Oral Curcumin With Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial
et al., Frontiers in Pharmacology, doi:10.3389/fphar.2021.669362, CTRI/2020/05/025482, May 2021
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 140 patients, 70 treated with curcumin and piperine (for absorption), and 70 treated with probiotics, showing faster recovery, lower progression, and lower mortality with curcumin.
This is the 3rd of 21 COVID-19 RCTs for curcumin, which collectively show efficacy with p=0.0000022.
This is the 6th of 28 COVID-19 controlled studies for curcumin, which collectively show efficacy with p=0.0000000061.
|
risk of death, 81.8% lower, RR 0.18, p = 0.02, treatment 2 of 70 (2.9%), control 11 of 70 (15.7%), NNT 7.8.
|
|
risk of death, 60.0% lower, RR 0.40, p = 0.39, treatment 2 of 15 (13.3%), control 5 of 15 (33.3%), NNT 5.0, severe group.
|
|
risk of death, 90.9% lower, RR 0.09, p = 0.05, treatment 0 of 25 (0.0%), control 5 of 25 (20.0%), NNT 5.0, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), moderate group.
|
|
risk of death, 66.7% lower, RR 0.33, p = 1.00, treatment 0 of 30 (0.0%), control 1 of 30 (3.3%), NNT 30, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm), mild group.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Pawar et al., 28 May 2021, Double Blind Randomized Controlled Trial, India, peer-reviewed, 8 authors, study period July 2020 - September 2020, this trial compares with another treatment - results may be better when compared to placebo, trial CTRI/2020/05/025482.
Oral Curcumin With Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial
Frontiers in Pharmacology, doi:10.3389/fphar.2021.669362
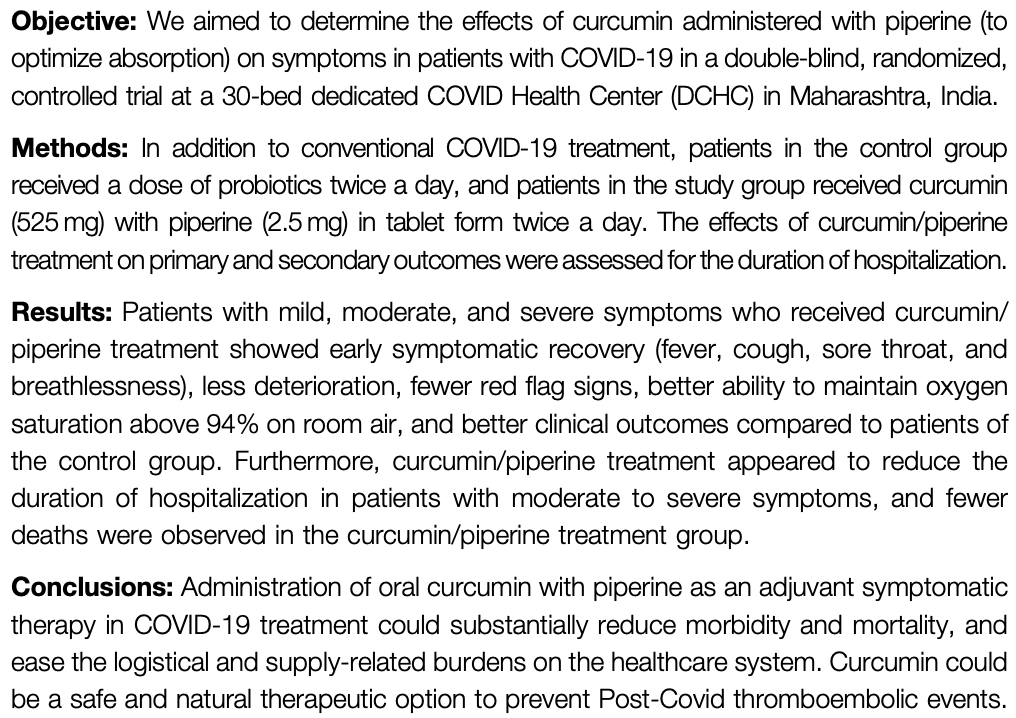
Background: Coronavirus disease-2019 (COVID-19) has a wide range of pathophysiological effects. Curcumin, an active constituent of Curcuma longa (turmeric), has several properties, including anti-inflammatory, antioxidant, antiviral, antithrombotic, and anti-proliferative effects, which make it a promising candidate for the symptomatic treatment of COVID-19. Objective: We aimed to determine the effects of curcumin administered with piperine (to optimize absorption) on symptoms in patients with COVID-19 in a double-blind, randomized, controlled trial at a 30-bed dedicated COVID Health Center (DCHC) in Maharashtra, India. Methods: In addition to conventional COVID-19 treatment, patients in the control group received a dose of probiotics twice a day, and patients in the study group received curcumin (525 mg) with piperine (2.5 mg) in tablet form twice a day. The effects of curcumin/piperine treatment on primary and secondary outcomes were assessed for the duration of hospitalization. Results: Patients with mild, moderate, and severe symptoms who received curcumin/ piperine treatment showed early symptomatic recovery (fever, cough, sore throat, and breathlessness), less deterioration, fewer red flag signs, better ability to maintain oxygen saturation above 94% on room air, and better clinical outcomes compared to patients of the control group. Furthermore, curcumin/piperine treatment appeared to reduce the duration of hospitalization in patients with moderate to severe symptoms, and fewer deaths were observed in the curcumin/piperine treatment group. Conclusions: Administration of oral curcumin with piperine as an adjuvant symptomatic therapy in COVID-19 treatment could substantially reduce morbidity and mortality, and ease the logistical and supply-related burdens on the healthcare system. Curcumin could be a safe and natural therapeutic option to prevent Post-Covid thromboembolic events.
ETHICS STATEMENT The studies involving human participants were reviewed and approved by The Royal Pune Independent Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
AUTHOR CONTRIBUTIONS KP contributed to study design, data interpretation, writing of the manuscript, and literature search; RM, SKP, and SSP contributed to data collection and literature search. RhB and RmB performed the literature search; and MK and AD performed the data analysis.
SUPPLEMENTARY MATERIAL The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.669362/ full#supplementary-material Conflict of Interest: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Aggarwal, Harikumar, Potential Therapeutic Effects of Curcumin, the Anti-inflammatory Agent, against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases, Int. J. Biochem. Cel Biol, doi:10.1016/j.biocel.2008.06.010
Anand, Kunnumakkara, Newman, Aggarwal, Bioavailability of Curcumin: Problems and Promises, Mol. Pharmaceutics, doi:10.1021/mp700113r
Biswas, Rahman, Modulation of Steroid Activity in Chronic Inflammation: a Novel Anti-inflammatory Role for Curcumin, Mol. Nutr. Food Res, doi:10.1002/mnfr.200700259
Conti, Caraffa, Gallenga, Ross, Kritas et al., IL-1 Induces Throboxane-A2 (TxA2) in COVID-19 Causing Inflammation and Micro-thrombi: Inhibitory Effect of the IL-1 Receptor Antagonist
Curcumin, Thrombosis, and Coagulation, J. Cel Physiol, doi:10.1002/jcp.26249
De Almeida Alvarenga, Leal, Borges, Silva De Aguiar, Faxén-Irving et al., Curcumin -A Promising Nutritional Strategy for Chronic Kidney Disease Patients, J. Funct. Foods, doi:10.1016/j.jff.2017.12.015
Ghosh, Gehr, Ghosh, Curcumin and Chronic Kidney Disease (CKD): Major Mode of Action through Stimulating Endogenous Intestinal Alkaline Phosphatase, Molecules, doi:10.3390/molecules191220139
Gupta, Patchva, Aggarwal, Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials, Aaps J, doi:10.1208/s12248-012-9432-8
Han, The Effects of Black Pepper on the Intestinal Absorption and Hepatic Metabolism of Drugs, Expert Opin. Drug Metab. Toxicol, doi:10.1517/17425255.2011.570332
Hewlings, Kalman, Curcumin: A Review of its Effects on Human Health, Foods, doi:10.3390/foods6100092
Huang, Wang, Li, Ren, Zhao et al., Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China, The Lancet, doi:10.1016/S0140-6736(20)30183-5
Keihanian, Saeidinia, Bagheri, Johnston, Sahebkar, None
Kim, Ku, Bae, Anticoagulant Activities of Curcumin and its Derivative, BMB Rep, doi:10.5483/bmbrep.2012.45.4.221
Leong, The Spice for Hypertension: Protective Role of Curcuma Longa, Biomed. Pharmacol. J, doi:10.13005/bpj/1555
Liu, Ying, The Inhibitory Effect of Curcumin on Virus-Induced Cytokine Storm and its Potential Use in the Associated Severe Pneumonia, Front Cel Dev Biol, doi:10.3389/fcell.2020.00479
Loeber, Buechner, Dissertatio Inauguralis Medica de Curcuma Officinarum Ejusque Genuinis Virtutibus
Maurya, Kumar, Prasad, Bhatt, Saxena, Structure-based Drug Designing for Potential Antiviral Activity of Selected Natural Products from Ayurveda against SARS-CoV-2 Spike Glycoprotein and its Cellular Receptor, VirusDis, doi:10.1007/s13337-020-00598-8
Mollazadeh, Cicero, Blesso, Pirro, Majeed et al., Immune Modulation by Curcumin: The Role of Interleukin-10, Crit. Rev. Food Sci. Nutr, doi:10.1080/10408398.2017.1358139
Moriyuki, Sekiguchi, Matsubara, Nishikawa, Kawabata, Curcumin Inhibits the Proteinase-Activated Receptor-2-Triggered Prostaglandin E2 Production by Suppressing Cyclooxygenase-2 Upregulation and Akt-dependent Activation of Nuclear Factor-Κb in Human Lung Epithelial Cells, J. Pharmacol. Sci, doi:10.1254/jphs.10126sc
Oppenheimer, Turmeric (Curcumin) in Biliary Diseases, The Lancet, doi:10.1016/s0140-6736(00)98193-5
Ovbiagele, Potential Role of Curcumin in Stroke Prevention, Expert Rev. Neurotherapeutics, doi:10.1586/14737175.8.8.1175
Poolsup, Suksomboon, Kurnianta, Deawjaroen, Effects of Curcumin on Glycemic Control and Lipid Profile in Prediabetes and Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis, PloS one, doi:10.1371/journal.pone.0215840
Ravindran, Prasad, Aggarwal, Curcumin and Cancer Cells: How many Ways Can Curry Kill Tumor Cells Selectively?, AAPS J, doi:10.1208/s12248-009-9128-x
Salehi, Stojanović-Radić, Matejić, Sharifi-Rad, Anil Kumar et al., The Therapeutic Potential of Curcumin: A Review of Clinical Trials, Eur. J. Med. Chem, doi:10.1016/j.ejmech.2018.12.016
Shanmugam, Rane, Kanchi, Arfuso, Chinnathambi et al., The Multifaceted Role of Curcumin in Cancer Prevention and Treatment, Molecules, doi:10.3390/molecules20022728
Sharma, Chopra, Kulkarni, Agrewala, Resveratrol and Curcumin Suppress Immune Response through CD28/CTLA-4 and CD80 Costimulatory Pathway, Clin. Exp. Immunol, doi:10.1111/j.1365-2249.2006.03257.x
Sharma, Nehru, Munshi, Jyothy, Antioxidant Potential of Curcumin against Oxidative Insult Induced by Pentylenetetrazol in Epileptic Rats, Methods Find Exp. Clin. Pharmacol, doi:10.1358/mf.2010.32.4.1452090
Shoba, Joy, Joseph, Majeed, Rajendran et al., Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers, Planta Med, doi:10.1055/s-2006-957450
Singh, Potential Role of Curcumin against Viral Infections with a View on Structure and Pathogenesis of COVID-19, AIJR Preprints, doi:10.21467/preprints.213
Suresh, Curcumin and Coagulopathy in the COVID19 Era, Ind. J. Clin. Biochem, doi:10.1007/s12291-020-00914-5
Suresh, Srinivasan, Tissue Distribution & Elimination of Capsaicin, Piperine & Curcumin Following Oral Intake in Rats, Indian J. Med. Res
Toden, Goel, The Holy Grail of Curcumin and its Efficacy in Various Diseases: Is Bioavailability Truly a Big Concern?, J Restorat Med, doi:10.14200/jrm.2017.6.0101
Venkatesan, Punithavathi, Babu, Protection from Acute and Chronic Lung Diseases by Curcumin, Adv. Exp. Med. Biol, doi:10.1007/978-0-387-46401-5_17
Vogel, Pelletier, Examen chimique de la racine de Curcuma, J. Pharm
Wongcharoen, Phrommintikul, The Protective Role of Curcumin in Cardiovascular Diseases, Int. J. Cardiol, doi:10.1016/j.ijcard.2009.01.073
Zahedipour, Hosseini, Sathyapalan, Majeed, Jamialahmadi et al., Potential Effects of Curcumin in the Treatment of COVID -19 Infection, Phytotherapy Res, doi:10.1002/ptr.6738
Zhang, -W, Fu, Gao, Liu, Curcumin and Diabetes: a Systematic Review. Evidence-Based Complement, Altern. Med, doi:10.1155/2013/636053
DOI record:
{
"DOI": "10.3389/fphar.2021.669362",
"ISSN": [
"1663-9812"
],
"URL": "http://dx.doi.org/10.3389/fphar.2021.669362",
"abstract": "<jats:p><jats:bold>Background:</jats:bold> Coronavirus disease-2019 (COVID-19) has a wide range of pathophysiological effects. Curcumin, an active constituent of Curcuma longa (turmeric), has several properties, including anti-inflammatory, antioxidant, antiviral, anti-thrombotic, and anti-proliferative effects, which make it a promising candidate for the symptomatic treatment of COVID-19.</jats:p><jats:p><jats:bold>Objective:</jats:bold> We aimed to determine the effects of curcumin administered with piperine (to optimize absorption) on symptoms in patients with COVID-19 in a double-blind, randomized, controlled trial at a 30-bed dedicated COVID Health Center (DCHC) in Maharashtra, India.</jats:p><jats:p><jats:bold>Methods:</jats:bold> In addition to conventional COVID-19 treatment, patients in the control group received a dose of probiotics twice a day, and patients in the study group received curcumin (525 mg) with piperine (2.5 mg) in tablet form twice a day. The effects of curcumin/piperine treatment on primary and secondary outcomes were assessed for the duration of hospitalization.</jats:p><jats:p><jats:bold>Results:</jats:bold> Patients with mild, moderate, and severe symptoms who received curcumin/piperine treatment showed early symptomatic recovery (fever, cough, sore throat, and breathlessness), less deterioration, fewer red flag signs, better ability to maintain oxygen saturation above 94% on room air, and better clinical outcomes compared to patients of the control group. Furthermore, curcumin/piperine treatment appeared to reduce the duration of hospitalization in patients with moderate to severe symptoms, and fewer deaths were observed in the curcumin/piperine treatment group.</jats:p><jats:p><jats:bold>Conclusions:</jats:bold> Administration of oral curcumin with piperine as an adjuvant symptomatic therapy in COVID-19 treatment could substantially reduce morbidity and mortality, and ease the logistical and supply-related burdens on the healthcare system. Curcumin could be a safe and natural therapeutic option to prevent Post-Covid thromboembolic events.</jats:p><jats:p><jats:bold>Clinicaltrials.gov identifier:</jats:bold><jats:ext-link>CTRI/2020/05/025482</jats:ext-link></jats:p>",
"alternative-id": [
"10.3389/fphar.2021.669362"
],
"author": [
{
"affiliation": [],
"family": "Pawar",
"given": "Kirti S",
"sequence": "first"
},
{
"affiliation": [],
"family": "Mastud",
"given": "Rahul N",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pawar",
"given": "Satheesh K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pawar",
"given": "Samragni S",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhoite",
"given": "Rahul R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bhoite",
"given": "Ramesh R",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kulkarni",
"given": "Meenal V",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deshpande",
"given": "Aditi R",
"sequence": "additional"
}
],
"container-title": "Frontiers in Pharmacology",
"container-title-short": "Front. Pharmacol.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2021,
5,
28
]
],
"date-time": "2021-05-28T07:58:34Z",
"timestamp": 1622188714000
},
"deposited": {
"date-parts": [
[
2021,
5,
28
]
],
"date-time": "2021-05-28T07:58:40Z",
"timestamp": 1622188720000
},
"indexed": {
"date-parts": [
[
2024,
4,
8
]
],
"date-time": "2024-04-08T09:14:36Z",
"timestamp": 1712567676758
},
"is-referenced-by-count": 105,
"issued": {
"date-parts": [
[
2021,
5,
28
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
5,
28
]
],
"date-time": "2021-05-28T00:00:00Z",
"timestamp": 1622160000000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2021.669362/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2021,
5,
28
]
]
},
"published-online": {
"date-parts": [
[
2021,
5,
28
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1016/j.biocel.2008.06.010",
"article-title": "Potential Therapeutic Effects of Curcumin, the Anti-inflammatory Agent, against Neurodegenerative, Cardiovascular, Pulmonary, Metabolic, Autoimmune and Neoplastic Diseases",
"author": "Aggarwal",
"doi-asserted-by": "publisher",
"first-page": "40",
"journal-title": "Int. J. Biochem. Cel Biol.",
"key": "B1",
"volume": "41",
"year": "2009"
},
{
"DOI": "10.1021/mp700113r",
"article-title": "Bioavailability of Curcumin: Problems and Promises",
"author": "Anand",
"doi-asserted-by": "publisher",
"first-page": "807",
"journal-title": "Mol. Pharmaceutics",
"key": "B2",
"volume": "4",
"year": "2007"
},
{
"DOI": "10.1002/mnfr.200700259",
"article-title": "Modulation of Steroid Activity in Chronic Inflammation: a Novel Anti-inflammatory Role for Curcumin",
"author": "Biswas",
"doi-asserted-by": "publisher",
"first-page": "987",
"journal-title": "Mol. Nutr. Food Res.",
"key": "B3",
"volume": "52",
"year": "2008"
},
{
"DOI": "10.23812/20-34-4EDIT-65",
"article-title": "IL-1 Induces Throboxane-A2 (TxA2) in COVID-19 Causing Inflammation and Micro-thrombi: Inhibitory Effect of the IL-1 Receptor Antagonist (IL-1Ra)",
"author": "Conti",
"doi-asserted-by": "publisher",
"first-page": "1623",
"journal-title": "J. Biol. Regul. Homeost Agents",
"key": "B4",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1016/j.jff.2017.12.015",
"article-title": "Curcumin - A Promising Nutritional Strategy for Chronic Kidney Disease Patients",
"author": "de Almeida Alvarenga",
"doi-asserted-by": "publisher",
"first-page": "715",
"journal-title": "J. Funct. Foods",
"key": "B5",
"volume": "40",
"year": "2018"
},
{
"DOI": "10.3390/molecules191220139",
"article-title": "Curcumin and Chronic Kidney Disease (CKD): Major Mode of Action through Stimulating Endogenous Intestinal Alkaline Phosphatase",
"author": "Ghosh",
"doi-asserted-by": "publisher",
"first-page": "20139",
"journal-title": "Molecules",
"key": "B6",
"volume": "19",
"year": "2014"
},
{
"DOI": "10.1208/s12248-012-9432-8",
"article-title": "Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials",
"author": "Gupta",
"doi-asserted-by": "publisher",
"first-page": "195",
"journal-title": "Aaps J.",
"key": "B7",
"volume": "15",
"year": "2013"
},
{
"DOI": "10.1517/17425255.2011.570332",
"article-title": "The Effects of Black Pepper on the Intestinal Absorption and Hepatic Metabolism of Drugs",
"author": "Han",
"doi-asserted-by": "publisher",
"first-page": "721",
"journal-title": "Expert Opin. Drug Metab. Toxicol.",
"key": "B8",
"volume": "7",
"year": "2011"
},
{
"DOI": "10.3390/foods6100092",
"article-title": "Curcumin: A Review of its Effects on Human Health",
"author": "Hewlings",
"doi-asserted-by": "publisher",
"first-page": "92",
"journal-title": "Foods",
"key": "B9",
"volume": "6",
"year": "2017"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"article-title": "Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"journal-title": "The Lancet",
"key": "B10",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1002/jcp.26249",
"article-title": "Curcumin, Hemostasis, Thrombosis, and Coagulation",
"author": "Keihanian",
"doi-asserted-by": "publisher",
"first-page": "4497",
"journal-title": "J. Cel Physiol",
"key": "B11",
"volume": "233",
"year": "2018"
},
{
"DOI": "10.5483/bmbrep.2012.45.4.221",
"article-title": "Anticoagulant Activities of Curcumin and its Derivative",
"author": "Kim",
"doi-asserted-by": "publisher",
"first-page": "221",
"journal-title": "BMB Rep.",
"key": "B12",
"volume": "45",
"year": "2012"
},
{
"DOI": "10.13005/bpj/1555",
"article-title": "The Spice for Hypertension: Protective Role of Curcuma Longa",
"author": "Leong",
"doi-asserted-by": "publisher",
"first-page": "1829",
"journal-title": "Biomed. Pharmacol. J.",
"key": "B13",
"volume": "11",
"year": "2018"
},
{
"DOI": "10.3389/fcell.2020.00479",
"article-title": "The Inhibitory Effect of Curcumin on Virus-Induced Cytokine Storm and its Potential Use in the Associated Severe Pneumonia",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "479",
"journal-title": "Front Cel Dev Biol",
"key": "B14",
"volume": "8",
"year": "2020"
},
{
"article-title": "Dissertatio Inauguralis Medica de Curcuma Officinarum Ejusque Genuinis Virtutibus",
"author": "Loeber",
"first-page": "28",
"key": "B15",
"year": "1748"
},
{
"DOI": "10.1007/s13337-020-00598-8",
"article-title": "Structure-based Drug Designing for Potential Antiviral Activity of Selected Natural Products from Ayurveda against SARS-CoV-2 Spike Glycoprotein and its Cellular Receptor",
"author": "Maurya",
"doi-asserted-by": "publisher",
"first-page": "179",
"journal-title": "VirusDis.",
"key": "B16",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1080/10408398.2017.1358139",
"article-title": "Immune Modulation by Curcumin: The Role of Interleukin-10",
"author": "Mollazadeh",
"doi-asserted-by": "publisher",
"first-page": "89",
"journal-title": "Crit. Rev. Food Sci. Nutr.",
"key": "B17",
"volume": "59",
"year": "2019"
},
{
"DOI": "10.1254/jphs.10126sc",
"article-title": "Curcumin Inhibits the Proteinase-Activated Receptor-2-Triggered Prostaglandin E2 Production by Suppressing Cyclooxygenase-2 Upregulation and Akt-dependent Activation of Nuclear Factor-Κb in Human Lung Epithelial Cells",
"author": "Moriyuki",
"doi-asserted-by": "publisher",
"first-page": "225",
"journal-title": "J. Pharmacol. Sci.",
"key": "B18",
"volume": "114",
"year": "2010"
},
{
"DOI": "10.1016/s0140-6736(00)98193-5",
"article-title": "Turmeric (Curcumin) in Biliary Diseases",
"author": "Oppenheimer",
"doi-asserted-by": "publisher",
"first-page": "619",
"journal-title": "The Lancet",
"key": "B19",
"volume": "229",
"year": "1937"
},
{
"DOI": "10.1586/14737175.8.8.1175",
"article-title": "Potential Role of Curcumin in Stroke Prevention",
"author": "Ovbiagele",
"doi-asserted-by": "publisher",
"first-page": "1175",
"journal-title": "Expert Rev. Neurotherapeutics",
"key": "B20",
"volume": "8",
"year": "2008"
},
{
"DOI": "10.1371/journal.pone.0215840",
"article-title": "Effects of Curcumin on Glycemic Control and Lipid Profile in Prediabetes and Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis",
"author": "Poolsup",
"doi-asserted-by": "publisher",
"first-page": "e0215840",
"journal-title": "PloS one",
"key": "B21",
"volume": "14",
"year": "2019"
},
{
"DOI": "10.1208/s12248-009-9128-x",
"article-title": "Curcumin and Cancer Cells: How many Ways Can Curry Kill Tumor Cells Selectively?",
"author": "Ravindran",
"doi-asserted-by": "publisher",
"first-page": "495",
"journal-title": "AAPS J.",
"key": "B22",
"volume": "11",
"year": "2009"
},
{
"DOI": "10.1016/j.ejmech.2018.12.016",
"article-title": "The Therapeutic Potential of Curcumin: A Review of Clinical Trials",
"author": "Salehi",
"doi-asserted-by": "publisher",
"first-page": "527",
"journal-title": "Eur. J. Med. Chem.",
"key": "B23",
"volume": "163",
"year": "2019"
},
{
"DOI": "10.3390/molecules20022728",
"article-title": "The Multifaceted Role of Curcumin in Cancer Prevention and Treatment",
"author": "Shanmugam",
"doi-asserted-by": "publisher",
"first-page": "2728",
"journal-title": "Molecules",
"key": "B24",
"volume": "20",
"year": "2015"
},
{
"DOI": "10.1111/j.1365-2249.2006.03257.x",
"article-title": "Resveratrol and Curcumin Suppress Immune Response through CD28/CTLA-4 and CD80 Co-stimulatory Pathway",
"author": "Sharma",
"doi-asserted-by": "publisher",
"first-page": "155",
"journal-title": "Clin. Exp. Immunol.",
"key": "B25",
"volume": "147",
"year": "2007"
},
{
"DOI": "10.1358/mf.2010.32.4.1452090",
"article-title": "Antioxidant Potential of Curcumin against Oxidative Insult Induced by Pentylenetetrazol in Epileptic Rats",
"author": "Sharma",
"doi-asserted-by": "publisher",
"first-page": "227",
"journal-title": "Methods Find Exp. Clin. Pharmacol.",
"key": "B26",
"volume": "32",
"year": "2010"
},
{
"DOI": "10.1055/s-2006-957450",
"article-title": "Influence of Piperine on the Pharmacokinetics of Curcumin in Animals and Human Volunteers",
"author": "Shoba",
"doi-asserted-by": "publisher",
"first-page": "353",
"journal-title": "Planta Med.",
"key": "B27",
"volume": "64",
"year": "1998"
},
{
"DOI": "10.21467/preprints.213",
"article-title": "Potential Role of Curcumin against Viral Infections with a View on Structure and Pathogenesis of COVID-19",
"author": "Singh",
"doi-asserted-by": "publisher",
"journal-title": "AIJR Preprints",
"key": "B28",
"year": "2020"
},
{
"article-title": "Tissue Distribution & Elimination of Capsaicin, Piperine & Curcumin Following Oral Intake in Rats",
"author": "Suresh",
"first-page": "682",
"journal-title": "Indian J. Med. Res.",
"key": "B29",
"volume": "131",
"year": "2010"
},
{
"DOI": "10.1007/s12291-020-00914-5",
"article-title": "Curcumin and Coagulopathy in the COVID19 Era",
"author": "Suresh",
"doi-asserted-by": "publisher",
"first-page": "504",
"journal-title": "Ind. J. Clin. Biochem.",
"key": "B30",
"volume": "35",
"year": "2020"
},
{
"DOI": "10.14200/jrm.2017.6.0101",
"article-title": "The Holy Grail of Curcumin and its Efficacy in Various Diseases: Is Bioavailability Truly a Big Concern?",
"author": "Toden",
"doi-asserted-by": "publisher",
"first-page": "27",
"journal-title": "J Restorat Med.",
"key": "B31",
"volume": "6",
"year": "2017"
},
{
"DOI": "10.1007/978-0-387-46401-5_17",
"article-title": "Protection from Acute and Chronic Lung Diseases by Curcumin",
"author": "Venkatesan",
"doi-asserted-by": "publisher",
"first-page": "379",
"journal-title": "Adv. Exp. Med. Biol.",
"key": "B32",
"volume": "595",
"year": "2007"
},
{
"article-title": "Examen chimique de la racine de Curcuma",
"author": "Vogel",
"first-page": "289",
"journal-title": "J. Pharm.",
"key": "B33",
"volume": "1",
"year": "1815"
},
{
"DOI": "10.1016/j.ijcard.2009.01.073",
"article-title": "The Protective Role of Curcumin in Cardiovascular Diseases",
"author": "Wongcharoen",
"doi-asserted-by": "publisher",
"first-page": "145",
"journal-title": "Int. J. Cardiol.",
"key": "B34",
"volume": "133",
"year": "2009"
},
{
"DOI": "10.1002/ptr.6738",
"article-title": "Potential Effects of Curcumin in the Treatment of COVID ‐19 Infection",
"author": "Zahedipour",
"doi-asserted-by": "publisher",
"first-page": "2911",
"journal-title": "Phytotherapy Res.",
"key": "B35",
"volume": "34",
"year": "2020"
},
{
"DOI": "10.1155/2013/636053",
"article-title": "Curcumin and Diabetes: a Systematic Review",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Evidence-Based Complement. Altern. Med.",
"key": "B36",
"volume": "2013",
"year": "2013"
}
],
"reference-count": 36,
"references-count": 36,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fphar.2021.669362/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": "Oral Curcumin With Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "12"
}
