=0.0000022.
|
risk of progression, 94.3% lower, RR 0.06, p = 0.001, treatment 0 of 21 (0.0%), control 8 of 20 (40.0%), NNT 2.5, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of no recovery, 38.4% lower, RR 0.62, p = 0.04, treatment 11 of 21 (52.4%), control 17 of 20 (85.0%), NNT 3.1.
|
|
hospitalization time, 44.8% lower, relative time 0.55, p < 0.001, treatment 21, control 20.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Saber-Moghaddam et al., 3 Jan 2021, prospective, Iran, peer-reviewed, 9 authors.
Oral nano‐curcumin formulation efficacy in management of mild to moderate hospitalized coronavirus disease ‐19 patients: An open label nonrandomized clinical trial
Phytotherapy Research, doi:10.1002/ptr.7004
Curcumin is proposed as a potential treatment option for coronavirus disease-19 by inhibiting the virus entrance, encapsulation and replication, and modulating various cellular signaling pathways. In this open-label nonrandomized clinical trial, efficacy of nano-curcumin oral formulation has been evaluated in hospitalized patients with mild-moderate COVID-19. Forty-one patients who fulfilled the inclusion criteria were allocated to nano-curcumin (n = 21) group (Sinacurcumin soft gel, contains 40 mg curcuminoids as nanomicelles, two capsules twice a day) or control (n = 20) group, for 2 weeks. Patients' symptoms and laboratory data were assessed at baseline and during follow-up period. Most of symptoms including fever and chills, tachypnea, myalgia, and cough resolved significantly faster in curcumin group. Moreover, SaO 2 was significantly higher in treatment group after 2, 4, 7, and 14 days of follow-up and lymphocyte count after 7 and 14 days. Duration of supplemental O 2 use and hospitalization was also meaningfully shorter in treatment group. It is also noteworthy to mention that no patient in treatment group experienced deterioration of infection during follow-up period, but it occurred in 40% of control group. Oral curcumin nano-formulation can significantly improve recovery time in hospitalized COVID-19 patients. Further randomized placebo controlled trials with larger sample size are recommended.
References
Abdollahi, Momtazi, Johnston, Sahebkar, Therapeutic effects of curcumin in inflammatory and immune-mediated diseases: A nature-made jack-of-all-trades?, Journal of Cellular Physiology, doi:10.1002/jcp.25778
Akbari, Kariznavi, Jannati, Elyasi, Tayarani-Najaran, Curcumin as a preventive or therapeutic measure for chemotherapy and radiotherapy induced adverse reaction: A comprehensive review, Food and Chemical Toxicology, doi:10.1016/j.fct.2020.111699
Avasarala, Zhang, Liu, Wang, London et al., Correction: Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome, PLoS One, doi:10.1371/journal.pone.0057285
Avasarala, Zhang, Liu, Wang, London et al., Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome, PLoS One, doi:10.1371/journal.pone.0057285
Biswas, Mcclure, Jimenez, Megson, Rahman, Curcumin induces glutathione biosynthesis and inhibits NF-κB activation and interleukin-8 release in alveolar epithelial cells: Mechanism of free radical scavenging activity, Antioxidants & Redox Signaling, doi:10.1089/ars.2005.7.32
Castro Rocha, Renato De Assis, Curcumin as a potential treatment for COVID-19, Phytotherapy Research, doi:10.1002/ptr.6745
Gupta, Patchva, Koh, Aggarwal, Discovery of curcumin, a component of golden spice, and its miraculous biological activities, Clinical and Experimental Pharmacology and Physiology, doi:10.1111/j.1440-1681.2011.05648.x
Hatamipour, Sahebkar, Alavizadeh, Dorri, Jaafari, Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids, Iranian Journal of Basic Medical Sciences, doi:10.22038/ijbms.2019.32873.7852
Ho, Bryson, Rumsfeld, Medication adherence: Its importance in cardiovascular outcomes, Circulation, doi:10.1161/circulationaha.108.768986
Jain, Rains, Croad, Larson, Jones, Curcumin supplementation lowers TNF-α, IL-6, IL-8, and MCP-1 secretion in high glucosetreated cultured monocytes and blood levels of TNF-α, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats, Antioxidants & Redox Signaling, doi:10.1089/ars.2008.2140
Jäger, Lowery, Calvanese, Joy, Purpura et al., Comparative absorption of curcumin formulations, Nutrition Journal, doi:10.1186/1475-2891-13-11
Kloesch, Becker, Dietersdorfer, Kiener, Steiner, Anti-inflammatory and apoptotic effects of the polyphenol curcumin on human fibroblast-like synoviocytes, International Immunopharmacology, doi:10.1016/j.intimp.2013.01.003
Kulkarni, Kumar, Tevethia, Premkumar, Arab et al., Systematic review with meta-analysis: Liver manifestations and outcomes in COVID-19, Alimentary Pharmacology & Therapeutics, doi:10.1111/apt.15916
Kumar-M, Mishra, Jha, Shukla, Choudhury et al., Coronavirus disease (COVID-19) and the liver: A comprehensive systematic review and meta-analysis, Hepatology International, doi:10.1007/s12072-020-10071-9
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: Consider cytokine storm syndromes and immunosuppression, Lancet, doi:10.1016/S0140-6736(20)30628-0
Moballegh Naseri, Abadi, Poormoghadam, Zarrabi, Keyhanvar et al., Curcumin delivery mediated by bio-Based nanoparticles, A Review. Molecules
Moghadamtousi, Abdul Kadir, Hassandarvish, Tajik, Abubakar et al., A review on antibacterial, antiviral, and antifungal activity of curcumin, BioMed Research International, doi:10.1155/2014/186864
Mollazadeh, Cicero, Blesso, Pirro, Majeed et al., Immune modulation by curcumin: The role of interleukin-10, Critical Reviews in Food Science and Nutrition, doi:10.1080/10408398.2017.1358139
Mounce, Cesaro, Carrau, Vallet, Vignuzzi, Curcumin inhibits Zika and chikungunya virus infection by inhibiting cell binding, Antiviral Research, doi:10.1016/j.antiviral.2017.03.014
Nelson, Dahlin, Bisson, Graham, Pauli et al., The essential medicinal chemistry of curcumin, Journal of Medicinal Chemistry
Noor, Islam, Prevalence and associated risk factors of mortality among COVID-19 patients: A meta-analysis, Journal of Community Health, doi:10.1007/s10900-020-00920-x
Praditya, Kirchhoff, Brüning, Rachmawati, Steinmann et al., Anti-infective properties of the golden spice curcumin, Frontiers in Microbiology
Prasad, Tyagi, Curcumin and its analogues: A potential natural compound against HIV infection and AIDS, Food & Function, doi:10.1039/c5fo00485c
Raflee, Nelson, Manley, Wellner, Floer et al., Effect of curcumin on acidic pH-induced expression of IL-6 and IL-8 in human esophageal epithelial cells (HET-1A): Role of PKC, MAPKs, and NF-ĸB, American Journal of Physiology-Gastrointestinal and Liver Physiology, doi:10.1152/ajpgi.90428.2008
Richardson, Griffin, Tucker, Smith, Oechsle et al., Baricitinib as potential treatment for 2019-nCoV acute respiratory disease, Lancet, doi:10.1016/S0140-6736(20)30304-4
Rodriguez-Morales, Cardona-Ospina, Gutiérrez-Ocampo, Villamizar-Peña, Holguin-Rivera et al., Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis, Travel Medicine and Infectious Disease, doi:10.1016/j.tmaid.2020.101623
Roy, Sarkar, Celik, Ghosh, Basu et al., Can concomitant use of zinc and curcumin with other immunityboosting nutraceuticals be the arsenal against COVID-19?, Phytotherapy Research, doi:10.1002/ptr.6766
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Medicine, doi:10.1007/s00134-020-05991-x
Shakoory, Carcillo, Chatham, Amdur, Zhao et al., Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of the macrophage activation syndrome: Re-analysis of a prior Phase III trial, Critical Care Medicine, doi:10.1097/CCM.0000000000001402
Song, Ge, Cai, Zhang, Curcumin protects mice from coxsackievirus B3-induced myocarditis by inhibiting the phosphatidylinositol 3 kinase/Akt/nuclear factor-κB pathway, Journal of Cardiovascular Pharmacology and Therapeutics, doi:10.1177/1074248413503044
Utomo, Meiyanto, Revealing the potency of citrus and galangal constituents to Halt SARS-CoV-2 infection, Preprints, doi:10.20944/preprints202003.0214.v1
Wang, Horby, Hayden, Gao, A novel coronavirus outbreak of global health concern, The Lancet, doi:10.1016/S0140-6736(20)30185-9
Whitehead, Julious, Cooper, Campbell, Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable, Statistical Methods in Medical Research, doi:10.1177/0962280215588241
Zahedipour, Hosseini, Sathyapalan, Majeed, Jamialahmadi et al., Potential effects of curcumin in the treatment of COVID-19 infection, Phytotherapy Research, doi:10.1002/ptr.6738
DOI record:
{
"DOI": "10.1002/ptr.7004",
"ISSN": [
"0951-418X",
"1099-1573"
],
"URL": "http://dx.doi.org/10.1002/ptr.7004",
"abstract": "<jats:p>Curcumin is proposed as a potential treatment option for coronavirus disease‐19 (COVID‐19) by inhibiting the virus entrance, encapsulation and replication, and modulating various cellular signaling pathways. In this open‐label nonrandomized clinical trial, efficacy of nano‐curcumin oral formulation has been evaluated in hospitalized patients with mild–moderate COVID‐19. Forty‐one patients who fulfilled the inclusion criteria were allocated to nano‐curcumin (<jats:italic>n</jats:italic> = 21) group (Sinacurcumin soft gel, contains 40 mg curcuminoids as nanomicelles, two capsules twice a day) or control (<jats:italic>n</jats:italic> = 20) group, for 2 weeks. Patients' symptoms and laboratory data were assessed at baseline and during follow‐up period. Most of symptoms including fever and chills, tachypnea, myalgia, and cough resolved significantly faster in curcumin group. Moreover, SaO<jats:sub>2</jats:sub> was significantly higher in treatment group after 2, 4, 7, and 14 days of follow‐up and lymphocyte count after 7 and 14 days. Duration of supplemental O<jats:sub>2</jats:sub> use and hospitalization was also meaningfully shorter in treatment group. It is also noteworthy to mention that no patient in treatment group experienced deterioration of infection during follow‐up period, but it occurred in 40% of control group. Oral curcumin nano‐formulation can significantly improve recovery time in hospitalized COVID‐19 patients. Further randomized placebo controlled trials with larger sample size are recommended.</jats:p>",
"alternative-id": [
"10.1002/ptr.7004"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2020-08-18"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2020-12-14"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-01-03"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Clinical Pharmacy, School of Pharmacy Mashhad University of Medical Sciences Mashhad Iran"
}
],
"family": "Saber‐Moghaddam",
"given": "Niloofar",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Clinical Pharmacy, School of Pharmacy Mashhad University of Medical Sciences Mashhad Iran"
}
],
"family": "Salari",
"given": "Soofia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rheumatic Diseases Research Center, School of Medicine Mashhad University of Medical Sciences Mashhad Iran"
}
],
"family": "Hejazi",
"given": "Sepideh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Internal Medicine Mashhad University of Medical Sciences Mashhad Iran"
}
],
"family": "Amini",
"given": "Mahnaz",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7185-970X",
"affiliation": [
{
"name": "Targeted Drug Delivery Research Center, School of Pharmacy Mashhad University of Medical Sciences Mashhad Iran"
}
],
"authenticated-orcid": false,
"family": "Taherzadeh",
"given": "Zhila",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medical Informatics, Faculty of Medicine Mashhad University of Medical Sciences Mashhad Iran"
}
],
"family": "Eslami",
"given": "Saeed",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmacology, School of Medicine Tehran University of Medical Sciences Tehran Iran"
}
],
"family": "Rezayat",
"given": "Seyed Mahdi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Nanotechnology Research Center Pharmaceutical Technology Institute, Mashhad University of Medical Sciences Mashhad Iran"
},
{
"name": "Department of Pharmaceutical Nanotechnology, School of Pharmacy Mashhad University of Medical Sciences Mashhad Iran"
}
],
"family": "Jaafari",
"given": "Mahmoud Reza",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9857-1175",
"affiliation": [
{
"name": "Department of Clinical Pharmacy, School of Pharmacy Mashhad University of Medical Sciences Mashhad Iran"
}
],
"authenticated-orcid": false,
"family": "Elyasi",
"given": "Sepideh",
"sequence": "additional"
}
],
"container-title": "Phytotherapy Research",
"container-title-short": "Phytotherapy Research",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2021,
1,
3
]
],
"date-time": "2021-01-03T23:05:08Z",
"timestamp": 1609715108000
},
"deposited": {
"date-parts": [
[
2023,
9,
2
]
],
"date-time": "2023-09-02T02:27:24Z",
"timestamp": 1693621644000
},
"indexed": {
"date-parts": [
[
2024,
3,
23
]
],
"date-time": "2024-03-23T10:58:36Z",
"timestamp": 1711191516750
},
"is-referenced-by-count": 80,
"issue": "5",
"issued": {
"date-parts": [
[
2021,
1,
3
]
]
},
"journal-issue": {
"issue": "5",
"published-print": {
"date-parts": [
[
2021,
5
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
1,
3
]
],
"date-time": "2021-01-03T00:00:00Z",
"timestamp": 1609632000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7004",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/ptr.7004",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7004",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "2616-2623",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2021,
1,
3
]
]
},
"published-online": {
"date-parts": [
[
2021,
1,
3
]
]
},
"published-print": {
"date-parts": [
[
2021,
5
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1002/jcp.25778",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_2_1"
},
{
"DOI": "10.1002/ptr.6745",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_3_1"
},
{
"DOI": "10.1016/j.fct.2020.111699",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_4_1"
},
{
"DOI": "10.1371/journal.pone.0057285",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_5_1"
},
{
"DOI": "10.1371/journal.pone.0134982",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_6_1"
},
{
"DOI": "10.1089/ars.2005.7.32",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_7_1"
},
{
"DOI": "10.1111/j.1440-1681.2011.05648.x",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_8_1"
},
{
"article-title": "Novel nanomicelle formulation to enhance bioavailability and stability of curcuminoids",
"author": "Hatamipour M.",
"first-page": "282",
"issue": "3",
"journal-title": "Iranian Journal of Basic Medical Sciences",
"key": "e_1_2_8_9_1",
"volume": "22",
"year": "2019"
},
{
"DOI": "10.1161/CIRCULATIONAHA.108.768986",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_10_1"
},
{
"DOI": "10.1186/1475-2891-13-11",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_11_1"
},
{
"DOI": "10.1089/ars.2008.2140",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_12_1"
},
{
"DOI": "10.1016/j.intimp.2013.01.003",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_13_1"
},
{
"DOI": "10.1111/apt.15916",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_14_1"
},
{
"DOI": "10.1007/s12072-020-10071-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_15_1"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_16_1"
},
{
"DOI": "10.3390/molecules25030689",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_17_1"
},
{
"DOI": "10.1080/10408398.2017.1358139",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_18_1"
},
{
"DOI": "10.1016/j.antiviral.2017.03.014",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_19_1"
},
{
"DOI": "10.1021/acs.jmedchem.6b00975",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_20_1"
},
{
"DOI": "10.1007/s10900-020-00920-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_21_1"
},
{
"DOI": "10.3389/fmicb.2019.00912",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_22_1"
},
{
"DOI": "10.1039/C5FO00485C",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_23_1"
},
{
"DOI": "10.1152/ajpgi.90428.2008",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_24_1"
},
{
"DOI": "10.1016/S0140-6736(20)30304-4",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_25_1"
},
{
"DOI": "10.1016/j.tmaid.2020.101623",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_26_1"
},
{
"DOI": "10.1002/ptr.6766",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_27_1"
},
{
"DOI": "10.1007/s00134-020-05991-x",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_28_1"
},
{
"DOI": "10.1097/CCM.0000000000001402",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_29_1"
},
{
"DOI": "10.1177/1074248413503044",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_30_1"
},
{
"article-title": "Revealing the potency of citrus and galangal constituents to Halt SARS‐CoV‐2 infection",
"author": "Utomo R. Y.",
"first-page": "2020030214",
"journal-title": "Preprints",
"key": "e_1_2_8_31_1",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30185-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_32_1"
},
{
"DOI": "10.1177/0962280215588241",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_33_1"
},
{
"DOI": "10.1002/ptr.6738",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_34_1"
},
{
"DOI": "10.1155/2014/186864",
"doi-asserted-by": "publisher",
"key": "e_1_2_8_35_1"
}
],
"reference-count": 34,
"references-count": 34,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/ptr.7004"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology"
],
"subtitle": [],
"title": "Oral nano‐curcumin formulation efficacy in management of mild to moderate hospitalized <scp>coronavirus disease</scp>‐19 patients: An open label nonrandomized clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "35"
}
